Abstract
Studies of feeding grounds are vital to understanding the ecology and conservation issues of sea turtles. The hypersaline, lake Bardawil, of North Sinai has recently been recognized as being a major feeding ground for sea turtles in the Mediterranean Sea. The objective of this research was to examine if the environmental variables (salinity, dissolved oxygen, and depth), distance to nearest Mediterranean inlet, and food availability (zooplankton and phytoplankton density) differed between areas according to species richness (green (Chelonia mydas) and loggerhead (Caretta caretta) turtles, single species, no species) and between areas in which loggerhead and green turtles were observed and not observed. Our results highlight the importance of environmental factors determining the distribution of endangered sea turtles as areas with high sea turtle richness had lower salinity, higher dissolved oxygen, were deeper and located closer to the nearest Mediterranean inlet. Our results support the suggestion that since the creation of the man-made inlets from the Mediterranean Sea, the environmental conditions of the hypersaline lake Bardawil have become less severe and more suitable for sea turtles as a feeding ground. The conservation of this biologically valuable lake will require active management to protect it from the increasing anthropogenic threats that will encroach upon the lake in the next decade.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Studies of sea turtle feeding grounds are vital to understanding the ecology and conservation issues of sea turtles. Sea turtles have high site fidelity and spend the majority of their life in feeding grounds (Seminoff et al. 2003; Senko et al. 2010; Mancini et al. 2015). Feeding grounds often occur in shallow, near shore or shoreline coastal areas, which are heavily threatened by human activity such as fishing, recreational activity, chemical and marine debris pollution, and physical habitat degradation (Groombridge and Luxmoore 1989). Studying the ecology of sea turtles in their feeding grounds often requires significant time and resources to overcome logistic challenges such as observing or capturing sea turtles underwater and locating turtles in geographically large areas. Most studies of sea turtle feeding grounds focus on sea turtle movement patterns, diving behavior, diet, population composition, and stranding rates while few examine environmental conditions associated with sea turtle distribution within a feeding ground (Houghton et al. 2003; Schofield et al. 2007; Arthur et al. 2008; Hazel et al. 2009; Senko et al. 2010; Kock et al. 2013).
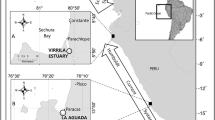
The Mediterranean coastline of Egypt and more specifically, lake Bardawil, of North Sinai have recently been recognized as being a major feeding ground and possible rookery for sea turtles in the Mediterranean Sea (Nada et al. 2013; Rabia and Attum 2015; Bradshaw et al. 2017). For example, Bradshaw et al. 2017, found that that majority of post-nesting green turtles (Chelonia mydas) in the northeastern Mediterranean basin originated from the North Sinai feeding grounds and that these females showed high feeding site fidelity. There is a concern regarding the conservation of sea turtles in lake Bardawil given high reported mortalities, possibly as a result of hypersaline conditions or by-catch from local fisherman (Nada et al. 2013). However, the population size of sea turtles in lake Bardawil may be increasing as a result of increased protection, habitat quality and food availability given the recent increasing number of female sea turtles nesting in the Mediterranean Sea (Bradshaw et al. 2017). Two man-made channels (Boghaz 1 and 2) in lake Bardawil (Fig. 1) were built to lessen the environmental severity of the very shallow, warm, and hypersaline lake in order to increase fisheries capacity by allowing water exchange with the Mediterranean Sea (Mehanna and Hegazi 2013). This water exchange has reduced maximum salinity and increased depth (Abd Ellah and Hussein 2009), while the sea grass Cymodocea nodosa, the primary dietary item of the green turtle within the Mediterranean, now dominates part of the lake (El-Bana et al. 2002; Cardona 2010; Abd Ellah and Hussein 2009). The decreases in salinity and increase in food availability in Lake Bardawil have been hypothesized to have created a new foraging ground for green (Bradshaw et al. 2017) and presumably loggerhead turtles.
The distribution of sea turtle richness, loggerhead, and green turtles in lake Bardawil. A. Distribution of sea turtle richness. The black circles = both sea turtle species (N = 37) grey circles = one sea turtle species (N = 39), and hollow circle = zero sea turtle species (N = 26). B. Distribution of loggerhead turtles. Black circles = loggerhead turtle observations (N = 70) and hollow circles = zero observations (N = 32). C. Distribution of green turtles. Black circles = green turtle observations (N = 38) and hollow circles = zero observations (N = 64)
Practically nothing is known regarding the population and ecology of sea turtles in the lake Bardawil. Loggerhead (Caretta caretta) and green turtles are known to inhabit lake Bardawil, while the sand bar that separates the lake from the Mediterranean Sea is an important nesting site for both species (Rabia and Attum 2015). Lake Bardawil is an important lake for the fisheries of the region and thus exists the potential for large scale mortality as a result of bycatch (Nada et al. 2013). Loggerhead and green turtles are the largest consumer and herbivore, respectively, in lake Bardawil and thus have an essential ecological and trophic role in the lake. For example, in typically nutrient poor hypersaline environments, sea turtles could play a vital role in nutrient cycling as occurs in other coastal feeding grounds (Moran and Bjorndal 2005, 2007). Despite the environmental severity, hypersaline lakes often demonstrate high biological productivity for reasons that are still unclear and thus are considered biologically valuable habitat (Shadrin et al. 2015).
Lake Bardawil is likely the most environmentally unique sea turtle feeding ground in the Mediterranean Sea. Lake Bardawil is a much smaller body of water and consists of the most extreme environmental conditions that sea turtles are known to occupy, as sea turtles typically occur in open and less saline bodies of water. Thus, lake Bardawil represents a convenient model to study the relationship between environmental conditions and sea turtle habitat use (Shadrin 2017).
The objective of this research was to examine if the mean value of the environmental variables (salinity, DO, and depth), distance to the nearest Mediterranean inlet, and food availability (zooplankton and phytoplankton density) differed between areas according to species richness (both species, single species, no species) and between areas in which loggerhead and green turtles were observed and not observed.
Methods
Lake Bardawil is located 35 km west of El Arish city and is considered among the least polluted lakes in Egypt. The extensive sea grass beds act as a nursery and spawning grounds of several commercially important fish species (Mehanna and Hegazi 2013). Lake Bardawil is entirely separated from the Mediterranean Sea by a long, narrow sand bar (width range 300 m – 2000 m) with the exception of a smaller, natural and seasonally open inlet and two man made channels (Boghaz 1 and 2) that allow water and organism exchange (Fig. 1). The environmental conditions in the lake are also different from the coastal Mediterranean Sea, with the lake being warmer, shallower (mean depth 1.2 m, maximum depth 6.5 m), and more saline (Krumgalz et al. 1981; Khalil et al. 2013, 2016; Anufriieva et al. 2018). The lake (600 km2) has a length of 90 km and an average width of 10 km (Anufriieva et al. 2018). Zaranik Protected Area (ZPA) protects the eastern corner of lake Bardawil. Vegetated coastal dunes and salt plains are the dominant mainland habitat surrounding the lake.
This study took place between August 5 to December 16, 2018. We created 68 random survey points in the lake using GIS. The minimum distance between the survey points was 800 m. We then navigated to these points on a boat and circled within a 50 m radius to survey for sea turtles for about 10 min. For each observation, we recorded the number and species. In addition, we recorded the location of opportunistic observations while travelling to survey points and while conducting other field work. We removed the opportunistic observations from the analysis if they were less than 500 m from any other observations. We then recorded post-hoc the environmental attributes, depth, salinity and dissolved oxygen (DO) by plotting our locations onto maps created by Khalil et al. (2016) and Abd Ellah and Hussein (2009). We created an interpolated map of phytoplankton and zooplankton density using the interpolated tool in ArcMap 10.8. The data interpolations were based on the data collected at 19 sampling points (Fouda et al. 1987). We considered plankton density as an indirect index of food availability. We believe that historic data collected locally data does a better job of capturing fine scale variation in the lake than satellite-based data. We were not able to include all possible environmental data in our analysis if locally collected data was unavailable.
We statistically examined if the environmental variables (salinity, dissolved oxygen, and depth), distance to nearest Mediterranean inlet, and food availability (zooplankton and phytoplankton density) differed between areas according to species richness (Green (Chelonia mydas) and loggerhead (Caretta caretta) turtles, single species, no species) and between areas in which loggerhead and green turtles were observed and not observed through separate multivariate analysis of variance (MANOVA). If the MANOVA was significant, we then examined the univariate analysis of variance (ANOVA) for each variable. We used post-hoc Tukey contrasts to examine if the mean value differed between the levels of species richness.
Results
Loggerhead turtles were the most observed species with 321 observations (77%) compared to the 96 observations of green turtles (23%) and loggerheads also had a more widespread distribution than green turtles occurring in 70 and 38 locations, respectively, out of the 102 total survey points (Fig. 1). The MANOVA showed that there was a significant difference in the mean value of at least one variable according to the level of species richness (F=6.76, df=5, P<0.0001). There was a significant difference between the three levels of richness regarding mean depth (ANOVA: F=5.90, df=2, P=0.004), DO (ANOVA: F=4.57, df=2, P=0.013), and distance to the nearest inlet (ANOVA: F=3.97, df=2, P=0.022; Fig. 1). Follow up Tukey contrasts showed that mean depth and DO of locations without turtles observed was significantly lower than areas with one (depth P=0.03; DO P=0.031) and two species observed (depth P=0.033; DO P=0.017), while the mean depth (P=0.66) and DO (P=0.96) was not significantly different between locations in which one and two species were observed (Fig. 2). Areas with no species of turtles observed were significantly further away from the nearest inlet than areas in which two species were observed (P=0.016) but not further away from locations with one species was observed (P=0.31). In addition, the distance to the nearest inlet was not different between locations in which two species and one species were observed (P=0.30). There was no significant difference between the mean salinity (ANOVA: F=2.35, df=2, P=0.10), zooplankton density (ANOVA: F=1.62, df=2, P=0.23), or phytoplankton density (ANOVA: F=1.78, df=2, P=0.17) according to species richness.
The mean value of at least one predictor was significantly different between sites in which loggerhead turtles were observed and not observed (MANOVA: F=5.34, df=5, P<0.0001). The areas in which loggerhead turtles were observed (Fig. 3) was significantly deeper (ANOVA: F=6.83, df=1, P=0.010), had more dissolved oxygen (ANOVA: F=7.77, df=1, P=0.06), had less salinity (ANOVA: F=4.70, df=1, P=0.033), and were closer to an inlet (ANOVA: F=5.64, df=1, P=0.020; Figs. 1 and 4) than areas in which loggerhead turtles were not observed. There was no significant difference between zooplankton (ANOVA: F=0.59, df=1, P=0.45) and phytoplankton (ANOVA: F=0.56, df=1, P=0.46) density in areas in which loggerhead turtles were observed (zooplankton: mean 103.43 + SE 2.27 m3; phytoplankton: mean 248.57 + SE 11.68 m3) and not observed.
The mean value of at least one predictor was significantly different between sites in which green turtles were observed and not observed (MANOVA: F=2.44, df=5, P=0.031). The areas in which green turtles were observed were significantly closer to the inlet than areas in which green turtles were not observed (ANOVA: F=5.91, df=1, P=0.07; Figs. 1 and 4). However, there was no significant difference in the mean depth (mean 2.95 + SE 0.22 m; ANOVA: F=0.70, df=1, P=0.41), DO (mean 5.92 + SE 0.047 mg/L; ANOVA: F=2.88, df=1, P=0.093), salinity (mean 52.84 + SE 1.33 ‰; ANOVA: F=1.88, df=1, P=1.73), zooplankton density (mean 106.32 + SE 3.55 m3; ANOVA: F=0.70, df=1, P=0.41), and phytoplankton density (mean 264.47 + SE 16.67 m3; ANOVA: F=3.26, df=1, P=0.074) between areas in which green turtles were observed and not observed.
Discussion
Our results support the suggestion that since the creation of the man-made inlets from the Mediterranean Sea, the environmental conditions of the hypersaline lake Bardawil have become less severe and become more suitable for sea turtles as a feeding ground (Bradshaw et al. 2017). In addition, our results highlight the importance of environmental factors determining the distribution of endangered sea turtles in the unique feeding grounds of lake Bardawil. Areas in which we observed loggerhead and green turtles were found closer to the artificial inlets in lake Bardawil, which are associated with water that is deeper, less saline, contains higher DO, and has more invertebrate biomass (Fouda et al. 1987; Geneid and El-Hady 2006; Abd Ellah and Hussein 2009; Khalil et al. 2016). Although salinity has decreased in parts of the lake, salinity is still much higher than the Mediterranean Sea and believed to an abiotic factor that determines the composition of the flora and fauna (Abd Ellah and Hussein 2009). In lake Bardawil there is a relationship between zooplankton and salinity, as zooplankton richness and density decrease with increased salinity (Mageed 2006). As the environmental conditions have changed, so has the composition of zooplankton community (Mageed 2006). High salinity is characteristic of an extreme environment in which halophilic or specialist species occur, while other less adapted species are selected against (Mageed 2006; Lamptey and Armah 2008; Shadrin et al. 2015; Shadrin 2017). In addition to the osmotic challenges as a result of high salinity, specific heat capacity decreases leading to a less stable thermal aquatic environment. Increases in salinity leads to faster heating and cooling of hypersaline waters, with higher day and lower night temperatures compared to less saline aquatic environments. Diffused oxygen also decreases with increasing salinity and can lead to differing levels of diffused oxygen in hypersaline waters (Shadrin 2017). Loggerhead turtles were observed in areas with less salinity presumably because of less potential physiological stress and the higher dissolved oxygen in those areas. Occasionally sea turtles become trapped in the more shallow and saline areas of the lake and appear to be under physiological shock (personal observation, BR). The areas utilized by loggerhead and green turtles in lake Bardawil have the highest known salinities of habitat used by these species.
We did not find any difference in zoo or phytoplankton density between areas in which sea turtles were observed and not observed. We believe food availability is an important attribute of sea turtle habitat use but that the plankton data used in our analysis may be outdated, as the plankton data was collected around 25 years prior to our study and may not be a suitable representation of current food availability as the density has increased and species composition of zoo plankton has changed in the last two decades (Anufriieva et al. 2018). Dissolved oxygen measurements can be used as another index of food availability. Dissolved oxygen is an important metric of aquatic environments as oxygen has lower solubility in water than air and reduced availability of dissolved oxygen limits the ability of aquatic life to exist as it is vital for fish and invertebrate respiration (Craig et al. 2001). Air breathers like sea turtles are indirectly impacted as sea turtles have been found to be less likely to be observed in areas with low dissolved oxygen because of the associated decrease in fish and invertebrate density (Craig et al. 2001). Although lake Bardawil is considered to be an oxygen rich body of water, we believe that areas with the highest dissolved oxygen had the highest sea turtle richness and were used by loggerheads because of increased food availability (Mageed 2006; Khalil et al. 2013; Anufriieva et al. 2018).
Lake Badawil is a very shallow and depth limited lake as there are only a few places where water depth is two meters or more (Abd Ellah and Hussein 2009). Loggerheads and areas of high turtle richness occurred among the deeper waters (roughly 3 m) that were closer to the inlets. Green (Hays et al. 2002; Hazel et al. 2009; Senko et al. 2010) and loggerhead turtles (Schofield et al. 2007) are known to use moderately (<10 m) and shallow (3 m) coastal waters for feeding grounds. In the above studies, turtles avoided the deeper waters because presumably those areas comprised of poorer feeding grounds. However, in our study site, shallower waters are believed to have lower food availability because of their more severe environmental conditions. Zooplankton density is higher in the lake’s sea grass beds which are located in the deeper waters (Geneid and El-Hady 2006; Abd El-Hady and Khalifa 2015). Surprisingly, we did not find that environmental conditions differed between areas in which green turtles were and not observed. Perhaps measurements of sea grass coverage would have been a stronger predictor of green turtle occurance than the environmental conditions we used. Whereas the environmental factors we examined may have a greater impact on the diet of loggerhead turtles.
Almost all of the sea turtle observations occurred outside Zaranik Protected Area, exposing the sea turtle population to the many anthropogenic disturbances that threaten lake Bardawil (Fig. 1). Although the man-made inlets have benefited sea turtles by creating less severe environmental conditions and a new feeding ground, the lake’s ecological integrity has likely been compromised through the likely loss of extreme halophytes that were adapted for the lake’s prior higher salinity, as evidenced by the change in composition of the lake’s flora and fauna. More drastically is the shrinkage of the lake as a result of channeling water for salt harvesting which has caused the lake to shrink by 13 % in thirteen years (Abd Ellah and Hussein 2009). Many new salt mines have been created recently which has further reduced the area of the lake. Increased salt mine development is regularly proposed as a way to address the lack of employment in North Sinai, especially given the recent political unrest in the area. Lake Bardawil’s sea grass is now harvested by local people as a food source for livestock (Mehanna and Hegazi 2013). The fisheries in the lake has recently declined due to overfishing and bycatch is believed to be one of the main sources of sea turtle mortality (Mehanna and Hegazi 2013; Nada et al. 2013). There is also increasing pressure to establish tourism developments along the shore of lake Bardawil and use freshwater inflow into the lake to allow for large scale agricultural projects.
Lake Bardawil and the Mediterranean coastline has been recognized for its high biodiversity. In addition to being an important feeding ground, Lake Bardawil is an important stopover on the migration route of Paleartic birds, has among the highest flora diversity in the region (El Bana et al. 2002), is home to an endemic lizard and contains a population of the smallest and most endangered tortoise species of the Mediterranean basin, the Egyptian tortoise, Testudo kleinmanni (Attum et al. 2007). The conservation of this biologically valuable and rich coastal lake will require active management to protect it from the increasing anthropogenic threats that will encroach upon the lake in the next decade.
References
Abd El-Hady HH, Khalifa N (2015) Phytoplankton biochemical contents and zooplankton composition in vegetated and non-vegetated regions in Bardawil Lagoon, North Sinai, Egypt. I J Fish Aquat 2:46–54
Abd Ellah RG, Hussein MG (2009) Physical Limnology of Bardawil Lagoon, Egypt. American-Eurasian J Agric. Environ Sci 5:331–336
Anufriieva E, El-Shabrawy G, Shadrin N (2018) Copepoda in the shallow hypersaline Bardawil coastal lake (Egypt): Are there long-term changes in composition and abundance. Oceanol Hydrobiol St 47:219–229
Arthur K, Boyle M, Limpus C (2008) Ontogenetic changes in diet and habitat use in green sea turtle (Chelonia mydas) life history. Mar Ecol Prog Ser 362:303–311
Attum O, Baha El Din M, Baha El Din S, Habinan S (2007) Egyptian tortoise conservation: a community-based, field research program developed from a study on a semi-captive population. Zoo Biol 26:397–406
Bradshaw P, Broderick A, Carreras C, Inger R, Fuller W, Snape R, Stokes K, Godley B (2017) Satellite tracking and stable isotope analysis highlight differential recruitment among foraging areas in green turtles. Mar Ecol Prog Ser 582:201–214
Cardona L, Campos P, Levy Y, Demetropoulos A, Margaritoulis D (2010) Asynchrony between dietary and nutri- tional shifts during the ontogeny of green turtles (Chelonia mydas) in the Mediterranean. J Exp Mar Bio Ecol 393:83–89
Craig KJ, Crowder LB, Gray CD, McDaniel CJ, Kenwood TA, Hanifen JG (2001) Ecological effects of hypoxia on fish, sea turtles and marine mammals in the northwestern Gulf of Mexico. In: Rabalais NN, Turner RE (eds) Coastal Hypoxia: Consequences for Living Resources and Ecosystems. pp 269–292. https://doi.org/10.1029/CE058p0269
El-Bana M, Khedr A, Hecke JV, Bogaert J (2002) Vegetation composition of a threatened hypersaline lake (Lake Bardawil). North Sinai. Plant Ecology 163:63–75
Fouda M, Saab M, Saleh M (1987) An extensive study of plankton in the Bardawil lagoon, Egypt. Leban Sci Bull 3:5–23
Geneid YA, El-Hady HH (2006) Distribution, biomass and biochemical contents of the seagrass species of Lake Bardawil, Mediterranean Sea, Egypt. Biol Mar Medit 13(4):225–229
Groombridge B, Luxmoree R (1989) The green turtle and hawksbill (Reptilia: Cheloniidae): World status, exploitation, and trade. CITES Secretariat, Lausanne, Switzerland, p 601
Hays G, Glen F, Broderick A, Godley B, Metcalfe J (2002) Behavioural plasticity in a large marine herbivore: contrasting patterns of depth utilization between two green turtle (Cheloniamydas) populations. Mar Biol 141:985–990
Hazel J, Lawler I, Hamann M (2009) Diving at the shallow end: Green turtle behaviour in near-shore foraging habitat. J Exp Mar Biol Ecol 371:84–92
Houghton JDR, Callow MJ, Hays GC (2003) Habitat utilization by juvenile hawksbille turtles (Eretmochelys imbricata, LInnaeus, 1766) around a shallow water coral reef. J Nat Hist 37:1269–1280
Khalil M, Saad A, Fishar M, Bedir T (2013) (2013) Ecological Studies on Macrobenthic Invertebrates of Bardawil Wetland, Egypt. World Environment 3(1):1–8. https://doi.org/10.5923/j.env.20130301.01
Khalil MT, Abd El-Halim AS, Ahmed MHM, El Kafrawy SB, Emam WWM (2016) Integrated Field Study, Remote Sensing and GIS Approach for Assessing and Monitoring Some Chemical Water Quality Parameters in Bardawil Lagoon. Egypt 5:14656–14669
Kock V, Peckham H, Mancini A, Eguchi T (2013) Estimating at-sea mortality of marine turtles from stranding frequencies and drifter experiments. PLOS One 8:E56776
Lamptey E, Armah A (2008) Factors affecting macrobenthic fauna in a tropical hypersaline coastal lagoon in Ghana, West Africa. Estuar Coast 31:1006–1019
Mageed A (2006) Spatio-temporal variations of zooplankton community in the hypersaline lagoon of Bardawil, north Sinai–Egypt. Egypt J Aquat Res 1687–4285 vol 32 no 1: 168–183
Mancini A, Elsadek I, Madon B (2015) When simple is better: Comparing two sampling methods to estimate green turtles abundance at coastal feeding grounds. J Exp Mar Biol Ecol 465:113–120
Mehanna S, Hegazi M (2013) Population dynamics of grey mullet Mugil cephalus associated with seagrass community in Bardawil lagoon, Northern Sinai, Egypt. In: Proceedings, INOC -IIUM- International Conference on “Oceanography & Sustainable Marine Production: A Challenge of Managing Marine Resources under Climate Change, ICOSMaP,”, vol 2013, Kuantan-Malaysia, pp 28, 530–30, 539
Moran K, Bjorndal K (2005) Simulated green turtle grazing affects structure and productivity of seagrass pastures. Mar Ecol Prog Ser 305:235–247
Moran K, Bjorndal K (2007) Simulated green turtle grazing affects nutrient composition of the seagrass Thalassia testudinum. Mar Biol 150:1083–1092
Nada MA, Boura L, Grimanis K, Schofield G, El-Alwany MA, Noor N, Ommeran, MM, Rabia B (2013). Egypt’s Bardawil Lake: safe haven or deadly trap for sea turtles in the Mediterranean? A report by MEDASSET, Suez Canal University and Nature Conservation Egypt, p 79
Rabia B, Attum O (2015) Distribution and status of sea turtle nesting and mortality along the North Sinai coast, Egypt (Reptilia: Cheloniidae). Zool Middle East 61:26–31
Schofield G, Bishop C, MacLean G, Brown P, Baker M, Katselidis K, Dimpoulos P, Pantis J, Hays G (2007) Novel GPS tracking of sea turtles as a tool for conservation management. J Exp Mar Biol Ecol 347:58–68
Seminoff JA, Jones TT, Resendiz A, Nichols WJ, Chaloupka ML (2003) Monitoring Green turtles (Chelonia mydas) at a coastal foraging area in Baja California, Mexico: multiple indices describe population status. J Mar Biol Assoc UK 83:1335–1362
Senko J, Koch V, Megill WM, Carthy RR, Templeton RP, Nichols WJ (2010) Fine scale daily movements and habitat use of East Pacific green turtles at a shallow coastal lagoon in Baja California Sur. Mexico. J Exp Mar Biol Ecol 391:92–100
Shadrin N, Anufriieva E, Amat F, Eremin O (2015) Dormant stages of crustaceans as a mechanism of propagation in the extreme and unpredictable environment in the Crimean hypersaline lakes. Chin J Oceanol Limn 33:1362–1367
Shadrin NV (2017) Hypersaline lakes as polyextreme habitats for life. In: Zheng M, Deng T, Oren A (eds) Introduction to salt lake sciences. Science Press, Beijing, pp 173–178
Acknowledgments
We would like to thank the Egyptian Environmental Affairs Agency and Indiana University Southeast for their continued support. We would like to thank Wessam ElShohty, Nature Conservation Sector and Hany Al Nagar, Zaranik Protected Area for their assistance in the field.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We explicitly declare that they have no conflict of interest. This research was funded internally and all necessary approvals have been obtained.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Attum, O., Rabia, B. Green (Chelonia mydas) and loggerhead (Caretta caretta) habitat use of the most environmentally extreme sea turtle feeding ground in the Mediterranean basin. J Coast Conserv 25, 4 (2021). https://doi.org/10.1007/s11852-020-00793-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11852-020-00793-1