Abstract
Meningeal solitary fibrous tumors (SFTs) and hemangiopericytomas (HPCs) had been combined into a single classification until 2016. Recurrence and metastases rates are still understudied, especially for spinal SFT/HPCs. Here, we describe CNS SFT/HPCs and predictors for recurrence, metastases, and death, in spinal and intracranial SFT/HPCs, separately. We collected data from studies with patient-level data available on primary SFT/HPCs from multiple online databases. Clinico-demographic data, surgical outcomes, recurrence, metastases, and death rates were abstracted. We used logistic and Cox regression models to identify predictors for recurrence, metastases, and death for spinal and intracranial SFT/HPCs. Twenty-nine studies (368 patients) were included. Higher histological grade and subtotal resection were associated with recurrence (p values < 0.05), while higher histological grade and recurrence (p values < 0.005) were associated with metastases formation. Time to recurrence (p < 0.005) and metastases (p < 0.001) formation were shorter for spinal SFT/HPCs. Death rates were higher among intracranial SFT/HPC patients (p value = 0.001). Among patients with higher histological grade, rates of metastases formation were different between intracranial and spinal SFT/HPCs. Risk of metastases was higher in the first 5 years from surgery for both intracranial and spinal SFT/HPCs. Meningeal SFT/HPCs patients have high rates of recurrence and metastasis, which occur mostly within the first 5 years after diagnosis. Spinal and intracranial SFT/HPCs show similar behavior, but spinal SFT/HPCs tend to develop metastases and recurrences in a shorter interval of time. Careful follow-up for spinal SFT/HPCs should be considered because spinal cases seem to be slightly more aggressive and require more attention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meningeal solitary fibrous tumors (SFTs) are uncommon neoplasms affecting the central nervous system (CNS). Although meningeal SFT and hemangiopericytomas (HPCs) were thought to represent distinct entities, the identification of NAB2–STAT6 fusion as a defining molecular alteration in both tumors has led to a combined classification of these tumors at both dural and extradural sites [16]. Before 2016, meningeal SFTs/HPCs had been categorized into two groups—classic, typically more benign SFTs, and HPCs, which are usually more aggressive tumors [31]. Previous literature has shown that SFT/HPCs account for 1.9 to 4% of intracranial tumors [4, 9, 23, 25, 35, 38, 47, 59]. Mean age at presentation ranges from 38 to 45 years [2, 6, 24, 54], and these tumors are diagnosed more frequently in men [12, 17, 20, 39, 40, 57]. The time between initial symptoms and diagnosis ranges from 3.1 to 7.5 months [1, 8, 15]. However, unlike with other primary neoplasms, the natural history of primary spinal and intracranial lesions and of related recurrences or metastases is still not fully understood. Because of the widely variable time interval between recurrences and metastases formation for primary SFT/HPCs, there are no clear indications for imaging follow-up, particularly for spinal SFT/HPCs [29, 45, 49, 58].
There is a paucity of evidence in the literature exploring characteristics and risk factors associated with patient outcomes in these tumors. Indeed, only case reports and case series of SFT/HPCs are available. Therefore, it is difficult to draw valid prognostic considerations from these studies with few patients, especially concerning the subpopulation of spinal SFT/HPCs. To our knowledge, a review aggregating data from these reports and series has yet to be published. To increase statistical power in this systematic review, we reviewed all of the literature on intracranial and spinal SFT/HPCs published in the last 20 years, updating tumor definitions to the most recent World Health Organization (WHO) guidelines [2]. Furthermore, we systematically compared characteristics and outcomes between spinal and intracranial SFT/HPCs.
We aimed to describe the behavior of this specific subset of neoplasm and determine risk factors for both recurrences and metastasis, in particular for spinal SFT/HPCs. We additionally compared spinal SFT/HPCs to a subset of patients with intracranial SFT/HPCs.
Methods
This systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA, 2009) guidelines (Fig. 1) [22].
Literature search, study selection, and eligibility criteria
An expert medical reference librarian conducted a comprehensive search of several databases from January 2000 to January 2020. Search terms were “CNS hemangiopericytoma,” “CNS solitary fibrous tumor,” “HPC metastasis,” “SFT metastasis,” “meningeal hemangiopericytoma,” “meningeal solitary fibrous tumor,” “spinal HPC,” “spinal SFT,” “recurrent HPC,” “recurrent SFT,” and “hemangiopericytoma metastasis,” used alone and in combination. Databases included Ovid, MEDLINE, Epub Ahead of Print, Ovid Medline In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, and Scopus. Controlled vocabulary supplemented with keywords was used to search for recurrent and metastatic SFT/HPCs and the correct timing for imaging follow-up. Further studies were identified through the review of references of the retrieved articles and relevant reviews.
The questions posed in this study were the following:
-
I.
What are the characteristics and outcomes of primary intracranial and spinal SFT/HPCs?
Ia. What are the factors associated with recurrence and metastases of primary.
spinal and intracranial SFT/HPCs?
Ib. What are the factors affecting survival in patients with primary SFT/HPCs?
-
II.
How do spinal SFT/HPCs behave in comparison to intracranial SFT/HPCs?
Because the primary aim of the study was focused on spinal SFT/HPCs, we tailored our research strategy to investigate these tumors; however, we additionally collected all available data on intracranial SFT/HPCs to allow for comparison between intracranial and spinal SFT/HPCs populations.
Inclusion criteria for the studies in this systematic review were the following:
-
1.
Prospective and retrospective longitudinal studies describing primary intracranial and spinal SFT/HPCs;
-
2.
Surgical resected SFT/HPCs;
-
3.
Non-recurrent or non-metastatic SFT/HPCs at presentation. Studies on recurrent SFT/HPCs in which no clinicopathological information about the primary tumor was available were excluded;
-
4.
Case series with individual patient-level data with usable information regarding recurrence and histologically confirmed metastases;
-
5.
Studies published in English;
-
6.
Studies published after 2000.
Case reports, editorials, technical notes, meta-analyses, review articles, and duplicative reports were excluded, along with studies published in any language other than English. One author (E.G.) reviewed and selected the included studies.
Data abstraction
For each study, we extracted the following data: age, sex, intracranial location (supratentorial vs. infratentorial), spinal location (cervical, thoracic, and lumbar), number of recurrences, number of metastases, metastases location, diameter, degree of resection (gross total resection (GTR) vs. subtotal resection (STR)), vertebral bone erosion (i.e., any degree of bone erosion), upfront adjuvant radiotherapy (RT) administration, histological grade, time to recurrence and metastases detection, death, time to death, and overall follow-up time in patient-years. These variables were defined, for the purposes of this study, accordingly:
-
1.
Recurrent disease was considered both as regrowth in the same site or a formation of an in situ de novo lesion;
-
2.
Histologically confirmed metastases as any extracranial and extra-spinal de novo lesion;
-
3.
Primary spinal and intracranial SFT/HPCs at diagnosis;
-
4.
Histopathological data were defined and included according to the most recent CNS (2016 [31]) classification scheme. For both intracranial and spinal SFT/HPCs, tumors with a classic SFT/HPC histopathological phenotype and fewer than five mitoses (× 10 HPF) were considered grade I; tumors with intermediate or HPC phenotype and fewer than five mitoses (× 10 HPF) were considered grade II; tumors with five or more mitoses (× 10 HPF) were considered grade III, irrespective of their histopathological phenotype.
-
5.
The major diameter measured in centimeters;
-
6.
Spinal location was classified according to the classification proposed by Liu et al.: type I as extradural tumor (IA–intracanal, IB–extracanal), type II as intradural type (IIA–extramedullary, IIB–intramedullary), and type III as intradural tumor with extension into the extradural and paravertebral area;
-
7.
Upfront radiation therapy data were considered eligible to be included in the analysis only if adjuvant to the primary tumor.
We extracted and analyzed primary intracranial and spinal SFT/HPCs patients separately, calculating the number of and mean time to recurrences and metastases for both samples individually. We reviewed pathological findings for each case described and carefully reviewed the methodology applied in each series for pathological diagnosis, to adhere as strictly as possible to the most recent WHO guidelines [16, 31]. When necessary, the grading was updated to actual scores. Not every study in this analysis reported all of these variables and outcomes; however, they were included in the patient-level analysis if recurrence and extracranial metastasis data were provided. The included studies are reported in Table 1.
Evaluation of methodological quality
For each study, the risk of bias was assessed with the modified Newcastle-Ottawa Scale for quality assessment. Bias was defined based on the following questions: did the study include all patients or consecutive patients with adequate clinical follow-up (at least 12 months)? Was the outcome assessment objective and replicable? What was the study design (prospective vs. retrospective)? Did the histological criteria overlap with or adhere to the 2016 WHO guidelines for CNS tumors?
Studies judged to have low risk of bias were defined as those with a predefined study protocol (prospective or randomized study), adequate clinical follow-up (≥ 12 months), objective and replicable outcome assessment, prospective design, and pathological diagnosis based on or re-codable to 2016 WHO classification of CNS tumors. Bias scores were ranked in Table 2, and studies with scores lower than 6 were considered as having a high risk of bias. If the scores for the articles were not the same between two reviewers, a further discussion between all authors was pursued until an agreement was reached. We found 16 studies with a low risk of bias (score ≥ 6), 8 with a moderate and (score = 6), and 5 with a high risk of bias (score ≤ 5).
Statistical analysis
Descriptive statistics were reported as median and range for continuous variables and number and percentage for categorical variables. The Mann Whitney U test was used for group comparison analysis of continuous variables and Fisher’s exact test for categorical. We then fit univariate logistic regression models specifying metastases, recurrences, and death as the dependent variables and clinicopathologic variables and patient demographics specified as the independent variables. Subsequently, we fit multivariable logistic regression models to identify independent risk factors of recurrence, metastases, and mortality with the variables that were found to be significant from the univariate models. For time-to-event analysis, Kaplan–Meier curves were plotted. The independent prognostic factors were identified by fitting Cox proportional hazards regression models. A p value of < 0.05 was regarded as statistically significant. All statistical tests were 2-tailed. All statistical analyses were performed using Stata Version 13.0 (StataCorp, College Station, TX).
Results
Literature search and study characteristics
The initial literature search yielded 675 articles. After reviewing abstracts and titles, 314 articles were excluded because they were not relevant to our study (Fig. 1). After reading the full text of the remaining studies, 117 additional articles were excluded, because they were not of interest or because they did not meet the inclusion criteria. The remaining 71 studies, with a total of 2013 patients, were included in the analysis. Patient-level data were available in 29 of those studies (N = 368). In this sample of 368 patients, 70.4% (259) of tumors were primary intracranial SFT/HPCs, while 29.6% (109) were primary spinal SFT/HPCs.
Characteristics of primary spinal SFT/HPCs
Median age at presentation was 43 years (range 2–73 years) and 40.4% of patients were female, with a male to female ratio of 1.48:1. Median follow-up time was 4.6 years (range 0.1–25 years). The majority of spinal SFT/HPCs were located at the thoracic level (45.3%), followed by cervical (32.1%) and lumbar (22.6%) levels. The median diameter at the time of recognition was 4 cm (1–15 cm). According to the Liu et al. criteria, spinal SFT/HPCs were, for the most part, classified as type III lesions (27.5%, intradural tumor with extension into extradural space and paravertebral location), followed by IIA (17.4%, intradural-extramedullary location), IA and IB (15.9% respectively, intracanal or intracanal and extracanal), and IIB (7.2%, intramedullary). For the remaining 16.1%, it was not possible to retrieve this information. Some degree of vertebral body erosion was found in 34% of cases of extradural and paravertebral subtypes.
GTR was achieved in 67.4%, while STR occurred in 32.6% of cases, and was followed by upfront RT in 84.4% of those cases. Forty-five percent of patients who underwent GTR had adjuvant RT. In 43.6% of cases, a recurrence developed. The rate of recurrence formation was higher among patients with a higher histological grade compared to those with lower histological grade (4.8%/patient-year vs. 1.9%/patient-year respectively), and was significantly higher within the first 5 years after detection (7.8%/patient-year vs. 2.4%/patient-year).
Metastases were relatively common (13.3%) and occurred a median of 3.25 years (range 0.5–12.3 years) after the primary lesion. The overall risk of metastasis formation was estimated to be approximately 3.1%/patient-year. Lung was the most frequent location of metastases (32.1%), followed by the brain (2.3%) and bones and distal spinal locations (41.6%). Multiple metastases were found in 13% of patients with metastases.
The rate of metastasis formation was higher for patients with a higher histological grade compared to those with lower histological grade (6.2%/patient-year WHO vs. 0.16%/patient-year respectively for WHO grade III and grade I, p value = 0.002) and was significantly higher within the first 5 years after detection (6.8%/patient-year vs. 1.8%/patient-year, p value < 0.001). Clinico-demographic results are summarized in Table 3.
Metastases and recurrence risk factor analysis
Using Fisher’s exact tests, we confirmed that metastases occurred more often among participants with SFT/HPCs with a higher histological grade (p value = 0.050), and among those who experienced recurrence (p value < 0.001). There were no significant differences in age (< 50 versus > 50 years old), sex, tumor location, size, volume, number of recurrences, or between GTR and STR. Recurrences were more common in patients who underwent STR (p value < 0.001), among those patients with STR who did not have adjuvant RT (p value = 0.021), and among those with a higher histological grade (WHO grade III, p value < 0.001).
In univariate models, lower histological grade was associated with lower risk of recurrence in both intracranial (WHO grade I/II vs. grade III; odds ratio (OR) = 0.30, 95% CI 0.11–0.84, p value = 0.022) and spinal (WHO grade II; OR = 1.86, 95% CI 0.34–10.40, p value = 0.472, and WHO grade III; OR = 4.29, 95% CI 0.81–22.8, p value = 0.087) SFT/HPCs. Conversely, STR, as compared with GTR, was associated with greater risk of recurrence in both intracranial and spinal SFT/HPCs (OR = 2.63, 95% CI 1.48–4.66, p value < 0.001 and OR = 6.67, 95% CI 2.60–17.13, p value < 0,001, respectively). However, age, sex, location, and tumor size were not significantly associated with risk of recurrence.
Recurrent disease was a risk factor for metastases formation in intracranial patients (OR = 12.89, 95% CI 1.08–153.81, p value = 0.043). Higher histological grade was a risk factor for metastases for both intracranial (and OR = 2.91, 95% CI 1.44–5.93, p value = 0.003, respectively) and spinal (WHO grade II; OR = 2.83, 95% CI 0.95–10.40, p value = 0.061, and WHO grade III; OR = 10.5, 95% CI 3.13–35.20, p value < 0.001) SFT/HPCs. Again, age, sex, location, size and, in this case, degree of resection, were not associated with metastases formation.
Based on the univariate analysis results, multivariate logistic regression models were built. In multivariate logistic regression models adjusted for age, sex, location and, depending on the variable studied, histological grade, and recurrence, higher histological grade (WHO grade III, OR = 18.80, 95% CI 4.66–75.77, p value = 0.045) was associated with higher risk of recurrence. Similarly, higher histological grade (WHO grade III, OR = 6.04, 95%, 95% CI 1.06–34.85, p value = 0.045) and STR (OR = 11.16 95% CI 3.63–34.27, p value <0.001) were associated with higher risk of metastases. The results of the univariate and multivariable analyses are reported in Tables 4 and 5.
Survival analysis
Overall, 10.3% of patients died. Of those, 1 (0.9%) died of causes unrelated to SFT/HPCs in the postoperative period (first 30 days), while 10 (9.4%) died due to disease progression over a median follow-up time of 4.8 years (range 0.7–25 years). The median survival time for patients with metastases was 4.38 years (range 0.7–22.0 years). Among those who died, patients with metastasis survived for a significantly shorter period compared with patients without (p value < 0.020).
The 3-, 5-, and 10-year overall survival rates were 89.7%, 79.4%, and 76.6%, respectively. Higher odds of death were found among patients who developed recurrences (OR = 15.95, 95% CI 1.65–153.71, p value = 0.017), among patients who developed metastases (OR = 6.79, 1.39–33.94, p value = 0.018), and among patients who underwent STR (OR = 16.4, 95% CI 1.63–165.79, p value = 0.018).
Metastases were not associated with a significantly shorter survival time (hazard ratio (HR) = 2.15, 95% CI 0.54–8.64, p value = 0.281), nor were recurrences (HR = 5.10, CI 0.62–41.85, p value = 0.129). Also, metastasis location (extra CNS vs. CNS metastases) was not associated with higher mortality (p value = 0.667).
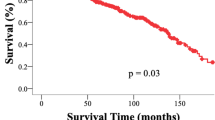
Higher histological grade was associated with significantly shorter survival time (HR = 9.23, 95% CI 1.74–48.92, p value = 0.009) (Fig. 2), as was STR (HR = 5.20, CI 1.03–26.13, p value = 0.045). By comparison, the adjunct of RT to STR (HR = 3.69, 95% CI 0.73–18.79, p value = 0.115) was associated with an improvement in survival time compared with STR alone (HR = 5.23, 95% CI 1.04–26.76, p value = 0.044) (Fig. 3). Other clinicopathological variables were not associated with survival.
Spinal vs. intracranial SFT/HPCs analysis
No significant differences were found between spinal and intracranial SFT/HPCs in terms of age at diagnosis and sex. We did not find significant differences in terms of rate of recurrence (p value = 0.538) or metastases formation (p value = 0.208) between spinal and intracranial SFT/HPCs. In both populations, higher histological grade and STR were the factors most strongly related to recurrence (p value < 0.05). Additionally, higher histological grade (p value = 0.030) and recurrences (p value = 0.002) were related to metastases formation. Finally, death rates were significantly higher among intracranial SFT/HPC patients, as compared with spinal SFT/HPC patients (26.5% vs. 9.4% respectively, p value = 0.001).
Although our analysis showed how SFT/HPCs had similar characteristics in both populations, the mean time to recurrence formation was overall shorter for spinal SFT/HPCs compared with intracranial SFT/HPCs (6.3 ± 4.8 vs. 8.1 ± 5.8 years, respectively, p value = 0.003). Again, mean time to metastasis formation was significantly shorter for spinal compared with intracranial SFT/HPCs (5.1 ± 3.3 vs. 8.7 ± 6.1 years, respectively, p value < 0.001). The risk of recurrence was not vastly different between intracranial (HR = 1.82, 95% CI 1.18–2.80, p value = 0.06) and spinal (HR = 2.09, 95% CI 0.97–4.49, p value = 0.059) tumors, nor among patients who had STR compared with GTR. However, the risk of recurrence was overall lower after the first 5 years from surgery for intracranial SFT/HPCs (see values in the section above).
The overall rate of metastases formation was different for patients with higher histological grade at diagnosis comparing intracranial (HR = 7.63, 95% CI 0.93–62.81, p value = 0.059) and spinal (HR = 9.15, 95% CI 2.32–36.09, p value = 0.002) SFT/HPCs. Risk of metastases formation was lower beyond the first 5 years from surgery (HR = 0.08, 95% CI 0.04–0.19, p value = 0.001) compared with the first 5 years. We found the same trend for spinal SFT/HPCs (HR after 5 years from surgery = 0.06, 95% CI 0.02–0.15, p value< 0.001).
A post hoc sensitivity analysis comparing data between studies with high and low risk of bias did not highlight statistically significant differences in terms of outcomes and risk factors, as considered above, between the two groups of studies.
Discussion
To the best of our knowledge, this is the most up-to-date systematic review on spinal and intracranial SFT/HPCs. After having reviewed and, when necessary, updated the histological grade of SFTs/HPCs reported in the last 20 years [16, 31], we estimated the rate of recurrence and metastases formation, defined risk factors, and analyzed patient survival for both spinal and intracranial populations. Compared with previous reviews [4, 18, 42], our study included primary intracranial spinal SFT/HPCs, and, for the first time, we analyzed and compared intracranial and spinal populations independently. Additionally, this is the first systematic review with patient-level time-to-event and survival analyses. We found that a higher histological grade and degree of resection (i.e., STR vs. GTR) are associated with recurrence. Additionally, higher histological grade and recurrence are associated with metastases formation, in both intracranial and spinal SFT/HPCs populations, particularly within the first 5 years. Age, sex, location, size, volume, degree of surgical resection, and adjuvant RT were not associated with metastases development. However, adjuvant RT after STR were negatively associated with recurrent disease. Metastatic and recurrent disease are associated with a significant decrease in survival. Building upon findings from recent studies [42], which agree that histological grade is strongly linked to metastases development, we additionally found that recurrence is a risk factor for metastases formation. Overall, these findings suggest that more aggressive disease impacts patient survival.
Tumor control is the first marker of success in treatment. SFTs/HPCs have a strong tendency to recur in the primary surgical site, and, not uncommonly, at a distal location [7, 17, 19, 21, 40]. Irrespective of histopathological classification, GTR during the first operation has been consistently identified as the most crucial factor for tumor control, with a substantially higher 5-year local control rate in patients treated with GTR (84%), as compared with STR (38%) [26]. Additionally, mitotic rate and adjuvant RT seem to be associated with better recurrence control [3, 10, 11, 37, 41, 43, 44, 46, 51,52,53], although adjuvant RT does not prevent metastases formation [3, 10, 43, 44, 51, 60]. Prevalence of metastases ranges from 11.1 to 57% [27, 28], and typically appears from 1 to 8 years after initial therapy; however, there have been cases where metastasis occurred more than 20 years after the initial treatment [14, 15, 48]. Metastases can be multiple, spreading to many organs and systems, although preferential sites were found to be lung, bone, soft tissue, and liver [1, 5, 17, 34] . Our data are consistent with previous studies and emphasize that most of the metastases develop within the first 5 years after treatment and most frequently in patients with high-grade lesions. Our findings support previous results that show tumor grade is the most significant risk factor for all outcomes. Thus, there does not appear to be a difference between grades I and II, while grade III is significantly associated with worse recurrence control. Overall, the findings support that a grading scheme incorporating mitotic rate is useful in stratifying SFT/HPCs. Moreover, a recent risk stratification model for non-meningeal tumors, proposed by Demicco et al., incorporates mitotic rate and necrosis, along with patient age and tumor size. It appears to be a robust prognostic factor in determining the propensity for metastatic disease [13, 32]. Failure to control recurrence rates and metastases is of primary concern, because both result in a significant reduction in survival [27].
In the literature, the 5-, 10-, and 15-year survival rates ranged from 83 to 65%, 77 to 40%, and 23 to 15%, respectively [1, 15, 44]. In high-grade tumors, however, survival rates decreased to only 88.9%, 66.7%, and 0% after 1, 3, and 5 years [60]. As demonstrated by our analysis, and by most studies, GTR was found to have a strong positive impact on survival [14, 30, 55]. GTR alone was associated with a median survival of 13 years, whereas STR has a median survival of less than 10 years [55]. When we compared patients with GTR and postoperative radiation to patients who with GTR alone, we did not observe any survival benefit for those who underwent adjuvant RT. However, among patients with a grade II SFT/HPC who underwent STR intervention, adjuvant RT was associated with survival. Again, when we compared patients who underwent GTR and postoperative RT to patients who went GTR alone, we observed a slight benefit with regard to recurrence among those who underwent adjuvant RT. The same trend was found among patients who underwent STR intervention—adjuvant RT was associated with a reduced risk of recurrence. Therefore, in the setting of STR—in those cases where GTR was considered impossible or risky—the addition of RT appears to be a reasonable alternative to GTR alone [52] . These findings corroborate those from Fritchie et al., who found that extent of surgery, radiotherapy/chemotherapy, mitotic rate, and necrosis were significant in predicting recurrence-free survival and disease-specific survival. Because CNS classification takes mitotic rate into account, it is not surprising that both appear to show association with recurrence-free survival.
We found that intracranial and spinal SFT/HPCs tend to exhibit similar behavior, although spinal tumors have an overall lower mortality rate. Additionally, spinal SFT/HPCs seem to develop recurrences and metastases over a significantly shorter time period compared with intracranial tumors. This finding could be reflective of a real difference in the progression of these two types of SFT/HPCs that must be taken into account when pursuing imaging follow-up. Alternatively, in our opinion, it may be secondary to the nature of the diagnosis. Spinal SFT/HPCs result in earlier symptom presentation, so are, therefore, likely get treated sooner than intracranial tumor patients. This may then result in higher survival rates, and, somewhat counterintuitively, higher rates of recurrence and metastases. However, again, this may be reflective of earlier detection and more radical treatment course, thus leading to more closely monitored follow-up and greater detection of recurrences and metastases. In both cases—spinal and intracranial—we believe that long-term clinical and radiographic follow-up is necessary [44, 47, 55]. It is important to detect extracranial metastasis in the initial stages [50], because providing close observation and timely assessment of the tumors is thought to prolong the patient survival [33, 36, 56].
Limitations
In this systematic review and meta-analysis, we performed a quantitative analysis of studies in which we were able to abstract individual patient data to carry out a prognostic analysis. Because we used data abstracted from previously published studies, there was a certain amount of missing data for some variables of interest (e.g., histological grade). Therefore, the findings investigating histological grade as a risk factor for recurrence or metastases must be interpreted with some caution, because power was limited and results may be overestimated. Indeed, while we were able to abstract full histological data for spinal SFT/HPCs, we categorized the histological grade abstraction for intracranial SFT/HPCs into grade III vs grade II/I to adhere to the 2016 WHO guidelines. However, the data presented here show that histological grade is a risk factor for tumor control and survival. Unfortunately, we were not able to retrieve enough reliable data to investigate other histological and molecular parameters. Surely future studies will and should study these aspects of the tumors associated with patient outcomes. Despite these limitations, this study represents an analysis of the largest sample of patients with intracranial and spinal SFT/HPCs, and provides a framework to guide decision making and consultation of patients about the possible natural history of this rare tumor.
Conclusion
Meningeal SFT/HPCs patients have a propensity for high rates of recurrence and metastasis, which occur mostly within the first 5 years after diagnosis. Spinal and intracranial SFT/HPCs show similar behavior, but with a tendency for spinal SFT/HPCs to exhibit metastases development and recurrence over a shorter interval of time. Clinicians should be more cautious in their planning for follow-up of spinal SFT/HPCs, because, even if the two populations exhibit similar overall behavior, spinal tumors seem to be slightly more aggressive and require more attention.
References
Alén JF, Lobato RD, Gómez PA, Boto GR, Lagares A, Ramos A, Ricoy JR (2001) Intracranial hemangiopericytoma: study of 12 cases. Acta Neurochir 143:575–586. https://doi.org/10.1007/s007010170062
Ambrosini-Spaltro A, Eusebi V (2010) Meningeal hemangiopericytomas and hemangiopericytoma/solitary fibrous tumors of extracranial soft tissues: a comparison. Virchows Arch 456:343–354. https://doi.org/10.1007/s00428-010-0888-6
Bastin KT, Mehta MP (1992) Meningeal hemangiopericytoma: defining the role for radiation therapy. J Neuro-Oncol 14:277–287. https://doi.org/10.1007/BF00172604
Bisceglia M, Dimitri L, Giannatempo G, Carotenuto V, Bianco M, Monte V, D’Angelo V, Magro G (2011) Solitary fibrous tumor of the central nervous system: report of an additional 5 cases with comprehensive literature review. Int J Surg Pathol 19:476–486. https://doi.org/10.1177/1066896911405655
Brunori A, Cerasoli S, Donati R, Giangaspero F, Chiappetta F (1999) Solitary fibrous tumor of the meninges: two new cases and review of the literature. Surg Neurol 51:636–640. https://doi.org/10.1016/S0090-3019(98)00115-3
Carneiro SS, Scheithauer BW, Nascimento AG, Hirose T, Davis DH (1996) Solitary fibrous tumor of the meninges: a lesion distinct from fibrous meningioma. A clinicopathologic and immunohistochemical study. Am J Clin Pathol 106:217–224
Caroli E, Salvati M, Orlando ER, Lenzi J, Santoro A, Giangaspero F (2004) Solitary fibrous tumors of the meninges: report of four cases and literature review. Neurosurg Rev 27:246–251. https://doi.org/10.1007/s10143-004-0331-z
Chamberlain MC, Glantz MJ (2008) Sequential salvage chemotherapy for recurrent intracranial hemangiopericytoma. Neurosurgery 63:720–726. https://doi.org/10.1227/01.NEU.0000325494.69836.51
Coffey RJ, Cascino TL, Shaw EG (1993) Radiosurgical treatment of recurrent hemangiopericytomas of the meninges: preliminary results. J Neurosurg 78:903–908. https://doi.org/10.3171/jns.1993.78.6.0903
Combs SE, Thilmann C, Debus J, Schulz-Ertner D (2005) Precision radiotherapy for hemangiopericytomas of the central nervous system. Cancer 104:2457–2465. https://doi.org/10.1002/cncr.21448
Copeland WR, Link MJ, Stafford SL, Pollock BE (2014) Single-fraction stereotactic radiosurgery of meningeal hemangiopericytomas. J Neuro-Oncol 120:95–102. https://doi.org/10.1007/s11060-014-1521-3
Das A, Singh PK, Suri V, Sable MN, Sharma BS (2015) Spinal hemangiopericytoma: an institutional experience and review of literature. Eur Spine J 24:606–613. https://doi.org/10.1007/s00586-015-3789-1
Demicco EG, Wagner MJ, Maki RG, Gupta V, Iofin I, Lazar AJ, Wang W-L (2017) Risk assessment in solitary fibrous tumors: validation and refinement of a risk stratification model. Mod Pathol 30:1433–1442. https://doi.org/10.1038/modpathol.2017.54
Dufour H, Métellus P, Fuentes S, Murracciole X, Régis J, Figarella-Branger D, Grisoli F (2001) Meningeal hemangiopericytoma: a retrospective study of 21 patients with special review of postoperative external radiotherapy. Neurosurgery 48:753–756
Fountas KN, Kapsalaki E, Kassam M, Feltes CH, Dimopoulos VG, Robinson JS, Smith JR (2006) Management of intracranial meningeal hemangiopericytomas: outcome and experience. Neurosurg Rev 29:145–153. https://doi.org/10.1007/s10143-005-0001-9
Fritchie K, Jensch K, Moskalev EA, Caron A, Jenkins S, Link M, Brown PD, Rodriguez FJ, Guajardo A, Brat D, Velázquez Vega JE, Perry A, Wu A, Raleigh DR, Santagata S, Louis DN, Brastianos PK, Kaplan A, Alexander BM, Rossi S, Ferrarese F, Haller F, Giannini C (2019) The impact of histopathology and NAB2–STAT6 fusion subtype in classification and grading of meningeal solitary fibrous tumor/hemangiopericytoma. Acta Neuropathol 137:307–319. https://doi.org/10.1007/s00401-018-1952-6
Galanis E, Buckner JC, Scheithauer BW, Kimmel DW, Schomberg PJ, Piepgras DG (1998) Management of recurrent meningeal hemangiopericytoma. Cancer 82:1915–1920. https://doi.org/10.1002/(SICI)1097-0142(19980515)82:10<1915::AID-CNCR15>3.0.CO;2-W
Ghose A, Guha G, Kundu R, Tew J, Chaudhary R (2017) CNS hemangiopericytoma. Am J Clin Oncol Cancer Clin Trials 40:223–227. https://doi.org/10.1097/COC.0000000000000146
Han N, Kim H, Min SK, Paek S-H, Park C-K, Choi S-H, Chae U-R, Park S-H (2016) Meningeal solitary fibrous tumors with delayed extracranial metastasis. J Pathol Transl Med 50:113–121. https://doi.org/10.4132/jptm.2015.10.30
Hayashi Y, Uchiyama N, Hayashi Y, Nakada M, Iwato M, Kita D, Higashi R, Hirota Y, Kai Y, Kuratsu J i, Hamada J i (2009) A reevaluation of the primary diagnosis of hemangiopericytoma and the clinical importance of differential diagnosis from solitary fibrous tumor of the central nervous system. Clin Neurol Neurosurg 111:34–38. https://doi.org/10.1016/j.clineuro.2008.07.010
Heiser MA, Waldron JS, Tihan T, Parsa AT, Cheung SW (2009) Temporal fossa hemangiopericytoma: a case series. Otol Neurotol 30:985–989. https://doi.org/10.1097/MAO.0b013e3181b76b58
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JPA, Straus S, Thorlund K, Jansen JP, Mulrow C, Catala-Lopez F, Gotzsche PC, Dickersin K, Boutron I, Altman DG, Moher D (2015) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 162:777–784. https://doi.org/10.7326/M14-2385
Jaaskelainen J, Servo A, Haltia M, Wahlstrom T, Valtonen S (1985) Intracranial hemangiopericytoma: radiology, surgery, radiotherapy, and outcome in 21 patients. Surg Neurol 23:227–236
Jallo GI, Roonprapunt C, Kothbauer K, Freed D, Allen J, Epstein F, Cooper PR, Brotchi J, Wang MY (2005) Spinal solitary fibrous tumors: a series of four patients: case report. Neurosurgery 57:195. https://doi.org/10.1227/01.NEU.0000163420.33635.9F
Jia Q, Zhou Z, Zhang D, Yang J, Liu C, Wang T, Wu Z, Yang C, Wei H, Zhao J, Liu T, Zhou W, Yang X, Xiao J (2017) Surgical management of spinal solitary fibrous tumor/hemangiopericytoma: a case series of 20 patients. Eur Spine J 27:891–901. https://doi.org/10.1007/s00586-017-5376-0
Kakkar A, Kumar A, Jha P, Goyal N, Mallick S, Sharma MC, Suri A, Singh M, Kale SS, Julka PK, Sarkar C, Suri V (2014) Meningeal hemangiopericytomas: a clinicopathological study with emphasis on MGMT (O6-methylguanine-DNA methyltransferase) promoter methylation status. Neuropathology 34:333–342. https://doi.org/10.1111/neup.12107
Kim JH, Jung HW, Kim YS, Kim CJ, Hwang SK, Paek SH, Kim DG, Kwun BD (2003) Meningeal hemangiopericytomas: long-term outcome and biological behavior. Surg Neurol 59:47–53. https://doi.org/10.1016/S0090-3019(02)00917-5
Kim KYA, Gonzalez I, McComb JG, Giannotta SL, Sandberg DI, Gutin PH, Rock JP, Sutton LN (2004) Unusual presentations of cerebral solitary fibrous tumors: report of four cases. Neurosurgery 54:1004–1009. https://doi.org/10.1227/01.NEU.0000115675.74366.87
Lang N, Zhang E, Xing X, Yuan H (2018) Solitary fibrous tumour of the spine: imaging features of a commonly misdiagnosed entity. Eur Radiol 28:3986–3995. https://doi.org/10.1007/s00330-018-5349-7
Liu H, Yang A, Chen N, Yang J, Qiu X, Zhang J (2013) Hemangiopericytomas in the spine. Neurosurgery 72:16–24. https://doi.org/10.1227/NEU.0b013e3182752f50
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Macagno N, Vogels R, Appay R, Colin C, Mokhtari K, Kusters B, Wesseling P, Figarella-Branger D, Flucke U, Bouvier C (2019) Grading of meningeal solitary fibrous tumors/hemangiopericytomas: analysis of the prognostic value of the Marseille grading system in a cohort of 132 patients. Brain Pathol 29:18–27. https://doi.org/10.1111/bpa.12613
Martin AJ, Fisher C, Igbaseimokumo U, Jarosz JM, Dean AF (2001) Solitary fibrous tumours of the meninges: case series and literature review. J Neuro-Oncol 54:57–69. https://doi.org/10.1023/A:1012553119349
Mekni A, Kourda J, Ben Hammouda K, Tangour M, Kchir N, Zitouna M, Haouet S (2009) Solitary fibrous tumour of the central nervous system: pathological study of eight cases and review of the literature. Pathology 41:649–654. https://doi.org/10.3109/00313020903071439
Menon GR, Patil A, Pisharody KK, Nair SN (2015) Meningeal hemangiopericytomas: review of an institutional series of 21 cases. Neurosurg Q 25:219–227. https://doi.org/10.1097/WNQ.0000000000000029
Metellus P, Bouvier C, Guyotat J, Fuentes S, Jouvet A, Vasiljevic A, Giorgi R, Dufour H, Grisoli F, Figarella-Branger D (2007) Solitary fibrous tumors of the central nervous system: clinicopathological and therapeutic considerations of 18 cases. Neurosurgery 60:715–722. https://doi.org/10.1227/01.NEU.0000255418.93678.AD
Noh SH, Lim JJ, Cho KG (2015) Intracranial hemangiopericytomas: a retrospective study of 15 patients with a special review of recurrence. J Korean Neurosurg Soc 58:211–216. https://doi.org/10.3340/jkns.2015.58.3.211
Osborne DR, Dubois P, Drayer B, Sage M, Burger P, Heinz ER (1981) Primary intracranial meningeal and spinal hemangiopericytoma: radiologic manifestations. Am J Neuroradiol 2:69–74
Pakasa NM, Pasquier B, Chambonnière ML, Morrison AL, Khaddage A, Perret AG, Dumollard JM, Barral FG, Péoc’h M (2005) Atypical presentations of solitary fibrous tumors of the central nervous system: an analysis of unusual clinicopathological and outcome patterns in three new cases with a review of the literature. Virchows Arch 447:81–86. https://doi.org/10.1007/s00428-005-1220-8
Park BJ, Kim Y II, Hong YK, Jeun SS, Lee KS, Lee YS (2013) Clinical analysis of intracranial hemangiopericytoma. J Korean Neurosurg Soc 54:309–316. https://doi.org/10.3340/jkns.2013.54.4.309
Payne BR, Prasad D, Steiner M, Steiner L (2000) Gamma surgery for hemangiopericytomas. Acta Neurochir 142:527–537
Ratneswaren T, Hogg FRA, Gallagher MJ, Ashkan K (2018) Surveillance for metastatic hemangiopericytoma-solitary fibrous tumors-systematic literature review on incidence, predictors and diagnosis of extracranial disease. J Neuro-Oncol 138:447–467. https://doi.org/10.1007/s11060-018-2836-2
Rutkowski MJ, Bloch O, Jian BJ, Chen C, Sughrue ME, Tihan T, Barani IJ, Berger MS, McDermott MW, Parsa AT (2011) Management of recurrent intracranial hemangiopericytoma. J Clin Neurosci 18:1500–1504. https://doi.org/10.1016/j.jocn.2011.04.009
Rutkowski MJ, Jian BJ, Bloch O, Chen C, Sughrue ME, Tihan T, Barani IJ, Berger MS, McDermott MW, Parsa AT (2012) Intracranial hemangiopericytoma: clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer 118:1628–1636. https://doi.org/10.1002/cncr.26411
Satayasoontorn K, Righi A, Brunocilla E, Vanel D (2014) Meningeal hemangiopericytoma only diagnosed at the time of late bone metastasis:1543–1549. https://doi.org/10.1007/s00256-014-1926-2
Schiariti M, Goetz P, El-Maghraby H, Tailor J, Kitchen N (2011) Hemangiopericytoma: long-term outcome revisited. Clinical article. J Neurosurg 114:747–755. https://doi.org/10.3171/2010.6.JNS091660
Schirmer CM, Heilman CB (2011) Hemangiopericytomas of the skull base. Neurosurg Focus 30:E10. https://doi.org/10.3171/2011.2.FOCUS119
Sheehan J, Ph D, Kondziolka D, Flickinger J, Al ET (2002) R Adiosurgery for T Reatment of R Ecurrent. 51:905–911. https://doi.org/10.1227/01.NEU.0000027979.72088.BB
Shirzadi A, Drazin D, Gates M, Shirzadi N, Banykh S, Fan X, Hunt L, Baron EM, King WA, Kim TT, Johnson JP (2013) Surgical management of primary spinal hemangiopericytomas: an institutional case series and review of the literature. Eur Spine J 22:450–459. https://doi.org/10.1007/s00586-012-2626-z
Someya M (2001) Four cases of meningeal hemangiopericytoma treated with surgery and radiotherapy. Jpn J Clin Oncol 31:548–552. https://doi.org/10.1093/jjco/hye116
Soyuer S, Chang EL, Selek U, McCutcheon IE, Maor MH (2004) Intracranial meningeal hemangiopericytoma: the role of radiotherapy. Report of 29 cases and review of the literature. Cancer 100:1491–1497. https://doi.org/10.1002/cncr.20109
Stessin AM, Sison C, Nieto J, Raifu M, Li B (2013) The role of postoperative radiation therapy in the treatment of meningeal hemangiopericytoma - experience from the SEER database. Int J Radiat Oncol Biol Phys 85:784–790. https://doi.org/10.1016/j.ijrobp.2012.05.042
Tsugawa T, Mori Y, Kobayashi T, Hashizume C, Shibamoto Y, Wakabayashi T (2014) Gamma knife stereotactic radiosurgery for intracranial hemangiopericytoma. J Radiosurg SBRT 3:29–35
Vassal F, Manet R, Forest F, Camdessanche JP, Péoc’h M, Nuti C (2011) Solitary fibrous tumors of the central nervous system: report of five cases with unusual clinicopathological and outcome patterns. Acta Neurochir 153:377–384. https://doi.org/10.1007/s00701-010-0866-4
Veeravagu A, Jiang B, Patil CG, Lee M, Soltys SG, Gibbs IC, Chang SD (2011) CyberKnife stereotactic radiosurgery for recurrent, metastatic, and residual hemangiopericytomas. J Hematol Oncol 4:26. https://doi.org/10.1186/1756-8722-4-26
Vuorinen V, Sallinen P, Haapasalo H, Visakorpi T, Kallio M, Jaaskelainen J (1996) Outcome of 31 intracranial haemangiopericytomas: poor predictive value of cell proliferation indices. Acta Neurochir 138:1399–1408
Wu W, Shi J x, Cheng H l, Wang H d, Hang C h, Shi QL, Yin H x (2009) Hemangiopericytomas in the central nervous system. J Clin Neurosci 16:519–523. https://doi.org/10.1016/j.jocn.2008.06.011
Yi X, Xiao D, He Y, Yin H, Gong G, Long X, Liao W, Li X (2017) Spinal solitary fibrous tumor/hemangiopericytoma: a clinicopathologic and radiologic analysis of eleven cases. World Neurosurg 104:318–329. https://doi.org/10.1016/j.wneu.2017.05.016
Yilmaz C, Kabatas S, Ozen OI, Gulsen S, Caner H, Altinors N (2009) Solitary fibrous tumor. J Clin Neurosci 16:1578–1581. https://doi.org/10.1016/j.jocn.2009.02.039
Zweckberger K, Jung CS, Mueller W, Unterberg AW, Schick U (2011) Hemangiopericytomas grade II are not benign tumors. Acta Neurochir 153:385–394. https://doi.org/10.1007/s00701-010-0877-1
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
None. No human participants are involved in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Giordan, E., Marton, E., Wennberg, A.M. et al. A review of solitary fibrous tumor/hemangiopericytoma tumor and a comparison of risk factors for recurrence, metastases, and death among patients with spinal and intracranial tumors.. Neurosurg Rev 44, 1299–1312 (2021). https://doi.org/10.1007/s10143-020-01335-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-020-01335-x