Abstract
Purpose
To determine whether an additional arterial phase (AP) leads to a change in the grade of splenic injury according to the 2018 revision of the AAST Organ Injury Scale, which has incorporated vascular injuries into the grading system and also to study its impact on management.
Methods
In this retrospective study, 527 patients who sustained blunt abdominal trauma and had underwent dual-phase CT (AP and portal venous phase (PVP)) from December 2014 to October 2016 (23 months) were included. Two experienced radiologists independently graded the splenic injury according to the revised system in 2 blinded ways (AP + PVP and PVP alone). Receiver operator characteristic (ROC) curves were generated for grade of injury on both the phases for all splenic interventions.
Results
Splenic injuries were detected in 154 patients, and splenic vascular injuries were detected in 52 of them. Of these, 22 vascular injuries were detected only on the AP, leading to a change in the grade of injury according to the new system in 18 patients. The AUC for ROC curves was generated for the grade of injury on AP + PVP vs. PVP alone for angioembolization (0.80 vs. 0.71, p value 0.002), and all splenic interventions (0.89 vs. 0.83, p value 0.003) showed higher AUC for AP + PVP.
Conclusion
Addition of AP leads to a significant change in the grading of splenic injuries according to the revised grading system due to increased detection of vascular injuries. Accurate classification of splenic injuries using additional AP would lead to better triage of patients for splenic interventions or conservative management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The spleen is one of the most commonly injured solid organs in blunt abdominal trauma. Being a highly vascular organ, it can lead to severe internal hemorrhage causing hemodynamic compromise even in patients with minimal external injuries [1,2,3]. The management of solid organ injury in hemodynamically stable patients has undergone a paradigm shift from predominantly surgical to non-operative management with or without angioembolization. The high diagnostic accuracy of computed tomography (CT) has led to its widespread use in the evaluation of blunt abdominal trauma in identifying, characterizing, and grading intra-abdominal injuries [4,5,6].
The Organ Injury Scale (OIS) published by the American Association for the Surgery of Trauma (AAST) was based on operative findings and has been widely used for grading splenic and other solid organ injuries [7, 8]. The 2018 revision of the OIS for the spleen, liver, and kidney was recently published by the AAST [9]. The 2018 revision has incorporated “CT diagnosed active bleeding or Active Extravasation of contrast (AE) and Contained Vascular Injury (CVI), defined as either a pseudoaneurysm or arteriovenous fistula.” Irrespective of the degree of the parenchymal injuries, the presence of AE/CVI would increase the grade of injury to grade 4, if CVI and AE confined within the splenic capsule, and to grade 5 if the AE extends beyond the splenic capsule into the peritoneum (Table 1). The presence of AEs and CVIs is associated with failure of non-operative management, and the 2018 revision further highlights their importance.
Despite the routine use of CT in the evaluation of blunt abdominal trauma, there is no clear consensus regarding the optimal CT protocol and there is widespread variation in the protocols used at various centers [10]. It has been well established that the portal venous phase (PVP) images are the most important for diagnosing parenchymal injuries in solid organs [11]. However, the diagnosis of AE/CVI poses a challenge on PVP CT. Recent retrospective studies have shown that a number of these vascular injuries are missed when only the PVP is used and suggested that the addition of arterial phase (AP) improves the overall sensitivity of CT for detecting AE and CVI [12,13,14,15].
In the background of the recent incorporation of AE and CVI in the 2018 revision of AAST scale, the improved sensitivity of the AP for detecting AE/CVI may lead to upgrading of splenic injuries and no studies have been published till date analyzing the same. This study was performed to evaluate the impact of adding AP to the CT protocol on the grading of splenic injuries according to the revised system.
Materials and methods
This study was approved by the Institutional Review Board (ref no. IESC/T-421), and the requirement for informed consent was waived. We retrospectively reviewed the records of all patients who presented to our level 1 trauma center from December 2014 to October 2016 (23 months). All patients in whom a splenic injury was diagnosed on CT and had underwent a dual-phase CT (AP and PVP) were included. As part of our institute protocol, a dual-phase CT was performed on all patients in whom fluid was detected in the abdomen on screening FAST (Focused Assessment with Sonography for Trauma) examination. Patients who had presented more than 48 h after the injury were excluded.
CT acquisition
All CT examinations were performed on SOMATOM Definition AS (64 detector row scanner, Siemens Medical, Forchheim, Germany) or SOMATOM Sensation scanner (40 detector row scanner, Siemens Medical, Forchheim, Germany). All patients received a bolus of 100 mL of intravenous contrast material (Iohexol—Omnipaque 350; GE Healthcare, Princeton, NJ; containing 350 mg iodine per ml) at a rate of 4 mL/s followed by a saline flush of 20 mL at 4 mL/s. A dual-phase (AP followed by PVP) CT was performed. Scans were acquired using automatic bolus tracking with the region of interest (ROI) set in the descending aorta just below the level of the diaphragm and above the level of origin of the renal arteries, and the threshold for initiation was set at 100 HU with a scan delay of 7 s. Following this, the PVP images were acquired with a standard delay of 35 s after the completion of AP (65–70 s from the time of initiation of the injection of the contrast). A 5-min delayed phase was not acquired in this study.
Patient management
Patient management was decided based on the hemodynamic status of the patient. Patients who developed severe hypotension or were non-responders to fluid resuscitation were managed with primary splenectomy. Patients who had recurrent hypotension (defined as systolic blood pressure less than 100 mmHg, heart rate greater than 120 beats per minute after initial response to fluids (transient responders)) were taken up for angiography and embolization irrespective of the presence of CVI or AE on the initial CT [16, 17]. The rest of the patients were managed with non-operative management without angioembolization.
DSA
DSA was done using GE Innova (GE Healthcare Technologies, Waukesha, WI) in selected cases based on hemodynamic status. Initial selective splenic angiography was done in anteroposterior position to look for CVI or AE. Non-selective proximal splenic artery embolization was done with endovascular coils if angiographic signs of CVI or AE were not visualized on the initial splenic angiography, while selective embolization of branch artery was done using coaxial microcatheter and microcoils if CVI or AE was visualized on the initial splenic angiography.
Image analysis
The images were reviewed independently by two attending radiologists (S.G., A.K., both with 12 years of experience in trauma radiology), who were blinded to the clinical information and the angiography findings. The images were analyzed in two blinded ways—PVP alone and AP + PVP together for grading the splenic injuries with an interim interval of 6 weeks between the two. The grading of splenic injury was done according to the 2018 revision of the AAST. Separate grades were assigned in a blinded manner on PVP and AP + PVP by both the radiologists. When AP + PVP were analyzed together, PVP was used for the analysis of the parenchymal injuries and both the phases were used for analyzing the presence of AE/CVI.
CVI was diagnosed when a well-circumscribed globular area(s) of contrast material of attenuation similar to an adjacent contrast-enhanced artery was seen. AE was diagnosed when a linear or irregular area of contrast material enhancement with an attenuation value similar to or greater than that of the aorta or an adjacent major artery was seen [9, 18,19,20].
Splenic angiography and surgery were used as the reference standard for confirming the diagnosis of CVI and AE in patients who had undergone angiography or surgery as a part of their management.
Statistical analysis
Inter-observer variability was assessed by calculating κ (Cohen’s kappa) coefficient. κ values greater than 0.7 were considered to indicate strong agreement, while those in the range of 0.4 to 0.7 were considered to indicate marginal agreement, and those less than 0.4 were considered to indicate poor agreement. The difference in the grade of injury based on PVP alone and AP + PVP was compared using Wilcoxon matched-pairs rank-sum test, and p values < 0.05 were considered to be significant.
Receiver operator characteristic (ROC) curves were generated for the grade of injury on PVP alone, AP + PVP combined for angioembolization, splenectomy separately, and for all splenic interventions combined using non-parametric methods as described by DeLong et al. [21]. The area under the curve (AUC) was calculated with 95% confidence intervals and compared to test for significant differences.
The sample size required was calculated assuming a power of 0.8, a significance level of 0.05, and a ratio of 1:2 between the positive and negative cases (the ones undergoing angioembolization or surgery to the ones without) for non-parametric analysis of the ROC curves and demonstrating a significant difference in the AUCs. The minimum required sample size was estimated to be 108 patients with splenic injuries with at least 36 patients undergoing surgery or angioembolization.
Results
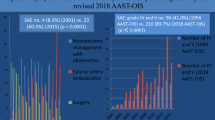
Of the 527 patients who had sustained blunt abdominal injury and had underwent a dual-phase CT, splenic injuries were diagnosed on 164 patients. Of these, 10 were excluded (presented more than 48 h after the injury), and the remaining 154 patients (aged 18–87 years with a median age of 26 years) were included. This included 131 males (85%), 23 females (15%). The modes of injury in the 154 patients were road traffic accidents (106 patients, 68.8%), fall from height (26 patients, 16.8%), assault (20 patients, 12.9%), and other modes in the remaining 2 patients.
The grades of splenic injuries according to the 2018 revision of AAST as graded by both the reviewers for PVP, AP + PVP combined are summarized in Table 2. The Cohen’s κ coefficient for inter-observer variability was 0.97 (95% CI − 0.94 to 0.99) for PVP and 0.94 (95% CI − 0.90 to 0.97) for the AP + PVP combined analysis.
Splenic vascular injuries (including both CVI and AE) were identified in 52 of the 154 (33.7%) patients and included 24 CVI (46.1%) and 28 AE (53.9%) when the AP + PVP were analyzed together. Of these 52, 22 (42.3%; 17 CVI and 5 AE) were identified only on AP, 4 (7.7%; all AE) only on PVP, and the remaining 26 (50%; 7 CVI and 19 AE) on both AP and PVP. There was a change in the grade of injury in 18 patients when the AP was reviewed along with the PVP because of the detection of vascular injuries not seen on PVP alone (Fig. 1). This included 15 CVIs and 3 AEs. The 18 patients, in whom the grade was upgraded to grade 4, included six with grade 2 and 12 with grade 3 injuries based on PVP alone (Table 3).
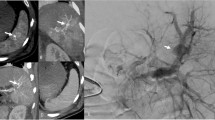
Splenic pseudoaneurysm detected only on the arterial phase CT in a 40-year-old male who had sustained blunt abdominal trauma. Contrast enhanced axial arterial phase CT (a) shows a contrast filled outpouching within the splenic parenchyma (arrow). Corresponding axial portal venous phase image at the same level (b) shows decreased conspicuity of the pseudoaneurysm (arrow). Digital subtraction angiography of the splenic artery (c) of the same patient confirms the presence of pseudoaneurysm (arrow), which was then coil embolized (arrow head in d)
Management
Of the total 154 patients, 25 (16.2%) underwent splenectomy while 41 (26.6%) underwent non-operative management with angioembolization and 88 (57.1%) were managed successfully with observation alone (Table 4).
Among the 52 patients with splenic vascular injuries detected on CT, 13 (25%) underwent splenectomy. When the CVIs and AEs were analyzed separately, only one of the 24 patients (4.1%) with CVI underwent splenectomy, while 12 of the 28 patients (42.8%) with AEs underwent splenectomy (p value < 0.001). The percentage of patients who underwent angioembolization was significantly higher in those with splenic vascular injuries on CT than in those with no vascular injuries on CT (30 of 52 (57.6%) vs. 11 of 102 (10.8%), p value < 0.001). Likewise, patients with higher grades of injury (grade 4 or more) underwent angioembolization more frequently than patients with low-grade injuries (40 of 80 (50%) vs. 1 of 74 (1.3%), p value < 0.001). One vascular injury was detected on DSA in a patient which was not detected on either the AP or the PVP by both the reviewers.
Of the 18 patients whose injuries were upgraded to grade 4 because of the detection of vascular injury on AP, 13 (72.2%) underwent angioembolization, 2 (11.1%) underwent surgery, and 3 were managed with observation.
Thirteen of these 18 (72.2%) vascular injuries were confirmed with a reference standard, 11 on DSA, and 2 on surgery. In 2 other patients who underwent DSA, no vascular injury was detected and there was severe spasm of the splenic vessels. The remaining 3 (16.7%) were managed with observation as they were hemodynamically stable throughout their course in the hospital. All these 18 injuries would have been graded as low-grade (grade 2/3) injuries if only the PVP was used.
The AUC for ROC curves generated for the AAST grade (2018 revision) on PVP, AP + PVP combined for angioembolization, and both splenic interventions showed statistically significant higher AUC for AP + PVP (Fig. 2). There was no statistically significant difference in the AUC for splenectomy. The AUC for AP + PVP for angioembolization and both splenic interventions combined were above 0.8.
Receiver operating characteristic (ROC) curves for splenic angioembolization (a), splenectomy (b), and both combined (c) for the grade of splenic injury according to the revised AAST on arterial phase (AP) + portal venous phase (PVP) combined and portal venous phase (PVP) alone with respective area under the curves (AUC). Significant difference seen in the AUC for splenic angioembolization (a, p value 0.002) and both interventions combined (c, p value 0.003)
Discussion
The results of this study highlight the impact of AP in the CT evaluation and grading of splenic injuries in the setting of blunt abdominal trauma. Over 40% of the splenic vascular injuries were detected only on the AP, and the addition of the AP leads to a change in the grade of injury in 11.7% of patients with splenic injuries.
In this study, vascular injuries were detected on CT in 34% of patients with splenic injuries. Similar rates of splenic vascular injuries were reported by Marmery et al. (86 out of 392, 22%) and Uyeda et al. (54 out of 146, 36.9%) [12, 18]. In the current study, 42.3% of the vascular injuries were detected only on the AP. In a study by Uyeda et al. on the role of AP in splenic injuries in blunt abdominal trauma, 25% (13 out of 52) of the vascular injuries were detected only on the AP. If only the CVIs are considered, in this study, 70.8% CVIs were detected only on the AP which is similar to 13 out of 22 (59.1%) in the study by Uyeda et al. [12]. Likewise, Boscak et al., Melikian et al., and Atluri et al. have shown better detection of vascular injuries, especially CVIs with the addition of AP [13, 14, 22].
A CVI results from injury to the intraparenchymal arterial vascular branches in direct communication with the arterial tree. Thus, contrast opacifies the CVI best in the AP and this is further enhanced by the surrounding un-opacified parenchyma. However, during the PVP, there is a relative washout of contrast within the pseudoaneurysm which decreases its attenuation and increase in the attenuation of the surrounding parenchyma which is now homogeneously opacified making the pseudoaneurysm less conspicuous.
AE occurs when the pressure in the vessel exceeds that of the surrounding tissues, and the rate of extravasation also depends on the pressure gradient. Injury to peripheral higher-order branches and relatively low pressure in the injured vessel secondary to vasospasm would result in slower rates of extravasation, which may not be detected on the AP. But an increasing amount of contrast extravasates until the time of acquisition of PVP, making AEs more apparent on PVP [19, 23]. In a few patients, AEs were detected only on AP. This could be due to the progressive dilution of extravasated contrast making it iso-dense to the surrounding parenchyma, masking them on the PVP.
The current study shows the impact of the addition of AP to the CT protocol on the 2018 revision of the AAST [9]. The splenic injury was classified to a higher grade (grade 4) in 11.7% of the patients due to the detection of vascular injuries on AP, and 83.3% of them had underwent some form of splenic intervention. There is no similar published data to this effect. The combination of AP + PVP having larger AUC suggests that the additional AP would help better predict the need for splenic interventions in a patient with splenic injury. None of the patients in the current study had an AE extending beyond the confines of the splenic capsule that was detected only on AP (grade 5). This could likely be attributed to the absence of surrounding enhancing parenchyma that could potentially obscure their detection on PVP.
Factors like the rate of injection of contrast, the time of acquisition of the AP, and the heterogenous enhancement of the spleen in the arterial phase are potential confounding factors in the study. A uniform protocol of 4 mL/s was used in this study. Bae et al. observed that an increase in rate of injection of contrast (up to 10 mL/s) steeply increases the magnitude of peak aortic enhancement [24]. Soto et al. had suggested rates of 3–5 mL/s to be used in the setting of blunt abdominal trauma [25]. An early AP CT is usually used for the study of the abdominal arterial tree, while a late AP is used in the evaluation of hyper-vascular hepatic masses. In the setting of trauma, late AP is used in some centers using a split bolus technique and a single acquisition [26]. Atluri et al. in their study demonstrated that the mean time from opacification of the aorta to pseudoaneurysm was 1.32 s in 15 out of 20 splenic pseudoaneurysms on angiography, thus favoring the use of an early AP in the trauma setting [22]. Heterogenous enhancement of the splenic parenchyma on the AP could lead to the appearance of hyperdense areas that could mimic a vascular injury [27]. There are no published studies thus far that describe CT features to reliably differentiate the two.
This study has a few limitations. Firstly, patient management was not randomized. Hence, the actual clinical outcome of different forms of management could not be ascertained. Also, a relatively small number of patients underwent angioembolization (26.6%). The mere presence of vascular injury was not considered an indication for angioembolization. The clinical status, especially the hemodynamic status, was taken into account before deciding the management. Patients with vascular injuries who were managed conservatively without angioembolization were hemodynamically stable throughout the hospital course. The need for angioembolization was decided on a case-by-case basis based on the clinical parameters. Since all the patients did not undergo angiography or surgery, the reference standard for the diagnosis of vascular injuries was not available in all patients, leading to a verification bias.
To conclude, this study not only reiterates that the addition of AP significantly increases the detection rate of splenic vascular injuries on CT but also shows that it leads to significant changes in the final grade on the organ injury scale of AAST according to the 2018 revision. Accurate classification of splenic injuries to appropriate AAST grades using additional AP could lead to better and prompt triage of patients for splenic interventions or conservative management.
Abbreviations
- AAST:
-
American Association for the Surgery of Trauma
- AE:
-
Active extravasation of contrast
- AP:
-
Arterial phase
- AUC:
-
Area under the curve
- CVI:
-
Contained vascular injury
- DSA:
-
Digital subtraction angiography
- OIS:
-
Organ Injury Scale
- PVP:
-
Portal venous phase
- ROC:
-
Receiver operator characteristic
References
Zarzaur BL, Kozar R, Myers JG, Claridge JA, Scalea TM, Neideen TA, Maung AA, Alarcon L, Corcos A, Kerwin A, Coimbra R (2015) The splenic injury outcomes trial: an American Association for the Surgery of Trauma multi-institutional study. J Trauma Acute Care Surg 79(3):335–342. https://doi.org/10.1097/TA.0000000000000782
D’Huyvetter C (2000) The trauma disease. J Trauma Nurs 7(1):5–12
Gad MA, Saber A, Farrag S, Shams ME, Ellabban GM (2012) Incidence, patterns, and factors predicting mortality of abdominal injuries in trauma patients. N Am J Med Sci 4(3):129–134. https://doi.org/10.4103/1947-2714.93889
Brillantino A, Iacobellis F, Robustelli U, Villamaina E, Maglione F, Colletti O, de Palma M, Paladino F, Noschese G (2015) Non operative management of blunt splenic trauma: a prospective evaluation of a standardized treatment protocol. Eur J Trauma Emerg Surg Published online September 28:593–598. https://doi.org/10.1007/s00068-015-0575-z
Chastang L, Bège T, Prudhomme M, Simonnet AC, Herrero A, Guillon F, Bono D, Nini E, Buisson T, Carbonnel G, Passebois L, Vacher C, le Moine MC (2015) Is non-operative management of severe blunt splenic injury safer than embolization or surgery? Results from a French prospective multicenter study. J Visc Surg 152(2):85–91. https://doi.org/10.1016/j.jviscsurg.2015.01.003
Watson GA, Hoffman MK, Peitzman AB (2015) Nonoperative management of blunt splenic injury: what is new? Eur J Trauma Emerg Surg 41(3):219–228. https://doi.org/10.1007/s00068-015-0520-1
Moore EE, Shackford SR, Pachter HL et al (1989) Organ injury scaling: spleen, liver, and kidney. J Trauma 29(12):1664–1666
Moore EE, Cogbill TH, Jurkovich GJ, Shackford SR, Malangoni MA, Champion HR (1995) Organ injury scaling: spleen and liver (1994 revision). J Trauma 38(3):323–324. https://doi.org/10.1097/00005373-199503000-00001
Kozar RA, Crandall M, Shanmuganathan K, Zarzaur BL, Coburn M, Cribari C, Kaups K, Schuster K, Tominaga GT, AAST Patient Assessment Committee (2018) Organ injury scaling 2018 update: spleen, liver, and kidney. J Trauma Acute Care Surg 85(6):1119–1122. https://doi.org/10.1097/TA.0000000000002058
Hinzpeter R, Boehm T, Boll D, Constantin C, del Grande F, Fretz V, Leschka S, Ohletz T, Brönnimann M, Schmidt S, Treumann T, Poletti PA, Alkadhi H (2016) Imaging algorithms and CT protocols in trauma patients: survey of Swiss emergency centers. Eur Radiol Published online September 5:1922–1928. https://doi.org/10.1007/s00330-016-4574-1
Becker CD, Spring P, Glättli A, Schweizer W (1994) Blunt splenic trauma in adults: can CT findings be used to determine the need for surgery? AJR Am J Roentgenol 162(2):343–347. https://doi.org/10.2214/ajr.162.2.8310923
Uyeda JW, LeBedis CA, Penn DR, Soto JA, Anderson SW (2014) Active hemorrhage and vascular injuries in splenic trauma: utility of the arterial phase in multidetector CT. Radiology. 270(1):99–106. https://doi.org/10.1148/radiol.13121242
Boscak AR, Shanmuganathan K, Mirvis SE, Fleiter TR, Miller LA, Sliker CW, Steenburg SD, Alexander M (2013) Optimizing trauma multidetector CT protocol for blunt splenic injury: need for arterial and portal venous phase scans. Radiology. 268(1):79–88. https://doi.org/10.1148/radiol.13121370
Melikian R, Goldberg S, Strife BJ, Halvorsen RA (2016) Comparison of MDCT protocols in trauma patients with suspected splenic injury: superior results with protocol that includes arterial and portal venous phase imaging. Diagn Interv Radiol 22(5):395–399. https://doi.org/10.5152/dir.2016.15232
Anderson SW, Soto JA, Lucey BC, Burke PA, Hirsch EF, Rhea JT (2008) Blunt trauma: feasibility and clinical utility of pelvic CT angiography performed with 64-detector row CT. Radiology. 246(2):410–419. https://doi.org/10.1148/radiol.2462070082
Kirkpatrick AW, Ball CG, D’Amours SK, Zygun D (2008) Acute resuscitation of the unstable adult trauma patient: bedside diagnosis and therapy. Can J Surg 51(1):57–69 Accessed Sept 16, 2019. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2386316/
Renzulli P, Gross T, Schnüriger B, Schoepfer AM, Inderbitzin D, Exadaktylos AK, Hoppe H, Candinas D (2010) Management of blunt injuries to the spleen. Br J Surg 97(11):1696–1703. https://doi.org/10.1002/bjs.7203
Marmery H, Shanmuganathan K, Mirvis SE, Richard H III, Sliker C, Miller LA, Haan JM, Witlus D, Scalea TM (2008) Correlation of multidetector CT findings with splenic arteriography and surgery: prospective study in 392 patients. J Am Coll Surg 206(4):685–693. https://doi.org/10.1016/j.jamcollsurg.2007.11.024
Hamilton JD, Kumaravel M, Censullo ML, Cohen AM, Kievlan DS, West OC (2008) Multidetector CT evaluation of active extravasation in blunt abdominal and pelvic trauma patients. Radiographics. 28(6):1603–1616. https://doi.org/10.1148/rg.286085522
Anderson SW, Varghese JC, Lucey BC, Burke PA, Hirsch EF, Soto JA (2007) Blunt splenic trauma: delayed-phase CT for differentiation of active hemorrhage from contained vascular injury in patients. Radiology. 243(1):88–95. https://doi.org/10.1148/radiol.2431060376
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 44(3):837–845
Atluri S, Richard HM, Shanmuganathan K (2011) Optimizing multidetector CT for visualization of splenic vascular injury. Validation by splenic arteriography in blunt abdominal trauma patients. Emerg Radiol 18(4):307–312. https://doi.org/10.1007/s10140-011-0961-8
Shanmuganathan K, Mirvis SE, Reaney SM (1995) Pictorial review: CT appearances of contrast medium extravasations associated with injury sustained from blunt abdominal trauma. Clin Radiol 50(3):182–187
Bae KT (2010) Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology. 256(1):32–61. https://doi.org/10.1148/radiol.10090908
Soto JA, Anderson SW (2012) Multidetector CT of blunt abdominal trauma. Radiology. 265(3):678–693. https://doi.org/10.1148/radiol.12120354
Rosenkrantz AB (2017) Genitourinary imaging, an issue of radiologic clinics of North America, E-Book. Elsevier Health Sciences
Donnelly LF, Foss JN, Frush DP, Bisset GS (1999) Heterogeneous splenic enhancement patterns on spiral CT images in children: minimizing misinterpretation. Radiology. 210(2):493–497. https://doi.org/10.1148/radiology.210.2.r99fe16493
Acknowledgments
We would like to acknowledge the contribution of Dr. Kathirkamanathan Shanmuganathan (deceased), professor, Diagnostic Radiology, University of Maryland School of Medicine, for his help in conceptualization of the paper and in editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Institutional Review Board (ref no. IESC/T-421), and the requirement for informed consent was waived.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hemachandran, N., Gamanagatti, S., Sharma, R. et al. Revised AAST scale for splenic injury (2018): does addition of arterial phase on CT have an impact on the grade?. Emerg Radiol 28, 47–54 (2021). https://doi.org/10.1007/s10140-020-01823-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-020-01823-z