Abstract
Background
A recent study demonstrated that tolvaptan slowed estimated glomerular filtration rate (eGFR) decline in later-stage autosomal dominant polycystic kidney disease (ADPKD) patients. However, Japanese patients were not included in that trial, therefore tolvaptan’s efficacy in Japanese patients with advanced chronic kidney disease (CKD) has remained unknown.
Methods
In this prospective cohort study, 54 patients with ADPKD who had eGFR ≥ 15 ml/min/1.73 m2 and total kidney volume (TKV) ≥ 750 ml were treated with tolvaptan. The primary endpoint was the change in height-adjusted total kidney volume (htTKV) and eGFR after 1-year treatment with tolvaptan. Then, we compared the primary endpoint between later CKD stage (baseline eGFR < 45 ml/min/1.73 m2) and earlier CKD stage (baseline eGFR ≥ 45 ml/min/1.73 m2).
Results
The rate of kidney growth during the 1-year treatment did not differ significantly between earlier and later CKD stages. The median and interquartile range of relative change in htTKV in later CKD stage was 8.2%/year [4.4, 26.6], as compared with 5.7%/year [1.6, 16.4] in earlier CKD stage (p = 0.17). Nor did the rate of eGFR decline between earlier and later CKD stages. The relative annual change in eGFR in later CKD stage was − 9.7%/year [− 15.9, − 2.1], as compared with − 6.8%/year [− 11.1, 0.1] in earlier CKD stage (p = 0.18).
Conclusion
This analysis indicates that the efficacy of tolvaptan for Japanese patients with later stage ADPKD was not significantly different from that of Japanese patients with earlier stage ADPKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is one of the most common hereditary disorders. It results in progressive cystic enlargement that causes decreased kidney function and massive nephromegaly, and is the fourth leading cause of end-stage renal disease in adults worldwide [1, 2]. In Japan, more than 30,000 ADPKD patients have been diagnosed, accounting for 3–5% of dialysis patients [3]. Although dramatic reduction in total kidney volume (TKV) and good survival was observed after arterial embolization [4, 5], these procedures are limited to patients on dialysis.
In the last 10 years, various clinical interventional trials for ADPKD have been published [6]. Tolvaptan—vasopressin V2-receptor antagonists—actually slowed the increase in TKV and the decline in kidney function in ADPKD patients aged 18–50 years and who had a TKV ≥ 750 ml and estimated creatinine clearance ≥ 60 ml/min [7]. However, since tolvaptan users always encounter polyuria, most patients are willing to start tolvaptan use when they become symptomatic—with greater TKV or worsened kidney function [8]. Recently in the Replicating Evidence of Preserved Renal Function: an Investigation of Tolvaptan Safety and Efficacy in ADPKD (REPRISE) trial, tolvaptan was shown to be effective for later-stage ADPKD patients (CKD stage G2 to early stage G4) as well [9]. But most of the enrolled patients in the study were of white race, and there were very few Asian patients and no Japanese patients. Furthermore, plasma arginine vasopressin levels are different between races [10], which could lead to racial differences in the therapeutic response to the vasopressin receptor antagonist. Therefore, a better understanding of the efficacy of tolvaptan for Japanese ADPKD patients with later CKD stages remains to be established. We herein compared the efficacy and safety of tolvaptan for later CKD stage patients, whose estimated glomerular filtration rate (eGFR) is less than 45 ml/min/1.73 m2, with that of earlier CKD stage whose eGFR is 45 ml/min/1.73 m2 or greater.
Materials and methods
Patients and study design
In this prospective cohort study, all patients who received tolvaptan for the treatment of ADPKD in our facilities from June 2014 through December 2015 were included after having given informed consent. Eligibility requirements included a diagnosis of ADPKD according to Ravine’s revised unified diagnostic criteria [11, 12], eGFR > 15 ml/min/1.73 m2, and a TKV of 750 ml or more as measured by magnetic resonance imaging (MRI) or computed tomography (CT). The eGFR was calculated by the formula for Japanese patients devised by Matsuo et al. [13]. The exclusion criterion was severe liver dysfunction (total bilirubin > 2.0 mg/dl). All patients initiated 60 mg of tolvaptan in a split-dose regimen with 45 mg in the morning and 15 mg in the evening. They were monitored for safety for at least 3 days, then they continued taking tolvaptan on their own. The dose was modified according to patients’ reports of their tolerance, with a maximum dose of 120 mg/day.
Patients’ demographic data, which included kidney volume, were obtained when they first used tolvaptan, then at 6 months and 12 months. All laboratory data—including serum total protein, serum albumin, serum creatinine, aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase—were collected before treatment, at 3 and 5 days, and 1, 3, 6, and 12 months afterwards. Kidney volumes were calculated by using ellipsoid volume equations with three axis measurements, as in our previous studies [14, 15]. All laboratory values were measured by the automated, standardized methods used in our hospital within 24 h after drawing blood samples. The primary endpoints of the study were change of eGFR and height-adjusted total kidney volume (htTKV) after 1-year treatment. The secondary endpoints were drug safety and tolerability, which were assessed every month throughout the follow-up. Patients were judged to tolerate their doses when the final dose of tolvaptan was equal to or higher than the initial dose (60 mg/day). Side effects predefined in our study included liver enzyme elevation (double the normal upper limit), hypernatremia (≥ 145 mEq/l), and any known side effects of tolvaptan that led to drug discontinuation. The study’s protocol adhered to the requirement of STROBE, and was approved by the institutional review board of Toranomon Hospital, Japan (IRB No. 840). This study was registered with the University Hospital Medical Information Network (No. UMIN000013880).
Statistical analyses
Data were summarized using proportions, means with standard deviation, or median with interquartile range (IQR) as appropriate. Categorical variables were analyzed with the chi-squared or Fisher’s exact test, and continuous variables were compared using Student’s t-test, the Mann–Whitney U test, ANOVA, or Kruskal–Wallis test. Changes in eGFR and htTKV after 1-year treatment were assessed by both absolute (1-year data minus baseline data) and relative values (absolute values divided by baseline). Associations of baseline renal function with change in htTKV and eGFR were assessed by Spearman’s rank correlation test. All analyses were performed with R version 3.3.2 (The R Foundation for Statistical Computing, Vienna, Austria) and Stata SE version 14.2 (StataCorp, College Station, Texas, USA).
Results
Patient demographics
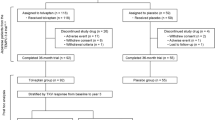
From June 2014 to December 2015, 56 patients received tolvaptan for the treatment of ADPKD in Toranomon Hospital and Toranomon Hospital Kajigaya (Fig. 1), with one patient excluded because he received partial renal transcatheter arterial embolization (TAE) before introduction of tolvaptan, while another patient died of subarachnoid hemorrhage during the observation period. Thus, 54 patients were analyzed for kidney function (of whom 28 patients were categorized as earlier CKD stage; 26 patients as later CKD stage) although two of them were excluded for kidney volume analyses because of lack of measurable follow-up MRI or CT scans. So, finally, 52 patients were analyzed for total kidney volume. Demographic and baseline characteristics (Table 1) did not differ in any CKD stages except for htTKV (p = 0.004) and eGFR (p < 0.001).
Study flowchart. Fifty-six patients received tolvaptan for the treatment of ADPKD, with one patient excluded because he received partial renal TAE before introduction of tolvaptan. Another patient died of subarachnoid hemorrhage during the observation period. Thus, 54 patients were analyzed for kidney function, although two of them were excluded from the kidney volume analyses because of lack of measurable follow-up MRI or CT scans. Finally, 52 patients were analyzed for total kidney volume. ADPKD autosomal dominant polycystic kidney disease, TAE transcatheter arterial embolization, MRI magnetic resonance imaging, CT computed tomography
Change in htTKV by CKD stage
We assessed the absolute and relative changes in htTKV at 1 year in all eligible patients. The median [IQR] value of absolute annual change in htTKV was 75 [44, 267] ml/year in later CKD stage, as compared with 36 [8, 136] ml/year in earlier CKD stage (p = 0.054). Relative annual change in htTKV (%/year) was 8.2 [4.4, 26.6] in later CKD stage, as compared with 5.7 [1.6, 16.4] in earlier CKD stage (p = 0.17) (Table 2; Fig. 2). Therefore, though there was a trend for change of htTKV to be higher in later CKD stage, the differences between earlier and later CKD stage were not significant in our cohort.
Change in height-adjusted total kidney volume (htTKV) after 1-year tolvaptan use among CKD stages. Changes in htTKV after 1-year treatment were assessed by both absolute (1-year data minus baseline data) and relative values (absolute values divided by baseline). a Absolute change in htTKV per year. Median [IQR] of absolute annual change (ml/year) in htTKV was 75 [44, 267] in later CKD stage vs. 36 [8, 136] in earlier CKD stage (p = 0.054). b Relative change in htTKV per year. Relative annual change (%/year) in htTKV was 8.2 [4.4, 26.6] in later CKD stage vs. 5.7 [1.6, 16.4] in earlier CKD stage (p = 0.17)
Change in eGFR by CKD stage
Next, we observed the absolute and relative change in eGFR at 1 year in our cohort. The median [IQR] absolute annual change in eGFR was − 2.8 [− 4.7, − 0.6] ml/min/1.73 m2 in later CKD stage, as compared with − 3.3 [− 5.7, 0.0] ml/min/1.73 m2 in earlier CKD stage (p = 0.64). The relative annual change (%/year) from baseline in eGFR was − 9.7 [− 15.9, − 2.1] in later CKD stage vs. − 6.8 [− 11.1, 0.0] in earlier CKD stage (p = 0.18) (Table 2; Fig. 3). Therefore, there was no significant difference in annual eGFR changes between earlier and later CKD stages.
Change in estimated glomerular filtration rate (eGFR) after 1-year tolvaptan use among CKD stages. Changes in eGFR after 1-year treatment were assessed by both absolute (1-year data minus baseline data) and relative values (absolute values divided by baseline). a Absolute change in eGFR per year by CKD stage in our cohort. Median [IQR] of absolute annual change (ml/min/1.73 m2) in eGFR was − 2.8 [− 4.7, − 0.6] in later CKD stage vs. − 3.3 [− 5.7, 0.0] in earlier CKD stage (p = 0.64). b Relative change in eGFR compared with the baseline eGFR. The relative annual change (%/year) from baseline in eGFR was − 9.7 [− 15.9, − 2.1] in later CKD stage vs. − 6.8 [− 11.1, 0.0] in earlier CKD stage (p = 0.18)
Correlation between baseline eGFR and primary outcome by CKD stage
We analyzed the correlation between the baseline eGFR and annual change in eGFR. Baseline eGFR was not significantly correlated with either absolute change in eGFR (p = 0.47) or relative change in eGFR (p = 0.19) at Spearman’s rank correlation test. These results were consistent with the result of the primary endpoint, i.e. no significant difference in eGFR change between earlier and later CKD stages.
On the other hand, there was a significant negative correlation between baseline eGFR and absolute annual change in htTKV (p = 0.03). This result supports the trend of the primary outcome that htTKV change in later CKD stage was higher than that of earlier CKD stage, although the difference was not significant. At the same time, there was no significant correlation between baseline eGFR and relative change in htTKV (p = 0.17); therefore, the correlation between baseline eGFR and htTKV change could not be determined.
Drug tolerance and safety
During the follow-up period, 3 patients had to decrease the dose of tolvaptan because of a difficulty in drinking enough water and 4 patients agreed to increase the tolvaptan dose to 90 mg/day because they reported tolerance for such a dose (Table 3). No patient wanted to increase the tolvaptan dose to 120 mg/day during the follow-up period.
Final doses of tolvaptan and the level of drug tolerance did not differ between earlier and later CKD stages. Proportion of tolerance in later CKD stage was 92.0%, as compared with 96.3% in earlier CKD stage (p = 0.60) (Table 3). Two patients discontinued tolvaptan during the 1-year follow-up period. One patient in earlier CKD stage died of subarachnoid hemorrhage, and another patient in later CKD stage stopped taking tolvaptan when hospitalized due to subarachnoid hemorrhage. The attending physicians reported that there was no causal relationship between tolvaptan use and the onset of subarachnoid hemorrhage.
Discussion
In this study, we found that the change in eGFR in patients with later CKD stage after 1-year treatment was similar to that in patients with earlier CKD stage. This finding suggests that even in Japanese ADPKD patients, the treatment effect of tolvaptan to slow eGFR decline in later CKD stage is similar to that of earlier CKD stages, as observed in the REPRISE trial. On the other hand, there was a trend for an increased growth in htTKV as CKD stage advances, as shown by the correlation test between baseline eGFR and htTKV growth; thus, further investigation in a larger number of patients could lead to a significant difference between earlier and later CKD stages in htTKV change. Torres et al.’s 1-year trial (REPRISE) reported that administration of tolvaptan in patients with later stage ADPKD slowed the eGFR decline, and the treatment effect was similar to that of early stage ADPKD patients. However, no Japanese patients and only a very small number of Asian patients were included in that study. In the present study, all patients were Japanese, and the change in total kidney volume and eGFR after 1-year treatment did not differ significantly between earlier and later CKD stage.
This study has some limitations. First, since the study’s aim was to clarify the change in eGFR and htTKV—and the safety of tolvaptan across CKD stages—this study did not have placebo control. So our results cannot directly prove the efficacy of tolvaptan in Japanese ADPKD patients with advanced CKD. Second, the number of participants is not large, which may have led to the risk of false positives. Larger studies with longer observation periods would be necessary to validate our findings. Finally, we calculated total kidney volume by using ellipsoid volume equations with three axis measurements, a technique that lacks accuracy in volume measurement compared with computed measurements. However, since kidney volume calculated by ellipsoid equations correlates highly with kidney volume measured by a 3D workstation, ellipsoid kidney volume is still reliable to follow up the relative change in total kidney volume [16].
In conclusion, our study suggests that the efficacy of tolvaptan for kidney function does not differ significantly in Japanese APKD patients between earlier and later CKD stages while the beneficial effect of kidney growth is more easily observed in those with better renal function. This study also highlights the fact that tolvaptan could be administered without increased frequency of adverse events in patients with reduced renal function. Our findings of a potential benefit of tolvaptan in Japanese patients, however, should be confirmed by a further, randomized study in Japanese patients.
References
Torres VE, Harris PC, Pirson Y (2007) Autosomal dominant polycystic kidney disease. Lancet 369:1287–1301
Grantham JJ (2008) Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med 359:1477–1485
Muto S, Kawano H, Higashihara E, Narita I, Ubara Y, Matsuzaki T et al (2015) The effect of tolvaptan on autosomal dominant polycystic kidney disease patients: a subgroup analysis of the Japanese patient subset from TEMPO 3:4 trial. Clin Exp Nephrol 19:867–877
Ubara Y, Tagami T, Sawa N, Katori H, Yokota M, Takemoto F et al (2002) Renal contraction therapy for enlarged polycystic kidneys by transcatheter arterial embolization in hemodialysis patients. Am J Kidney Dis 39(3):571–579
Junichi Hoshino T, Suwabe N, Hayami, Keiichi S, Yoshifumi U et al (2015) Survival after arterial embolization therapy in patients with polycystic kidney and liver disease. J Nephrol 28(3):369–377
Iliuta IA, Kitchlu A, Pei Y (2017 Jun) Methodological issues in clinical trials of polycystic kidney disease: a focused review. J Nephrol 30(3):363–371
Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E et al (2012) Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367:2407–2418
Grantham JJ, Torres VE, Chapman AB et al (2006) Volume progression in polycystic kidney disease. N Engl J Med 354:2122–2130
Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G et al (2017) Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med 377:1930–1942
Bakris G, Bursztyn M, Gavras I, Bresnahan M, Gavras H (1997) Role of vasopressin in essential hypertension: racial differences. J Hypertens 15(5):545–550
Pei Y, Obaji J, Dupuis A, Paterson AD, Magistroni R, Dicks E, Parfrey P, Cramer B, Coto E, Torra R, San Millan JL, Gibson R, Breuning M, Peters D, Ravine D (2009) Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 20:205–212
Pei Y (2006) Diagnostic approach in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 1:1108–1114
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53:982–992
Ubara Y, Tagami T, Sawa N, Katori H, Yokota M, Takemoto F, Inoue S, Kuzuhara K, Hara S, Yamada A (2009) Renal contraction therapy for enlarged polycystic kidneys by transcatheter arterial embolization in hemodialysis patients. Am J Kidney Dis 39:571–579
Suwabe T, Ubara Y, Mise K, Ueno T, Sumida K, Yamanouchi M, Hayami N, Hoshino J, Kawada M, Imafuku A, Hiramatsu R, Hasegawa E, Sawa N, Takaichi K (2015) Suitability of patients with autosomal dominant polycystic kidney disease for renal transcatheter arterial embolization. J Am Soc Nephrol 27:2177–2187
Higashihara E, Nutahara K, Okegawa T, Tanbo M, Hara H, Miyazaki I, Kobayasi K, Nitatori T (2015) Kidney volume estimations with ellipsoid equations by magnetic resonance imaging in autosomal dominant polycystic kidney disease. Nephron 129:253–262
Acknowledgements
This work was supported by JH’s research grants from Grants-in-Aid for Scientific Research (JSPS KAKENHI) Grant number 15K08719 and 18K08227, the Okinaka Memorial Institute, The Kidney Foundation Japan (JKFB 15–23), and Toranomon Hospital. No funding bodies had any role in study design, data analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interst
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee at which the studies were conducted (IRB approval number 840) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Oguro, M., Kogure, Y., Hoshino, J. et al. Tolvaptan in Japanese patients with later-stage autosomal dominant polycystic kidney disease. J Nephrol 31, 961–966 (2018). https://doi.org/10.1007/s40620-018-0545-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-018-0545-8