Abstract
We carried out an experimental investigation of cartilage endplate vascularity of degenerated intervertebral discs produced by exogenous melatonin (MEL) treatment. Adult Swiss albino rats were divided into three groups: control, operated degeneration, and MEL treatment. There were five rats in each group and, using a posterior approach, cuts were made parallel to the endplates in the posterior annulus fibrosus in five consecutive intervertebral discs between the 5th and 10th vertebral segments of the rats' tails. At 8 weeks, five of these animals were treated with exogenous MEL (s.c. injection of 30 μg/100 g body weight daily for 4 weeks). In each experimental group, one animal was examined using CT scanner to study the density of the cartilage endplate of the disc. To evaluate the bone growth and vascularity of the cartilage endplate region, the animals were killed for subsequent histopathological evaluation. We found that the vascular channel counts and percentage areas from animals treated with MEL were significantly lower than from the operated degeneration animals. Accordingly, the density histogram in the MEL group showed a spike profile for both the vertebral body and the cartilage endplate, indicating an increase in the amount of higher density tissues in these regions. Our results demonstrate that the use of MEL reduces the cartilage endplate vascularity of degenerated intervertebral discs, suggesting that it may have an osteoinductive effect on bone formation. Further studies are needed to characterize fully the relevance of our findings for the treatment of disorders such as postmenopausal osteoporosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intervertebral disc degeneration is the most common age-related condition in spines of elderly people with low back pain [14]. It is well known that a variety of spinal problems such as disc herniation, scoliosis, and spondylosis cause changes in the cartilage endplates of discs [17, 39]. Several studies have shown a correlation between intervertebral disc degeneration and increased bone density [47, 52]. In 1999, Roth et al. [43] demonstrated that the pineal hormone, melatonin (N-acetyl-5-methoxytryptamine) (MEL), was capable of promoting osteoblast differentiation and bone formation, and they suggested that this hormone might play an essential role in regulating bone growth. Recently, it was reported that MEL stimulates the proliferation and type I collagen synthesis of human bone cells in vitro, suggesting that MEL may act to stimulate bone formation [33]. To our knowledge, no experimental study in the literature specifically addresses the effects of MEL administration on the bone mineral density (mass per unit volume) and, in particular, cartilage endplate vascularity.
The objective of the present study was to reproduce experimental disc degeneration in order to study the radiological and histopathological features and investigate the effects of MEL on vascular channels in the cartilage endplates of degenerated intervertebral discs.
Materials and methods
Animals and surgical procedures
Fifteen adult, male, Swiss albino rats weighing 120–160 g were entered into the study. All experiments were performed according to the guidelines for ethical treatment of animals of the Ege University School of Medicine Animal Care and Use Committee. The rats were kept in specially prepared cages, where they had rat chow and water ad libitum in an air-conditioned environment. They were divided randomly into three groups (five animals in each) according to the experimental procedures. The first nonoperated control group consisted of five sham animals.
In the other two groups (operated degeneration and MEL treatment), after i.m. injection of 50 mg/kg body weight of ketamine (Alfamine) (EGE VET, İzmir, Turkey) plus 8–10 mg/kg xylasine (Alfazyne) (EGE VET, İzmir, Turkey) (0.6 ml/kg), the spines of the rats' tails were exposed using a posterior approach under aseptic conditions, and in five consecutive intervertebral discs between the 5th and 10th vertebral segments of the tails, cuts were made parallel to the endplates in the posterior annulus fibrosus [21]. Following surgery, all wounds were closed in a standard manner with absorbable sutures. At 8 weeks, five of these animals (MEL treatment group) were given subcutaneous injections of MEL at a dose of 30 μg per 100 g of body weight daily at 5:00 PM to 6:00 PM for 4 weeks.
Radiological evaluation
In each experimental group, one animal was examined using a Hitachi W450 CT scanner to study the density of the cartilage endplates of the intervertebral discs. The profile of the density histograms is related to the sum of the pixels with the same degree of gray as in the CT image. The white pixels represent cortical tissue, gray pixels cancellous bone, and black pixels water or air. If either white, gray, or black pixels are prevalent, the profile of the histogram will be spike-shaped. If the numbers of white, gray, and black pixels are balanced, the profile will be plateau-shaped [57]. For this reason, histograms concerning normal vertebral bodies have spike profiles, which indicates prevalently homogeneous bone. In contrast, plateau profiles are typical of bone in which cancellous areas and cortical tissue are quantitatively balanced. Three months after onset of the experiment, all rats were fasted overnight and then killed by decapitation for subsequent histopathological evaluation.
Histomorphometric analysis
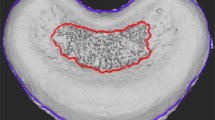
Each spine, including five consecutive intervertebral discs between the 5th and 10th vertebral segments of the tail, was fixed in 10% neutral buffered formalin and decalcified in 5% hydrochloric acid. A midsagittal section was done in each specimen, and each slice was stained with hematoxylin and eosin for microscopic examination. The vascular channels in the cranial and caudal cartilage endplates of the five consecutive intervertebral discs of each animal in each group were counted. For each endplate region, vascular channel area was determined using a square grid placed on the cartilage endplate [6, 53] (Fig. 1). Then it was quantified as a percentage of the total area of the cartilage endplate, and a mean value for each disc level was calculated. Thus, a total of 25 measurement values was obtained in every group. All measurements were made by two histologists who were blinded to the animal groups separately.
Statistical analysis
The results were expressed as mean±SD. Statistical analysis was performed by one-way analysis of variance (ANOVA), which was followed by Duncan's post-hoc test for pairwise comparisons. P<0.05 was considered as statistically significant.
Results
Radiological findings
In this study, data were collected on CT density from cartilage endplate regions and vertebral bodies at each disc level in each animal. In both nonoperated control and MEL-treated animals, the density histograms showed a spike profile (Fig. 2a, c). On the other hand, the profile of the histogram in the operated degeneration group showed a wide plateau (Fig. 2b). Thus, the addition of MEL therapy following surgical disc degeneration procedures apparently caused an increase in the amount of higher density tissues in the cranial and caudal cartilage endplate regions.
Quantitative morphometry
Vascular channel counts
The vascular channel counts of the cranial and caudal cartilage endplates of all groups were measured. It was found that they were identical. In other words, there was no significant difference between the cranial and caudal groups (P=0.54). In the operated degeneration group, vessel counts were significantly higher than in the nonoperated control animals (P<0.05) (Fig. 3a, b). In animals treated with MEL following degeneration, the counts were found to have decreased from a mean value of 21.02±3.43 in the operated degeneration group to a mean of 17.04±2.88 (Fig. 3c). Thus, the counts from animals treated with MEL were significantly lower than those from the operated degeneration animals (P<0.05) (Table 1).
Vascular channel area
The percentage of area of vascular channels in the cranial and caudal cartilage endplates were calculated in all animals. As summarized in Table 2, the percentage of the region of interest was significantly higher in the operated degeneration group than in the nonoperated control group (P<0.05). Furthermore, it was significantly lower in the MEL therapy group than in the operated degeneration group (25.71±5.92 vs 41.91±7.03, respectively) (P<0.05).
Discussion
Histopathological studies have demonstrated that vascular channels disappear with concomitant degeneration of the disc in the spines of elderly people with low back pain [16, 32, 39]. It is well known that the avascular intervertebral disc relies for nutrition on vascular channels in the cartilage endplate [11, 14, 15, 29, 32]. On the other hand, various experimental models have been described to produce disc degeneration [3, 20, 21, 22, 34, 39, 48, 49, 54]. Some researchers [3, 23] have used direct action on the intervertebral disc by traumatically damaging the annulus fibrosus. In these experimental models, the discal height is slightly reduced, as in human disc herniations. This diminution in height is associated with protrusions, and the typical intervertebral disc herniation is posterior, due to variations in structure of the annular fibers and different types of insertion of the fibers into the vertebral border [16, 23, 34, 54, 55, 56]. In human discs, there is similarly decreased cartilage endplate vascularity in all the experimental herniation models [51]. In our study, cartilage endplate vascularity was significantly higher than in nonoperated controls. This finding is in accordance with those reported for the human disc by Roberts et al. [42]. According to the present study, there was no significant difference in the vascularization between cranial and caudal cartilage endplates in rats (P=0.54).
The pineal hormone MEL is synthesized in the pinealocytes of the pineal gland in an endogenous rhythm, and its secretion is age-dependent in humans. At present, a number of conditions are said to be improved by administration of MEL [4, 7, 8, 9, 10, 12, 13, 18, 19, 31, 38, 41, 46, 50]. Postoperative sleep disturbance, which is related to MEL suppression after surgery, might be prevented by MEL replacement [7]. Recently, Shilo et al. [46] suggested that MEL administration to patients in intensive care units (ICUs) may be indicated in the treatment of ICU syndrome for sleep induction. It is shown to play an important role as a protective agent against a wide variety of processes that damage tissues by free radicals [4, 8, 10, 19, 31, 41, 50]. At present, it is speculated that the antioxidative enzymes such as superoxide dismutase, glutathione peroxidase, and glutathione reductase are also stimulated by MEL [41]. Experimental data provide information supporting the use of MEL in the treatment of neurodegenerative disorders and oxidative neuronal damage following ischemia or trauma [8, 12, 19, 31, 50]. Several authors claimed that it has clinical application in the neuroimmunotherapy of advanced cancer patients [5, 24, 35, 36]. On the other hand, Lissoni et al. [26] suggested that the concomitant administration of MEL during chemotherapy may prevent some chemotherapy-induced side effects. A few recent publications indicated an effect of MEL in bone metabolism [1, 27, 33, 35, 36, 37, 43, 44]. However, its role in bone mineralization is not yet clearly established. The current investigation was undertaken to study the effects of MEL on endplate vascularity of degenerated intervertebral discs. It is apparent that the rat is a useful experimental model for investigating these effects. To the authors' knowledge, no such study yet exists.
In the current study, exogenous MEL treatment decreased the vascularization of the cartilage endplate of degenerated intervertebral discs. This suggests that MEL has a positive effect on osteoblastic activity in the bone, causing mineralization of matrix and bone growth. The density histograms of the animals in all groups were compatible with the histopathological findings. In our present study, a direct action of MEL could be responsible for osteogenesis and increased bone mineral density following exogenous MEL administration. It is likely that MEL's receptors in the craniospinal axis play a role in the effects we observed.
In the last decade, a number of studies confirmed that MEL is a broad-spectrum antioxidant and a potent endogenous free radical scavenger [1, 4, 8, 10, 31, 41, 50]. It is possible that these effects of MEL may have protected the bone and cartilage cells of free radical-mediated toxicity and thus caused the higher density of the bones in the treated group in the current experiment. At present, there is experimental evidence of a role of MEL in immunological reactions and inflammation [25, 26, 28, 40]. Lissoni et al. [25] reported that it may inhibit the acute inflammatory reaction and contribute to generation of the immune reaction by removing the immunosuppression related to the activation of the inflammatory response. The data from the present study suggest that bringing MEL to a normal level may regulate the immune systems of the treated animals.
Furthermore, it can indirectly influence bone metabolism by the secretion of some hormones with osteoinductive activity. One such hormone is estrogen; it has been shown that estrogen deficiency is associated with bone degradation and advanced vertebral osteoporosis [30], and its secretion might be stimulated by MEL. Other hormones that could affect osteoblast cells and bone blood flow may include progesterone, thyroxine, androgen, cortisol, and calcitonin [2]. However, it remains to be investigated whether MEL's receptors are involved in the MEL-regulated secretion of these hormones. At present, it remains to be investigated whether such effects are involved in the pathogenesis of different kinds of osteoporosis.
How strong is the evidence presented here? The current study has certain limitations. First, the sample size was not large, although five consecutive intervertebral discs of each animal in each group were investigated. Second, not all the animals in the study could be examined because CT is expensive. Third, the orientation of the disc within the body differs between rats and humans, rats being quadrupedal and humans bipedal. Also, measurement of the collagen content of intervertebral disc tissue would provide some data regarding bone mineralization, as it is considered to play a regulatory role in osteoblastic growth and differentiation [45]. Future studies will involve use of the serum osteocalcin level as an indicator of osteoblast function in the investigation of effects of MEL on cartilage endplate and vertebral bodies. Thus it could be proven if MEL has an inhibitory effect on the disc degeneration process.
In conclusion, our results suggest that exogenous MEL may play an osteoinductive role in bone formation and that MEL deficiency might be a pathophysiological mechanism in degenerative spinal diseases. Based on our results, it is thus possible to postulate that MEL treatment can be utilized to improve some disorders such as postmenopausal and senile osteoporosis. However, further experimental and clinical studies are needed before MEL can be widely recommended, because of many unanswered questions.
References
Abdel-Wanis ME, Kawahara N, Tomita K (2001) The association of neurofibromatosis 1 and spinal deformity with primary hyperparathyroidism and osteomalacia: might melatonin have a role? J Orthop Sci 6:193–198
Aloia JF, Cohn SH, Vaswani A, Yeh JK, Yuen K, Ellis K (1985) Risk factors for postmenopausal osteoporosis. Am J Med 78:95–100
Brinckmann P, Porter RW (1994) A laboratory model of lumbar disc protrusion. Spine 19:228–235
Bromme HJ, Morke W, Peschke D, Ebelt H, Peschke D (2000) Scavenging effect of melatonin on hydroxyl radicals generated by alloxan. J Pineal Res 29:201–108
Brzezinski A (1997) Melatonin in humans. N Engl J Med 336:186–195
Chalkey J (1943) Methods for quantitative morphological analysis of tissue. J Natl Cancer Inst 4:47–53
Cronin AJ, Keifer JC, Davies MF, King TS, Bixler EO (2000) Melatonin secretion after surgery. Lancet 356:1244–1245
Cuzzocrea S, Costantino G, Gitto E, Mazzon E, Fulia F, Serraino I, Cordaro S, Barberi I, De Sarro A, Caputi AP (2000) Protective effects of melatonin in ischemic brain injury. J Pineal Res 29:217–227
Fauteck JD, Schmidt H, Lerchl A, Kurlemann G, Wittkowski W (1999) Melatonin in epilepsy: first results of replacement therapy and first clinical results. Biol Signals Recept 8:105–110
Fujimoto T, Nakamura T, Ikeda T, Takagi K (2000) Potent protective effects of melatonin on experimental spinal cord injury. Spine 25:769–775
Fukayama S, Tashjian AH Jr (1990) Stimulation by parathyroid hormone of 45Ca2+ uptake in osteoblast-like cells: possible involvement of alkaline phosphatase. Endocrinology 126:1941–1949
Gitto E, Tan DX, Reiter RJ, Karbownik M, Manchester LC, Cuzzocrea S, Fulia F, Barberi I (2001) Individual and synergic antioxidative actions of melatonin: studies with vitamin E, vitamin C, glutathione and desferioxamine (desferoxamine) in rat liver homogenates. J Pharm Pharmacol 53:1393–1401
Gordon N (2000) The therapeutics of melatonin: a paediatric perspective. Brain Dev 22:213–217
Harada A, Okuizumi H, Miyagi N, Genda E (1998) Correlation between bone mineral density and intervertebral disc degeneration. Spine 23:857–862
Holm S (1993) Pathophysiology of disc degeneration. Acta Orthop Scand 64:13–15
Holm S, Maroudas A, Urban JPG, Selstam G, Nachemson A (1981) Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res 8:101–119
James JIP (1970) The etiology of scoliosis. J Bone Joint Surg Br 52:410–419
Jan JE, Espezel H, Freeman RD, Fast DK (1998) Melatonin treatment of chronic sleep disorders. J Child Neurol 13:98
Kim YS, Joo WS, Jin BK, Cho YH, Baik HH, Park VW (1998) Melatonin protects 6-OHDA-induced neuronal death of nigrostriatal dopaminergic system. Neuroreport 9:2387–2390
Lane-Petter W, Pearson AEG (1971) The laboratory animal: principles and practice. Academic Press, London
Latorre A, Albareda J, Castiella T, Lasierra JM, Seral F (1998) Experimental model of multidirectional disc hernia in rats. Int Orthop 22:44–48
Lindblom K (1952) Experimental ruptures of intervertebral disc in rat's tails. J Bone Joint Surg Am 34:123–128
Lipson SJ, Muir H (1981) Experimental intervertebral disc degeneration. Morphologic and proteoglycan changes over time. Arthritis Rheum 24:12–21
Lissoni P, Fumagalli L, Paolorossi F, Rovelli F, Roselli MG, Maestroni GJ (1997) Anticancer neuroimmunomodulation by pineal hormones other than melatonin: preliminary phase II study of the pineal indole 5-methoxytryptophol inassociation with low-dose IL-2 and melatonin. J Biol Regul Homeost Agents 11:119–122
Lissoni P, Rovelli F, Meregalli S, Fumagalli L, Musco F, Brivio F, Brivio O, Esposti G (1997) Melatonin as a new possible anti-inflammatory agent. J Biol Regul Homeost Agents 11:157–159
Lissoni P, Tancini G, Barni S, Paolorossi F, Ardizzoia A, Conti A, Maestroni G (1997) Treatment of cancer chemotherapy-induced toxicity with the pineal hormone melatonin. Support Care Cancer 5:126–129
Machida M (1999) Cause of idiopathic scoliosis. Spine 24:2576–2583
Maestroni GJM (1993) The immunoendocrine role of melatonin. J Pineal Res 14:1–10
McFadden KD, Taylor JR (1989) End plate lesions of the lumbar spine. Spine 14:867–869
Meema S, Bunkler ML, Meema HE (1975) Preventive effect of estrogen on postmenopausal bone loss. Arch Intern Med 135:1436–1440
Mesenge C, Margaill I, Verrecchia C, Allix M, Boulu RG, Plotkine M (1998) Protective effect of melatonin in a model of traumatic brain injury in mice. J Pineal Res 25:41–46
Nachemson A, Lewin T, Maroudas A, Freeman MAR (1970) In vitro diffusion of dye through the endplates and the annulus fibrosus of human intervertebral discs. Acta Orthop Scand 41:589–607
Nakade O, Koyama H, Ariji H, Yajima A, Kaku T (1999) Melatonin stimulates proliferation and type I collagen synthesis in human bone cells in vitro. J Pineal Res 27:106–110
Neufeld JH, Machado T, Margelin L (1991) Variables affecting disc size in the lumbar spine of rabbits: anesthesia, paralysis and disc injury. J Orthop Res 9:104–112
Ostrowska Z, Kos-Kudla B, Marek B, Kajdaniuk D, Staszewicz P, Szapska B, Strzelczyk J (2002) The influence of pinealectomy and melatonin administration on the dynamic pattern of biochemical markers of bone metabolism in experimental osteoporosis in the rat. Neuroendocrinol Lett 23:104–109
Ostrowska Z, Kos-Kudla B, Marek B, Swietochowska E, Gorski J (2001) Assessment of the relationship between circadian variations of salivary melatonin levels and type I collagen metabolism in postmenopausal obese women. Neuroendocrinol Lett 22:121–127
Ostrowska Z, Kos-Kudla B, Swietochowska E, Marek B, Kajdaniuk D, Gorski J (2001) Assessment of the relationship between dynamic pattern of nighttime levels of melatonin and chosen biochemical markers of bone metabolism in a rat model of postmenopausal osteoporosis. Neuroendocrinol Lett 22:129–136
Peres MFP, Seabra MLV, Zukerman E, Tufik S (2000) Cluster headache and melatonin. Lancet 355:147
Pritzker KPH (1977) Aging and degeneration in the lumbar intervertebral disc. Orthop Clin North Am 8:65–77
Reiter RJ, Calvo JR, Karbownik M, Qi W, Tan DX (2000) Melatonin and its relation to the immune system and inflammation. Ann N Y Acad Sci 917:376–386
Reiter RJ, Tan DX, Osuna C, Gitto E (2000) Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci 7:444–458
Roberts S, Menage J, Urban JPG (1989) Biochemical and structural properties of the cartilage end-plate and its relation to the intervertebral disc. Spine 14:166–173
Roth JA, Kim BG, Lin WL, Cho MI (1999) Melatonin promotes osteoblast differentiation and bone formation. J Biol Chem 274:22041–22047
Sadat-Ali M, al-Habdan I, al-Othman A (2000) Adolescent idiopathic scoliosis. Is low melatonin a cause? Joint Bone Spine 67:62–64
Shi S, Kırk M, Kahn AJ (1996) The role of type I collagen in the regulation of the osteoblast phenotype. J Bone Miner Res 11:139–145
Shilo L, Dagan Y, Smorjik Y, Weinberg U, Dolev S, Komptel B, Shenkman L (2000) Effect of melatonin on sleep quality of COPD intensive care patients: a pilot study. Chronobiol Int 17:71–76
Silberberg R (1988) Histologic and morphometric observations on vertebral bone of aging sand rats. Spine 13:202–208
Stokes IA, Counts DF, Frymoyer JW (1989) Experimental instability in rabbit lumbar spine. Spine 14:68–72
Takenaka Y, Revel M, Kahan A, Amor B (1987) Experimental model of disc herniations in rats for study of nucleolytic drugs. Spine 12:556–560
Tan DX, Manchester LC, Reiter RJ, Cabrera J, Burkhardt S, Phillip T, Gitto E, Karbownik M, Li QD (2000) Melatonin suppresses autoxidation and hydrogen peroxide-induced lipid peroxidation in monkey brain homogenate. Neuroendocrinol Lett 21:361–365
Trueta J (1963) The role of vessels in osteogenesis. J Bone Joint Surg Am 45:402–418
Verstraeten A, Ermen HV, Haghebaert G, Nijs J, Geusens P, Dequeker J (1991) Osteoarthritis retards the development of osteoporosis: observations of coexistence of osteoarthrosis and osteoporosis. Clin Orthop 264:169–177
Weibel ER (1979) Stereological methods. Academic Press, New York
Yamada K (1962) The dynamics of experimental posture. Experimental study of intervertebral disc herniation in bipedal animals. Clin Orthop 25:20–31
Yasuma T, Makino E, Saito S, Inui M (1986) Histological development of intervertebral disc herniation. J Bone Joint Surg Am 68:1066–1072
Yu S, Haughton VM, Sether LA, Wagner M (1988) Annulus fibrosus in bulging intervertebral disks. Radiology 169:761–763
Zagra A, Lamartina C, Pace A, Ramondetta V, Vercellesi E (1990) Computer-assisted tomography of scoliosis operated with or without Harrington's rod. Biomechanics aspects of the fusion. Spine 15:796–802
Acknowledgements
We are indebted to Fatma Özdemir, İsmail Zonguldak, Namık Tavus, Feyzi Subaşı, and Çağlar Uz for their technical assistance and suggestions during the investigation and preparation of this article. We also thank the anonymous reviewers of Neurosurgical Review for their constructive comments and help with the manuscript. The authors indicated in the byline made substantial contributions to the following research tasks: initial conception (M.T.), design (M.T., A.U.), provision of resources (M.T., M.E.Y.), collection of data (M.T., A.U., S.U.), statistical analysis (H.Ü.), laboratory analysis and interpretation of data (M.T., A.U., S.U., H.Ü.), drafting of the manuscript (M.T., S.U.), critical revision of the manuscript for important intellectual content (M.T., A.U., H.Ü., M.E.Y.), and study supervision (M.T., M.E.Y.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turgut, M., Uslu, S., Uysal, A. et al. Changes in vascularity of cartilage endplate of degenerated intervertebral discs in response to melatonin administration in rats. Neurosurg Rev 26, 133–138 (2003). https://doi.org/10.1007/s10143-003-0259-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-003-0259-8