Abstract
Purpose
Tolvaptan, a vasopressin V2 receptor antagonist, slows the decline in renal function in autosomal dominant polycystic kidney disease (ADPKD). However, it increases urine output such that patient adherence could be compromised. In a cohort of patients with ADPKD on tolvaptan, we aimed to identify the contribution of sodium and urea excretion rate to daily urine output, and to evaluate the effectiveness of dietary counseling on sodium and urea excretion rates.
Methods
Retrospective analysis of 30 ADPKD patients who underwent a single session of personalized dietary counseling to reduce sodium and protein intake before initiation of tolvaptan. Creatinine and 24-h urine were obtained regularly on treatment. Generalized estimation equations were used.
Results
Mean age and median eGFR were 44 ± 11 years and 52 (43–74) ml/min/1.73 m2. Tolvaptan increased diuresis from 2.5 to 5.2 l/day. After adjusting for the dose of tolvaptan, an increase in sodium and urea excretion rate by 50 mmol/day was associated with an estimated additional urine volume of 0.6 l/day (95% CI 0.4–0.8 l/day; P < 0.001) and 0.25 l/day (95% CI 0.11–0.39 l/day; P < 0.001), respectively. Dietary counseling resulted in a transient reduction of sodium excretion by 19 mmol/day during the first 4 months (P = 0.016) but resulted in a more sustained reduction in urea excretion by 69 mmol/day (P = 0.008).
Conclusion
Both sodium and urea excretion rates contribute significantly to daily urine volume in patients treated with tolvaptan, and a single session of dietary counseling was transiently effective in reducing sodium intake but achieved a more sustained reduction in protein intake. Dietary counseling should be considered in the management of ADPKD patients treated by tolvaptan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common monogenic cause of kidney failure and the fourth most common renal disease requiring renal replacement therapy [1]. It is predominantly caused by a mutation involving the PKD1 gene (~ 85%) and the PKD2 gene (~ 15% of cases) which encode the proteins polycystin-1 (PC1) and -2 (PC2), respectively, leading to cyst formation and progressive cyst growth [2, 3]. Renal manifestations include hypertension, hematuria, proteinuria, mild urine concentrating defect, renal pain, kidney stones and renal insufficiency [4]. These manifestations are produced by the progressive and continuous enlargement and proliferation of fluid-filled cysts, leading to enlargement of the kidney years before development of kidney failure. Tolvaptan is a vasopressin V2 receptor antagonist that has been shown to reduce the rate of growth of cysts and the rate of decline in renal function in patients with ADPKD and has become part of the treatment strategies for the management of patients with ADPKD [5,6,7,8]. However, one the major side effects of these drugs is the extremely high urine output that affects both quality of life and social acceptability, hampering long-term adherence to treatment. Since there are no other treatment options available, reducing the side effect profile of tolvaptan seems urgent. Due to reduced urine osmolarity with V2 receptor antagonists, the total daily urine output also depends on the amount of urine osmols that need to be eliminated daily [9]. Therefore, reducing daily sodium and protein intake may be beneficial in reducing daily urine volume in patients treated with tolvaptan and, therefore, improve tolerability.
In a cohort of ADPKD patients who underwent a single session of personalized dietary counseling to reduce sodium and protein intake, and were treated by tolvaptan, we aimed to identify the contribution of sodium and urea excretion rates to daily urine output and to evaluate the effectiveness of a single dietary counseling session on daily sodium and urea excretion. Secondarily, we examined the rate of decline in eGFR before and after introduction of tolvaptan, and treatment tolerance in this cohort.
Methods
Study design and patient population
This is a retrospective single-center cohort study of patients with ADPKD followed within a structured specialized clinic to initiate tolvaptan at the CHU de Québec Hospital between January 2016 and December 2018. All subjects were adults and were approved to receive tolvaptan by their private insurer or by the Québec government insurance program based on the diagnosis of ADPKD and bilateral disease (class I of Mayo Clinic Image Classification). Total kidney volume (TKV) was determined using kidney volume calculator based on ellipsoid equation (π/6 × L × W × D) from MRI, which was adjusted for height (ml/m), and then plotted against age to categorize each patient according to the Mayo Clinic Image Classification [10]. In four subjects, MRI was not performed and the clear classification was not possible, but these four subjects had bilaterally enlarged kidneys of > 15 cm by ultrasound. Reasons for exclusion were non-adherence to monthly blood samples for surveillance of liver enzymes, possibility of pregnancy during treatment, eGFR < 30 ml/min/1.73 m2, and subjects unwilling to be exposed to polyuria. We also retrieved other variables such as blood pressure, antihypertensive drugs, height and weight. To evaluate the rate of changes in eGFR before and after introduction of tolvaptan, we further excluded patients who did not have at least three serum creatinine measurements over a period of > 3 months before, and at least three serum creatinine measurements over a period of at least 3 months after the introduction of tolvaptan. To evaluate the slope of decline in kidney function before and after initiation of tolvaptan, we used creatinine levels of up to 5 years prior to initiation of tolvaptan, and all creatinine levels after initiation of tolvaptan up to the last follow-up. Before introduction of tolvaptan, 248 serum creatinine measurements were performed in 28 patients over a median duration of 30 months (4.7–29.6), with a median number of samples per patient of 7 (6–9). After the introduction of tolvaptan, 310 measurements of creatinine over a median period of 9.5 (4.5–15.2) months were available for calculation of the slope of eGFR.
Clinical protocol
Subjects underwent two 24-h urine collections and were referred to a dietitian for a single session of personalized dietary counseling of 45 min, with the aim of reducing dietary sodium and protein intake. Patients then began tolvaptan with a starting dose of 45 mg in the morning and 15 mg in the evening, which was then gradually titrated to the maximum tolerated dose over the ensuing 6–12 months. After initiation of tolvaptan, patients were followed regularly for serum liver enzymes, creatinine, sodium and repeated 24-h urine collections.
Biochemical analysis
Creatinine measurements were based on IDMS-calibrated enzymatic method, and eGFR was determined using CKD-EPI formula [11]. Urine creatinine was measured using enzymatic method that had calibration traceable to an IDMS reference. Plasma and urine urea concentrations were determined by urease method. Electrolytes were measured using ion-specific electrodes.
Statistical analysis
Values presented are mean (SD), median (25–75th percentiles), or n (%) as appropriate. Bars in figures represent 95% confidence intervals. Because of variability of urine collection duration, we first calculated the median “24-h” urine creatinine and then adjusted 24-h urine volume, sodium and urea for all other urine collections using each individual’s median creatinine excretion rate, prior to detailed analysis. We then used generalized estimating equations (GEE) to estimate the contribution of sodium and urea excretion rate to the excess urine volume after adjustment for the dose of tolvaptan given at each time point. Using GEE to account for repeated measurements within each individual, we evaluated the impact of dietary intervention on daily sodium excretion overall, and according to the period after intervention (0–4 months, 4–8 months, 8–12 months and > 12 months) with corrected P value (Sidak) for multiple comparisons between each period and baseline. We used a similar procedure for daily urea excretion. To further examine if a dietary intervention was effective in those with higher baseline sodium intake, we separated the group into those with sodium excretion above and below median. To evaluate the differences in the slope of the decline in renal function before and after initiation of tolvaptan, we included all patients who had at least three measurement of creatinine over a period of > 3 months. Then we used GEE to build a model to estimate the slope of eGFR before and after initiation of treatment. As part of sensitivity analysis, we also included the percentage of the maximal daily dose of angiotensin converting enzyme inhibitors and/or angiotensin receptor blockers (ACEi/ARB) at the time of eGFR measurement as a covariate into the model. All statistical analyses were performed using SPSS 25.0 (SPPS Inc., Chicago, IL, USA).
Results
Figure 1 shows the study flowchart. Table 1 shows the baseline characteristics of the 30 subjects with respect to demographic, renal function, Mayo Clinic Image Classification of ADPKD, and urinary parameters. There were 66 24-h urine collections before dietary intervention at baseline, and 98 urine collections during follow-up after dietary counseling and treatment with tolvaptan. The coefficient of variation of the 24-h urine creatinine excretion was 11 ± 5%.
Determinants of urine volume
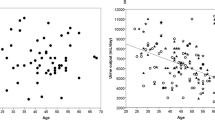
Figure 2a shows the maximal tolerated dose of tolvaptan. Only 30% tolerated the dose of 120 mg daily. As shown, even in patients with lower doses of tolvaptan, the daily urine volume was between 4.9 and 5.8 l/day (Fig. 2b). After adjusting for the dose of tolvaptan, an increase in sodium excretion rate by 50 mmol/day was associated with an estimated additional urine volume of 0.6 l/day (95% CI 0.4–0.8 l/day; P < 0.001), while an increase in urea excretion by 50 mmol/day was associated with an estimated additional urine volume of 0.25 l/day (95% CI 0.11–0.39 l/day; P < 0.001).
Impact of dietary counseling on sodium and urea excretion rates
Accounting for multiple urine collections before and after dietary counseling, Fig. 3a shows the estimate of daily sodium excretion at baseline and during treatment with tolvaptan after dietary counseling. Overall, there was a reduction of 13 mmol/day (95% CI 0.2–26.6; P = 0.046) after dietary counseling. However, further analysis shows that the reduction was more important and statistically significant during the first 4 months after dietary counseling, but then sodium excretion gradually returned to near baseline values subsequently (Fig. 3b).
Sodium excretion rate after dietary counseling. a Changes in sodium excretion when all urine collections were considered after dietary counseling. b Changes in sodium excretion after dietary counseling over time. c Changes in sodium excretion after dietary counseling according to baseline level of sodium excretion. d Changes in sodium excretion after dietary counseling according to baseline level of sodium excretion over time
Then we examined the impact of the dietary counseling according to the baseline level of sodium excretion below and above median level of 153 mmol/day. We found that the efficacy of dietary intervention was more apparent in patients with higher levels of baseline sodium excretion (Fig. 3c, d).
The number of available urine collections with daily urea excretion was lower (n = 138). Figure 4a shows that daily urea excretion declined after dietary intervention by 69 mmol/day (95% CI 18–121, P = 0.008). Figure 4b shows a more consistent reduction in urea excretion over time.
Loss of eGFR before and after tolvaptan
During a median observation period of 30 months before tolvaptan, the annual change in eGFR was − 5.5 ml/min/1.73 m2/year (95% CI − 8.2 to − 2.7; P < 0.001), and during a median observation period of 9.5 months after tolvaptan, the annual change in eGFR was − 1.3 ml/min/1.73 m2/year (95% CI − 2.8 to 0.06; P = 0.060), with a statistically significant difference between slopes of eGFR (P = 0.02). As part of sensitivity analysis, we examined whether changes in the doses of ACEi/ARBs could have affected the changes in the slope of the eGFR. We, therefore, incorporated into the model a covariable representing the percentage of maximal daily dose of an ACEi or ARB. By doing so, the results remained similar with ∆eGFR of − 5.4 ml/min/1.73 m2/year (95% CI − 8.3 to − 2.6; P < 0.001) before and of − 1.4 (95% CI − 2.8 to 0.029; P = 0.055) after initiation of tolvaptan, with a statistically significant difference between the slopes (P = 0.018).
Treatment tolerance and modification
Treatment modification occurred in six patients (20%), one (3%) in whom the dose was reduced due to important degree of nocturia, 2 (7%) who underwent temporary interruption of tolvaptan, and 3 (10%) who discontinued the medication permanently. The cause of permanent discontinuation of the medication was mostly related to dehydration symptoms and the inability of subjects to chronically drink large quantities of water. There were only four patients (13%) with hypernatremia, but all serum sodium levels were < 149 mmol/l. One patient who had a slightly elevated ALT level before tolvaptan initiation maintained a fluctuating level of ALT during treatment, which was always < 1.5 times the upper limit of normal.
Discussion
This cohort study shows that sodium and urea excretion rates contribute significantly to the daily urine output in patients treated with tolvaptan and that a single session of dietary counseling was effective in reducing sodium and urea excretion rates. Osmol excretion is the major determinant of urine volume in patients taking V2 receptor antagonists [9]. Restriction of osmol intake may, therefore, limit V2 receptor antagonist-induced polyuria, giving patients more control over urine volume-related side effects and improving the tolerability of these drugs. In our study, the aim of dietary intervention was reducing sodium and protein intake. Globally, the effect of dietary intervention did not last beyond the first 4 months for sodium intake. However, in patients with higher baseline level of sodium excretion, the dietary intervention seemed to be more effective. This seems logical as when the baseline sodium intake is already low, it becomes harder to further reduce it. However, repeated dietary intervention may be necessary to maintain a long-term adherence to lowering of sodium intake. In addition, a dietary intervention to reduce the 24-h osmolar load by means of reducing protein intake seemed to be more effective and more persistent over time.
In addition, in this small cohort, there was a significant attenuation of decline in renal function even though 36% of the subjects received a lower than recommended dose of tolvaptan.
The annualized rate of loss of renal function before introduction of tolvaptan was greater in this cohort (− 5.5 ml/min/1.73 m2) than previously reported in the placebo group of TEMPO3:4 (− 3.70 ml/min/1.73 m2) [6]. However, compared to the TEMPO3:4 trial, despite similar age, the TKV in our subjects was greater (2230 ml versus 1668 ml) and the eGFR was much lower (52 versus 82 ml/min/1.73 m2). Compared to Reprise Trial where the loss of eGFR in the placebo group was − 3.61 ml/min/1.73 m2, our subjects were younger (44 vs 47), had a higher baseline eGFR (52 versus 41 ml/min/1.73 m2), but the TKV was not reported in the Reprise trial [7]. The apparent greater benefit of tolvaptan in our study may be related to the fact that the patients were more at risk of progression of kidney disease. Indeed, there was some indication in the TEMPO3:4 Trial that the benefits were nominally greater in patients older than 35 years of age and those with TKV > 1500 ml. Based on Mayo Clinic Image Classification, 26 of the patients who underwent imaging studies were classified as 1C-E, who are at greater risk of eGFR loss [10, 12]. Finally, the rate of decline in renal function during the tolvaptan period was surprisingly small. However, this could be explained by a rapid reduction of the size of the cysts early during treatment, which is mediated through reduced fluid secretion into the cysts [5, 6, 13, 14]. Therefore, a longer duration of follow-up is necessary to evaluate whether the rate of decline in renal function remains the same.
The study has several strengths and limitations. First, the conclusions are based on a higher number of urine collections and many data points for the evaluation of eGFR both before and after initiation of tolvaptan. However, one of the disadvantages of 24-h urine collections is their potential unreliability; but in our study, the coefficient of variation of 24-h creatinine excretion was 11%. In addition, care was taken to adjust all urinary-related parameters for each individual’s median creatinine excretion, providing assurance that the results are reliable. The rate of decline in renal function was not an indication for starting tolvaptan, and hence the findings are relatively robust against regression towards mean phenomenon. Nevertheless, in four patients, total kidney volume was not available, and the study has the inherent limitations of a retrospective cohort study with relatively small number of subjects. In addition, the reliability of determination of sodium and urea in a very dilute urine was not specifically addressed in this study. Finally, the duration of the follow-up on tolvaptan was rather limited with a median of 9.5 months and, therefore, extrapolation of the slope of decline in eGFR needs to be confirmed on longer duration follow-up.
In conclusion, this cohort study shows that sodium and urea excretion rates are important determinants of daily urine output in patients taking V2 receptor antagonists, and that repeated dietary counseling may be effective in reducing both sodium and urea excretion rates, and potentially reduce the inconveniences related to urine volume. This study supports that dietary counseling to reduce osmole excretion rate should be incorporated in the management of ADPKD treated by tolvaptan.
References
Spithoven EM, Kramer A, Meijer E, Orskov B, Wanner C, Abad JM, Areste N, de la Torre RA, Caskey F, Couchoud C, Finne P, Heaf J, Hoitsma A, de Meester J, Pascual J, Postorino M, Ravani P, Zurriaga O, Jager KJ, Gansevoort RT, Registry E-E, Euro CC (2014) Renal replacement therapy for autosomal dominant polycystic kidney disease (adpkd) in Europe: prevalence and survival—an analysis of data from the era-edta registry. Nephrol Dial Transplant 29(Suppl 4):iv15–iv25
The European Polycystic Kidney Disease Consortium (1994) The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell 78:725
Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJM, Somlo S (1996) Pkd2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272:1339–1342
Torres VE, Harris PC (2009) Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int 76:149–168
Reif GA, Yamaguchi T, Nivens E, Fujiki H, Pinto CS, Wallace DP (2011) Tolvaptan inhibits erk-dependent cell proliferation, cl(-) secretion, and in vitro cyst growth of human adpkd cells stimulated by vasopressin. Am J Physiol Renal Physiol 301:F1005–1013
Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS, Investigators TT (2012) Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367:2407–2418
Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, Ouyang J, McQuade RD, Blais JD, Czerwiec FS, Sergeyeva O, Investigators RT (2017) Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med 377:1930–1942
Soroka S, Alam A, Bevilacqua M, Girard LP, Komenda P, Loertscher R, McFarlane P, Pandeya S, Tam P, Bichet DG (2018) Updated Canadian expert consensus on assessing risk of disease progression and pharmacological management of autosomal dominant polycystic kidney disease. Can J Kidney Health Dis 5:2054358118801589
Kramers BJ, van Gastel MDA, Boertien WE, Meijer E, Gansevoort RT (2019) Determinants of urine volume in adpkd patients using the vasopressin v2 receptor antagonist tolvaptan. Am J Kidney Dis 73:354–362
Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, Bae KT, Chapman AB, Grantham JJ, Mrug M, Hogan MC, El-Zoghby ZM, Harris PC, Erickson BJ, King BF, Torres VE, Investigators C (2015) Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol 26:160–172
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Irazabal MV, Blais JD, Perrone RD, Gansevoort RT, Chapman AB, Devuyst O, Higashihara E, Harris PC, Zhou W, Ouyang J, Czerwiec FS, Torres VE (2016) Prognostic enrichment design in clinical trials for autosomal dominant polycystic kidney disease: the tempo 3:4 clinical trial. Kidney Int Rep 1:213–220
Higashihara E, Torres VE, Chapman AB, Grantham JJ, Bae K, Watnick TJ, Horie S, Nutahara K, Ouyang J, Krasa HB, Czerwiec FS (2011) Tempoformula, Study I. Tolvaptan in autosomal dominant polycystic kidney disease: three years' experience. Clin J Am Soc Nephrol 6:2499–2507
Irazabal MV, Torres VE, Hogan MC, Glockner J, King BF, Ofstie TG, Krasa HB, Ouyang J, Czerwiec FS (2011) Short-term effects of tolvaptan on renal function and volume in patients with autosomal dominant polycystic kidney disease. Kidney Int 80:295–301
Funding
F Mac-Way holds scholarships from the Fonds de Recherche Québec-Santé.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
FM has received conference honoraria from Amgen and Sanofi, and received consultative honoraria from Otsuka; CL and MA have received consultative honoraria from Otsuka; PRC has received honoraria for consultation and CMEs from Otsuka Canada.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Côté, G., Asselin-Thompstone, L., Mac-Way, F. et al. Sodium and urea excretion as determinants of urine output in autosomal dominant polycystic kidney disease patients on V2 receptor antagonists: impact of dietary intervention. Int Urol Nephrol 52, 343–349 (2020). https://doi.org/10.1007/s11255-020-02384-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-020-02384-3