Abstract
Background
Tolvaptan, a vasopressin V2 receptor antagonist, is used to treat autosomal-dominant polycystic kidney disease (ADPKD). Although tolvaptan curbs disease progression, a few reports have examined factors related to treatment response. The estimated glomerular filtration rate (eGFR) decreases soon after tolvaptan is initiated. We investigated whether initial eGFR decline affects renal prognosis of patients.
Methods
This was a single-center, retrospective observational cohort study. Eighty-three patients with ADPKD who initiated tolvaptan were selected. We analyzed the relationship of the initial eGFR change with clinical parameters and analyzed the annual eGFR change in terms of renal prognostic value using univariable and multivariable linear regression analyses.
Results
The initial eGFR change was − 4.6 ± 8.0%/month. The initial eGFR change correlated significantly with the annual eGFR change in multivariable analysis, suggesting that the larger decline in the initial eGFR change, the better the renal prognosis. Furthermore, the change in fractional excretion (FE) of free water (FEH2O) correlated positively with initial eGFR change. FEH2O and urea nitrogen FE (FEUN) increased significantly; however, sodium FE (FENa) level remained unchanged. In approximately half of the patients, FENa unexpectedly decreased.
Conclusions
The initial eGFR decline might be caused by suppressing glomerular hyperfiltration, due to the pharmacological effect of tolvaptan, and/or by reducing renal plasma flow, due to potential volume depletion. The initial eGFR change reflects the tolvaptan effect, can be easily evaluated in clinical practice, and may be useful as one of the clinical indicator for predicting renal prognosis in patients under tolvaptan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autosomal-dominant polycystic kidney disease (ADPKD) is the most common hereditary renal disease. In individuals with ADPKD, multiple renal cysts are generated/enlarged due to mutations in PKD1 or PKD2 [1]. As the kidney enlarges, renal dysfunction progresses because of a decrease in the renal parenchyma. Approximately 50% of patients reach end-stage renal failure by the age of 60 years [1].
A clinical trial of tolvaptan, a vasopressin V2 receptor antagonist, in ADPKD patients with creatinine clearance (Ccr) ≥ 60 mL/min/1.73 m2 and TKV ≥ 750 mL showed that tolvaptan was able to suppress the renal volume increase and renal function decline (TEMPO3:4) [2]. Therefore, in 2014, tolvaptan was approved in Japan for patients with TKV ≥ 750 mL and an annual kidney volume increase rate ≥ 5%. The subsequent REPRISE trial in ADPKD patients with relatively low renal function (eGFR 25‒65 mL/min/1.73 m2) showed the effectiveness of tolvaptan [3]. These clinical trials reported that tolvaptan may delay the progression of renal damage [4]. Specifically, the time from an eGFR of 60 mL/min/1.73 m2 to an eGFR of 15 mL/min/1.73 m2 in the tolvaptan group was extended by approximately 4.4 years in the TEMPO trial and 6.8 years in the REPRISE trial, when compared with their respective placebo groups [4].
Renal function commonly declines soon after initiation of tolvaptan administration, i.e., an initial decline is observed [5]. A 1-week study of tolvaptan showed an 8.6% eGFR reduction [6], and a 3-week study showed a 5.4% eGFR reduction, with recovery after discontinuation [7, 8]. In the REPRISE trial, which was conducted in consideration of this initial decline, the eGFR in the tolvaptan group had recovered by 1 month after ceasing administration [3]. A similar initial decline was also observed in patients receiving renin‒angiotensin system (RAS) inhibitors [9, 10]. A large-scale study of the angiotensin receptor blocker losartan reported that a more substantial initial decline was associated with better long-term renal prognosis [11].
Tolvaptan is currently used worldwide, but a few predictors of response to tolvaptan have been identified. In the TEMPO study, the higher the baseline urine osmolality and the greater the decrease in urine osmolality 3 weeks after initiation of tolvaptan, the better the renal prognosis [12]. In addition, the baseline copeptin and the change in copeptin after 3 weeks of tolvaptan predicted future disease progression assessed for eGFR decline [13]. Recently, we reported that initial decrease in urinary aquaporin 2 in the first month of tolvaptan treatment reflects the pharmacologic effect of and could be an indicator of renal prognosis [14]. However, urinary osmolality is an unstable examination in outpatient clinic, and copeptin and aquaporin 2 are not widely used in routine medical care. In this study, to explore the predictors in daily clinical practice, we investigated factors affecting renal prognosis, including the initial eGFR change, in ADPKD patients who initiated tolvaptan.
Methods
Patients
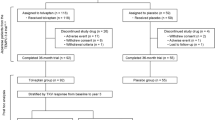
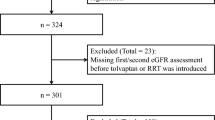
A flow chart of the study population is shown in Fig. 1. We enrolled 96 patients diagnosed with ADPKD [15], who initiated tolvaptan at our hospital from September 2014 to May 2019. The inclusion criteria were as follows: Patients with a TKV greater than 750 mL and more than 5% annualized TKV growth, and with eGFRs more than 15 mL/min/1.73 m2. Thirteen patients whose observation periods were less than 6 months were excluded. Eighty-three patients were eligible for the present study. Among the 83 patients, 75 patients for whom data of urinary osmolarity (U-Osm), urinary sodium (U-Na), and urinary urea nitrogen (U-UN) were available, were analyzed for the short-term effect of tolvaptan administration (Study A). Moreover, to determine the long-term effect of tolvaptan administration (Study B), renal prognosis (annual change in eGFR) was evaluated in 83 patients. This analysis was also performed with baseline TKV as a variable, in 78 patients for whom these data were available.
Study design
This was a single-center, retrospective observational cohort study. In Japan, patients need to be hospitalized to start tolvaptan. On the day of admission, we performed blood tests and urinalysis for all patients before initiation of tolvaptan and started a 3-day inpatient management program involving tolvaptan administration. According to the Japanese package insert, the recommended starting dose of tolvaptan is 60 mg/day (45 mg in the morning and 15 mg in the evening), although it is preferable to start at a lower dose for patients whose creatinine clearance is below 30 mL/min. After discharge, patients visited the outpatient department on a monthly basis for follow-up examinations. The dose was adjusted with 30 to 120 mg daily depending on renal function and tolerability. Tolvaptan was discontinued in patients with eGFR < 15 mL/min/1.73m2 or liver enzyme elevation during follow-up, because tolvaptan is contraindicated for such patients in Japan.
eGFR was calculated based on the serum creatine value using the formula applied as a standard in Japan [16]. Annual changes in eGFR (%/year) were calculated using all eGFR values from 1 month after initiation of tolvaptan to the final follow-up by the least squares methods which have been reported to be useful in predicting renal prognosis [17]. As performed in the TEMPO3:4 trial [2] and recent studies [18, 19], we used the value at 1 month after initiation of tolvaptan as a baseline data for calculating annual change. Independent variables included fractional excretions of free water, sodium, and urea nitrogen, which were evaluated as FEH2O, FENa, and FEUN, respectively. The fractional excretions (FE) were calculated by the following formula:
FEUN (%) = (urinary urea nitrogen/serum urea nitrogen)/(urinary creatinine/serum creatinine) × 100. Three-dimensional-reconstruction computed tomography (3D-CT) was performed within 3 months before initiation of tolvaptan to measure TKV. All kidney volumes were measured using the 3D workstation of the Ziostation2 (Version 2.4.2.3; Ziosoft, Tokyo, Japan).
Statistical analysis
Continuous variables are reported as the mean ± standard deviation. Categorical variables are reported as percentages (%) unless otherwise stated. The data were evaluated using the chi-square test or Student’s t test as appropriate. Pearson’s correlation coefficient® was used to assess bivariate relationships. Univariable and multivariable linear regression analyses were performed to investigate the factors related to the outcomes. A p value < 0.05 was considered statistically significant. All statistical analyses were performed using JMP software (v.15.0; SAS Institute, Cary, NC, USA).
Results
The baseline patient characteristics are presented in Table 1.
Study A: short-term effect following tolvaptan initiation
The factors implicated in the initial change in eGFR between tolvaptan initiation and 1 month thereafter were assessed in 75 patients. The change in FEH2O and high baseline eGFR correlated negatively with initial changes in eGFR (Table 2), suggesting that the higher the aquatic diuresis and the better the renal function, the greater the initial changes in eGFR. The change in FENa correlated negatively with annual changes in TKV in univariable analysis, however, no variables in fractional excretions correlated significantly with annual changes in TKV in multivariable analysis (Supplementary Table 1).
Despite the initial decline in eGFR and BUN declined significantly, FEH2O and FEUN increased significantly, but FENa remained unchanged (Fig. 2). When compared with the degree of initial changes in FEH2O (ΔFEH2O), FENa (ΔFENa), and FEUN (ΔFEUN), the degree of mean initial change in FENa (ΔFENa) was almost zero (Fig. 3). Therefore, we divided patients into two groups: one showing an increase in FENa at 1 month after initiation of administration (ΔFENa ≥ 0 group), and the other in which this increase was absent (ΔFENa < 0 group).
Changes in the clinical parameters between baseline and one month after initiation of tolvaptan A eGFR, B BUN, C FEH2O, D FENa, and E FEUN. The vertical axes are absolute values. eGFR and BUN decreased significantly at 1 month after initiation of tolvaptan. FEH2O and FEUN are increased significantly at 1 month after initiation of tolvaptan. However, FENa remained unchanged. eGFR, estimated glomerular filtration rate; BL, baseline; 1 M, 1 month after tolvaptan initiation; BUN, blood urea nitrogen; FEH2O, fractional excretion of free water; FENa, fractional excretion of sodium; FEUN, fractional excretion of urea nitrogen. *P < 0.0001
The degree of initial changes in eGFR, BUN, FEH2O, FENa, and FEUN A Δ eGFR, B Δ BUN, C Δ FEH2O, D Δ FENa, and E Δ FEUN. The vertical axes are the differences between the absolute values. Δ eGFR and Δ BUN were negative. Δ FEH2O and Δ FEUN were positive. However, Δ FENa was almost zero. Δ eGFR, the degree of initial change in estimated glomerular filtration rate; Δ BUN, the degree of initial change in blood urea nitrogen; Δ FEH2O, the degree of initial change in fractional excretion of free water; Δ FENa, the degree of initial change in fractional excretion of sodium; ΔFEUN, the degree of initial change in fractional excretion of urea nitrogen
The baseline characteristics of two groups are presented in Table 3. The proportion of males and baseline FENa values were significantly different between two groups. We compared eGFR, BUN, FEH2O, FENa, and FEUN at initiation of tolvaptan and at 1 month thereafter between the two groups (Fig. 4). The increase in FEH2O and FEUN in ΔFENa ≥ 0 group was greater than that in ΔFENa < 0 group (Fig. 5). FENa was increased in ΔFENa ≥ 0 group, but was decreased in ΔFENa < 0 group at 1 month after initiation of tolvaptan (Fig. 5). The changes in mean values of these clinical parameters between baseline and one month after tolvaptan initiation in entire population and in ΔFENa < 0 group and ΔFENa ≥ 0 group were shown in Fig. 6.
Changes in the clinical parameters between baseline and 1 month after initiation of tolvaptan in the ΔFENa ≥ 0 group and the ΔFENa < 0 group. A eGFR, B BUN, C FEH2O, D FENa, and E FEUN. The vertical axes were absolute values. eGFR and BUN decreased significantly at 1 month after initiation of tolvaptan in both groups. FEH2O and FEUN increased significantly at 1 month after initiation of tolvaptan in both groups. FENa increased significantly in the ΔFENa ≥ 0 group and FENa decreased significantly in the ΔFENa < 0 group. eGFR, estimated glomerular filtration rate; BL, baseline; 1 M, 1 month after tolvaptan initiation; BUN, blood urea nitrogen; FEH2O, fractional excretion of free water; FENa, fractional excretion of sodium; FEUN, fractional excretion of urea nitrogen; Δ FENa, the degree of initial change in fractional excretion of sodium. *P < 0.0001
The degree of initial changes in the clinical parameters between baseline and one month after initiation of tolvaptan in the ΔFENa ≥ 0 group and the ΔFENa < 0 group. A Δ eGFR, B Δ BUN, C Δ FEH2O, D Δ FENa, and E Δ FEUN. The vertical axes are the differences between the absolute values. There were significant differences in changes in eGFR, FEH2O, FENa, and FEUN between in the ΔFENa ≥ 0 group and in the ΔFENa < 0 group by 1 month after initiation of tolvaptan. eGFR, estimated glomerular filtration rate; BL, baseline; 1 M, 1 month after tolvaptan initiation; BUN, blood urea nitrogen; FEH2O, fractional excretion of free water; FENa, fractional excretion of sodium; FEUN, fractional excretion of urea nitrogen. * P < 0.0001
The changes in mean values of clinical parameters between baseline and one month after tolvaptan initiation in entire population (upper panel) and in the ΔFENa < 0 group and the ΔFENa ≥ 0 group (lower panel). eGFR (A) and BUN (B) decreased significantly at 1 month after initiation of tolvaptan. FEH2O (C) and FEUN (E) are increased significantly. FENa (D) remained unchanged in entire population. There were no significant differences in changes in eGFR (F), BUN (G), but FEH2O (H) and FEUN (J) in the ΔFENa ≥ 0 group increased more markedly than those in the ΔFENa < 0 group. FENa (I) in the ΔFENa ≥ 0 group increased, whereas the FENa in the ΔFENa < 0 group reduced by 1 month after initiation of tolvaptan. The orange line indicates the ΔFENa < 0 group, and the blue line indicates the ΔFENa ≥ 0 group. All numbers show average values. eGFR, estimated glomerular filtration rate; BL, baseline; 1 M, 1 month after initiation of tolvaptan; BUN, blood urea nitrogen; FEH2O, fractional excretion of free water; FENa, fractional excretion of sodium; FEUN, fractional excretion of urea nitrogen
Furthermore, we investigated the changes in eGFR, BUN, FEH2O, and FENa from baseline to 3 months later, including the third day after initiation of tolvaptan (Fig. 7). FEH2O increased markedly on the third day after initiation of tolvaptan, and FEH2O reduced in ΔFENa < 0 group from 1 month after initiation of tolvaptan. Baseline FENa in ΔFENa < 0 group was higher than that in ΔFENa ≥ 0 group. FENa on the third day was less than 1% in both groups. Thereafter, FENa increased at 1 month after initiation of tolvaptan in ΔFENa ≥ 0 group, but FENa remained unchanged in ΔFENa < 0 group. However, FENa increased at 2 and 3 months after initiation of tolvaptan in ΔFENa < 0 group.
Changes in the clinical parameters from baseline to 3 months, including the third day, after tolvaptan initiation in the ΔFENa < 0 and ΔFENa ≥ 0 groups. A, B eGFR and BUN decreased largely on the third day after initiation of tolvaptan in both groups. C FEH2O increased largely on the third day in both groups, but reduced in the ΔFENa < 0 group from 1 month after initiation of tolvaptan. D Baseline FENa in the ΔFENa < 0 group was higher than that in ΔFENa ≥ 0 group. In both groups, FENa was less than 1% on the third day. However, FENa was increased at 1 month after treatment initiation in the ΔFENa ≥ 0 group, and at 2 and 3 months after treatment initiation in the ΔFENa < 0 group. The orange lines indicate the ΔFENa < 0 group, and the blue line indicates the ΔFENa ≥ 0 group. All numbers show average values. eGFR, estimated glomerular filtration rate; BL, baseline; 1 M, 1 month after tolvaptan initiation; BUN, blood urea nitrogen; FEH2O, fractional excretion of free water; FENa, fractional excretion of sodium; FEUN, fractional excretion of urea nitrogen
Study B: long-term effect following tolvaptan initiation
We investigated whether the initial eGFR change influenced the annual eGFR change in 83 patients. The initial eGFR change was significantly and negatively correlated with the annual eGFR change (Fig. 8), suggesting that the larger decline in the initial eGFR change, the better the renal prognosis. This was significant, even when the patients were divided into ΔFENa < 0 and ΔFENa ≥ 0 groups (Supplementary Fig. 1).
In the multivariable analysis, the baseline eGFR, baseline urinary protein, and the initial eGFR change were significantly associated with the annual change in eGFR (Table 4). Another multivariable analysis with available baseline TKV as a variable (n = 78) revealed that baseline eGFR, proteinuria, and the initial eGFR change were also significantly associated with the annual change in eGFR (Table 4).
Discussion
In this study, we found that the initial eGFR decline which occurs during the first month of tolvaptan administration could be an indicator of renal prognosis in ADPKD patients.
A similar initial GFR decline has been observed with administration of RAS inhibitors. RAS inhibitors expand the efferent arterioles more than the afferent arterioles and reduce intraglomerular pressure to suppress glomerular hyperfiltration, resulting in reduced urinary protein and protecting renal function [11]. On the other hand, tolvaptan inhibits arginine vasopressin (AVP). AVP causes reabsorption of water in the collecting tubules; consequently, urea is gradually concentrated. AVP increases urea permeability of the terminal part of the medullary collecting duct via the urea transporter UT-A1/3 [20], allowing urea to diffuse into the interstitium. It is further reabsorbed by the descending limb of the loop of Henle via the ascending vasa recta, in a phenomenon known as urea recycling [21]. Tolvaptan inhibits AVP and suppresses urea recycling, thereby reducing urea reabsorption in the descending limb of the loop of Henle. Urea concentration in the ascending limb of the loop of Henle decreases, promoting water reabsorption. The intraluminal sodium chloride concentration increases and chloride concentration in the macula densa increases. This causes a reduction in the intraglomerular pressure due to tubulo-glomerular feedback (TGF), leading to a decrease in GFR [5, 22, 23]. Recently, Bankir et al. suggested that AVP may induce glomerular hyperfiltration as an indirect mechanism, in addition to a direct influence of AVP on cyst growth [24]. In this study, despite the initial decline in eGFR, BUN reduced and FEUN increased after the initiation of tolvaptan, supporting this “urea recycling” theory. Indeed, BUN decreases were reported previously [25, 26]. Taken together, the initial GFR decline in tolvaptan can be caused by suppressing glomerular hyperfiltration like RAS inhibitors.
Moreover, vasopressin increases sodium reabsorption via the epithelial sodium channels (ENaC) in collecting tubules [27]. Conversely, V2 receptor antagonist increased the rate of sodium excretion in normal rats in a dose-dependent manner [28]. Therefore, tolvaptan theoretically increase urinary excretion of sodium as previously reported [25]. However, in this study, approximately half of the patients showed decreased FENa at 1 month after initiation of tolvaptan. Boertin et al. showed that the initial decline in eGFR was due to a reduction of both GFR and renal plasma flow (RPF) [7]. Combined with our results, this suggests another mechanism for the initial change in GFR after initiation of tolvaptan (Fig. 9). GFR is reduced due to TGF caused by inhibition of urea recycling, leading to an increase in FEUN. On the other hand, RPF is reduced due to potential volume depletion, which activate RAS via the juxta glomerular apparatus (JGA), leading to a decrease in FENa. The initial changes in GFR are thought to occur due to the involvement of both TGF and reduced RPF, in various proportions. Specifically, on the third day, FEH2O was largely increased while FENa was less than 1%. This may be because sudden aquatic diuresis caused potential volume depletion, reducing RPF and suppressing urinary sodium excretion by activation of the RAS. By 1 month later, although aquatic diuresis continued, urinary sodium excretion increased in ΔFENa ≥ 0 group, suggesting improved potential volume depletion by sufficient water intake. Furthermore, the more largely increased FEUN in ΔFENa ≥ 0 group suggests that the initial GFR change is due to TGF, rather than to potential volume depletion. However, there was no difference in serum sodium values at 1 month after tolvaptan initiation between both groups, probably because the decrease in aquaresis occurred simultaneously with the decrease in natriuresis. Moreover, aquatic diuresis decreased and urinary sodium excretion did not increase in ΔFENa < 0 group, suggesting continuation of potential volume depletion. By 3 months after initiation of tolvaptan, there was no difference in FENa between two groups, suggesting improvement of potential volume depletion in most patients.
Conceptual diagram of the initial decline in the GFR after tolvaptan initiation. The antagonistic effect of tolvaptan on the V2R causes aquatic diuresis, leading to increased FEH2O. Euvolemia due to sufficient water intake demonstrates the pharmacological effects such as inhibition of urea recycling, followed by a NaCl concentration increase in the macula densa, reducing GFR via TGF, and leading to the initial decline in GFR. Inhibition of urea recycling leads to increased FEUN and FENa and decreased BUN. Euvolemia also decreases sodium reabsorption via NKCC2 and ENaC, leading to increased FENa. On the other hand, potential volume depletion due to insufficient water intake reduces RPF, also leading to an initial decline in GFR. RPF reduction activates the RAS via the JGA, leading to decreased FENa. GFR, glomerular filtration rate; FEH2O, fractional excretion of free water; FEUN, fractional excretion of urea nitrogen; BUN, blood urea nitrogen; NaCl, sodium chloride; TGF, tubulo-glomerular feedback; Na, sodium; NKCC2, Na–K–Cl cotransporter; ENaC, epithelial sodium channels; FENa, fractional excretion of sodium; RPF, renal plasma flow; RAS, renin‒angiotensin system; JGA, juxta glomerular apparatus; V2R, vasopressin V2 receptor
The significantly higher baseline FENa in ΔFENa < 0 group may indicate high sodium excretion, implying a high salt intake [29]. Kramers et al. reported that high urinary osmolar and sodium excretions caused a greater urine volume [30]. Cote et al. also reported that sodium and urea excretion determined the urine output in ADPKD patients on tolvaptan [31]. Therefore, the patients in ΔFENa < 0 group are prone to potential volume depletion due to a greater urine volume. Based on these results, restriction of salt intake and sufficient water intake are important in these patients, to avoid the effect of potential volume depletion and to ensure the efficacy of tolvaptan [31].
We observed a correlation between the initial eGFR change and the annual eGFR change, even when we divided patients into ΔFENa < 0 group and ΔFENa ≥ 0 group. Renal prognosis was better when the initial eGFR decline was greater. The initial GFR decline seen with administration of RAS inhibitors is thought to be associated with long-term renal prognosis, and is reversible after drug discontinuation [9, 10]. In a 3-week tolvaptan administration study, urinary biomarker excretion changed with tolvaptan use, including decreases in urinary albumin [8]. In the TEMPO trial, urinary albumin levels were also significantly decreased in the tolvaptan group as compared to the placebo group, suggesting that glomerular injury was suppressed [32]. These results suggest that tolvaptan has the similar renal protective effect as RAS inhibitors.
This study has several limitation: first, as it was a single-center clinical study, the number of participants was relatively small. Second, the observation period was relatively short for assessing the effect of treatment. Verification of our findings is necessary in larger studies. In this context, the results of large-scale clinical trials that have recently been completed are awaited.
In conclusion, the initial eGFR change might be caused by suppressing glomerular hyperfiltration, due to the pharmacological effect of tolvaptan, and/or by reducing RPF, due to potential volume depletion. The initial eGFR change significantly affected the annual eGFR change. This initial change, which can easily be evaluated in clinical practice, could be useful as one of the clinical indicator predicting renal prognosis in patients under tolvaptan.
References
Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359(14):1477–85. https://doi.org/10.1056/NEJMcp0804458.
Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367(25):2407–18. https://doi.org/10.1056/NEJMoa1205511.
Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med. 2017;377(20):1930–42. https://doi.org/10.1056/NEJMoa1710030.
Chebib FT, Perrone RD, Chapman AB, Dahl NK, Harris PC, Mrug M, et al. A practical guide for treatment of rapidly progressive ADPKD with tolvaptan. J Am Soc Nephrol. 2018;29(10):2458–70. https://doi.org/10.1681/ASN.2018060590.
Reddy B, Chapman AB. Acute response to tolvaptan in ADPKD: a window to predict long-term efficacy? Am J Kidney Dis. 2015;65(6):811–3. https://doi.org/10.1053/j.ajkd.2015.03.004.
Irazabal MV, Torres VE, Hogan MC, Glockner J, King BF, Ofstie TG, et al. Short-term effects of tolvaptan on renal function and volume in patients with autosomal dominant polycystic kidney disease. Kidney Int. 2011;80(3):295–301. https://doi.org/10.1038/ki.2011.119.
Boertien WE, Meijer E, de Jong PE, Bakker SJ, Czerwiec FS, Struck J, et al. Short-term renal hemodynamic effects of tolvaptan in subjects with autosomal dominant polycystic kidney disease at various stages of chronic kidney disease. Kidney Int. 2013;84(6):1278–86. https://doi.org/10.1038/ki.2013.285.
Boertien WE, Meijer E, de Jong PE, ter Horst GJ, Renken RJ, van der Jagt EJ, et al. Short-term effects of tolvaptan in individuals with autosomal dominant polycystic kidney disease at various levels of kidney function. Am J Kidney Dis. 2015;65(6):833–41. https://doi.org/10.1053/j.ajkd.2014.11.010.
Hansen HP, Rossing P, Tarnow L, Nielsen FS, Jensen BR, Parving HH. Increased glomerular filtration rate after withdrawal of long-term antihypertensive treatment in diabetic nephropathy. Kidney Int. 1995;47(6):1726–31. https://doi.org/10.1038/ki.1995.238.
Apperloo AJ, de Zeeuw D, de Jong PE. A short-term antihypertensive treatment-induced fall in glomerular filtration rate predicts long-term stability of renal function. Kidney Int. 1997;51(3):793–7. https://doi.org/10.1038/ki.1997.111.
Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011;80(3):282–7. https://doi.org/10.1038/ki.2011.79.
Devuyst O, Chapman AB, Gansevoort RT, Higashihara E, Perrone RD, Torres VE, et al. Urine osmolality, response to tolvaptan, and outcome in autosomal dominant polycystic kidney disease: results from the TEMPO 3:4 trial. J Am Soc Nephrol. 2017;28(5):1592–602. https://doi.org/10.1681/ASN.2016040448.
Gansevoort RT, van Gastel MDA, Chapman AB, Blais JD, Czerwiec FS, Higashihara E, et al. Plasma copeptin levels predict disease progression and tolvaptan efficacy in autosomal dominant polycystic kidney disease. Kidney Int. 2019;96(1):159–69. https://doi.org/10.1016/j.kint.2018.11.044.
Makabe S, Manabe S, Kataoka H, Akihisa T, Yoshida R, Ushio Y, et al. Urinary Aquaporin 2 as a potential indicator predicting tolvaptan response in patients with ADPKD. Kidney Int Rep. 2021;6(9):2436–44. https://doi.org/10.1016/j.ekir.2021.06.033.
Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet. 1994;343(8901):824–7. https://doi.org/10.1016/s0140-6736(94)92026-5.
Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. Collaborators developing the Japanese equation for estimated GFR GFR estimation using standardized serum cystatin C in Japan. Am J Kidney Dis. 2013;61(2):197–203. https://doi.org/10.1053/j.ajkd.2012.07.007.
Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311(24):2518–31. https://doi.org/10.1001/jama.2014.6634.
Inker LA, Heerspink HJL, Tighiouart H, Levey AS, Coresh J, Gansevoort RT, et al. GFR slope as a surrogate end point for kidney disease progression in clinical trials: a meta-analysis of treatment effects of randomized controlled trials. J Am Soc Nephrol. 2019;30(9):1735–45. https://doi.org/10.1681/ASN.2019010007.
Greene T, Ying J, Vonesh EF, Tighiouart H, Levey AS, Coresh J, et al. Performance of GFR slope as a surrogate end point for kidney disease progression in clinical trials: a statistical simulation. J Am Soc Nephrol. 2019;30(9):1756–69. https://doi.org/10.1681/ASN.2019010009.
Yang B, Bankir L. Urea and urine concentrating ability: new insights from studies in mice. Am J Physiol Ren Physiol. 2005;288(5):F881–96. https://doi.org/10.1152/ajprenal.00367.2004.
Bankir L, Bouby N, Ritz E. Vasopressin: a novel target for the prevention and retardation of kidney disease? Nat Rev Nephrol. 2013;9(4):223–39. https://doi.org/10.1038/nrneph.2013.22.
Bouby N, Ahloulay M, Nsegbe E, Dechaux M, Schmitt F, Bankir L. Vasopressin increases glomerular filtration rate in conscious rats through its antidiuretic action. J Am Soc Nephrol. 1996;7(6):842–51.
Zittema D, Boertien WE, van Beek AP, Dullaart RP, Franssen CF, de Jong PE, et al. Vasopressin, copeptin, and renal concentrating capacity in patients with autosomal dominant polycystic kidney disease without renal impairment. Clin J Am Soc Nephrol. 2012;7(6):906–13. https://doi.org/10.2215/CJN.11311111.
Bankir L, Bichet DG. What can copeptin tell us in patients with autosomal dominant polycystic disease? Kidney Int. 2019;96(1):19–22. https://doi.org/10.1016/j.kint.2019.02.037.
Minami S, Hamano T, Iwatani H, Mizui M, Kimura Y, Isaka Y. Tolvaptan promotes urinary excretion of sodium and urea: a retrospective cohort study. Clin Exp Nephrol. 2018;22(3):550–61. https://doi.org/10.1007/s10157-017-1475-9.
Kajimoto K, Abe T. Blood urea nitrogen as a marker of the acute response to addition of tolvaptan to standard therapy in patients hospitalized for acute heart failure syndromes. Int J Cardiol. 2014;177(2):589–91. https://doi.org/10.1016/j.ijcard.2014.08.147.
Bankir L, Fernandes S, Bardoux P, Bouby N, Bichet DG. Vasopressin-V2 receptor stimulation reduces sodium excretion in healthy humans. J Am Soc Nephrol. 2005;16(7):1920–8. https://doi.org/10.1681/ASN.2004121079.
Perucca J, Bichet DG, Bardoux P, Bouby N, Bankir L. Sodium excretion in response to vasopressin and selective vasopressin receptor antagonists. J Am Soc Nephrol. 2008;19(9):1721–31. https://doi.org/10.1681/ASN.2008010021.
Nowson CA, Lim K, Campbell NRC, O’Connell SL, He FJ, Daly RM. Impact of fractional excretion of sodium on a single morning void urine collection as an estimate of 24-hour urine sodium. J Clin Hypertens (Greenwich). 2019;21(12):1763–70. https://doi.org/10.1111/jch.13725.
Kramers BJ, van Gastel MDA, Boertien WE, Meijer E, Gansevoort RT. Determinants of urine volume in ADPKD patients using the vasopressin V2 receptor antagonist tolvaptan. Am J Kidney Dis. 2019;73(3):354–62. https://doi.org/10.1053/j.ajkd.2018.09.016.
Cote G, Asselin-Thompstone L, Mac-Way F, Rene de Cotret P, Lacroix C, Desmeules S, et al. Sodium and urea excretion as determinants of urine output in autosomal dominant polycystic kidney disease patients on V2 receptor antagonists: impact of dietary intervention. Int Urol Nephrol. 2020;52(2):343–9. https://doi.org/10.1007/s11255-020-02384-3.
Gansevoort RT, Meijer E, Chapman AB, Czerwiec FS, Devuyst O, Grantham JJ, et al. Albuminuria and tolvaptan in autosomal-dominant polycystic kidney disease: results of the TEMPO 3:4 Trial. Nephrol Dial Transplant. 2016;31(11):1887–94. https://doi.org/10.1093/ndt/gfv422.
Acknowledgements
We express our sincere appreciation to all the patients, collaborating physicians, and other medical staff for their important contributions to the study. This study was supported in part by a Grant-in-Aid for Intractable Renal Diseases Research, Research on rare and intractable diseases, Health and Labor Sciences Research Grants from the Ministry of Health, Labor and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Toshio Mochizuki received honoraria for lectures from Otsuka Pharmaceutical Co. Toshio Mochizuki and Hiroshi Kataoka belong to an endowed department sponsored by Otsuka Pharmaceutical Co, Chugai Pharmaceutical Co, Kyowa Kirin Co, and JMS Co.
Ethical approval
All procedures involving human participants were performed in accordance with the ethical standards of the institutional research committee of Tokyo Women’s Medical University (IRB approval number 5118) and with the tenets of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Akihisa, T., Kataoka, H., Makabe, S. et al. Initial decline in eGFR to predict tolvaptan response in autosomal-dominant polycystic kidney disease. Clin Exp Nephrol 26, 540–551 (2022). https://doi.org/10.1007/s10157-022-02192-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-022-02192-2