Abstract
Purpose
To determine the relationship between multidetector computed tomography (MDCT) findings, management strategies, and ultimate clinical outcomes in patients with splenic injuries secondary to blunt trauma.
Materials and methods
This Institutional Review Board-approved study collected 351 consecutive patients admitted at the Emergency Department (ED) of a Level I Trauma Center with blunt splenic trauma between October 2002 and November 2015. Their MDCT studies were retrospectively and independently reviewed by two radiologists to grade splenic injuries according to the American Association for the Surgery of Trauma (AAST) organ injury scale (OIS) and to detect intraparenchymal (type A) or extraparenchymal (type B) active bleeding and/or contained vascular injuries (CVI). Clinical data, information on management, and outcome were retrieved from the hospital database. Statistical analysis relied on Student’s t, chi-squared, and Cohen’s kappa tests.
Results
Emergency multiphase MDCT was obtained in 263 hemodynamically stable patients. Interobserver agreement for both AAST grading of injuries and vascular lesions was excellent (k = 0.77). Operative management (OM) was performed in 160 patients (45.58% of the whole cohort), and high-grade (IV and V) OIS injuries and type B bleeding were statistically significant (p < 0.05) predictors of OM. Nonoperative management (NOM) failed in 23 patients out of 191 (12.04%). In 75% of them, NOM failure occurred within 30 h from the trauma event, without significant increase of mortality. Both intraparenchymal and extraparenchymal active bleeding were predictive of NOM failure (p < 0.05).
Conclusion
Providing detection and characterization of parenchymal and vascular traumatic lesions, MDCT plays a crucial role for safe and appropriate guidance of ED management of splenic traumas and contributes to the shift toward NOM in hemodynamically stable patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trauma is a leading cause of mortality and disability especially for people under 35 years of age, and the spleen is the most frequently injured organ following blunt abdominal trauma [1]. During the last century, the management of blunt splenic trauma substantially evolved from routine splenectomy to the current era of splenic preservation whenever feasible [2, 3]. Currently, the combination of awareness of physiological splenic functions and advancements in diagnostic and interventional radiology resulted in nonoperative management (NOM) becoming the standard of care in hemodynamically stable patients. NOM with close patient observation and splenic artery embolization (SAE) in selected patients represents a safe and effective treatment for both minor and severe splenic injuries, achieving an overall success rate of 90–95%. The key advantages of NOM include lower costs, earlier discharge and possibility to obviate surgery, related complications, and delayed asplenic morbidity [3,4,5,6,7,8].
Failure of NOM (F-NOM) after splenic trauma refers to the need for operative intervention and is reported to occur in 10–30% of patients, a figure which seems to be closely related to patient selection [7, 8]. For this reason, due to its high accuracy in the trauma setting, contrast-enhanced computed tomography (CT) is strongly recommended in hemodynamically stable patients to identify and assess severity of traumatic injuries [9,10,11,12,13].
Along with other advancements in emergency and intensive care medicine, the advent of multidetector CT (MDCT) contributed to the shift toward NOM in hemodynamically stable patients. By increasing the speed of image acquisition, MDCT better visualizes organs and vascular structures in different phases of contrast enhancement, resulting in increased sensitivity for detecting active bleeding and contained vascular injuries (CVI) which can lead to hemodynamic deterioration [14]. Currently, the severity of splenic injury according to the organ injury scale (OIS) proposed by the American Association for the Surgery of Trauma (AAST) no longer represents a contraindication for NOM. However, the clinical implications of vascular injuries in the management of patients with splenic trauma remain controversial [3].
In this setting, the main objective of this study was to retrospectively assess the role of MDCT in the management of patients with post-traumatic splenic injuries, in order to determine the relationship between imaging findings, management strategies, and ultimate clinical outcome.
Materials and methods
This single-center, retrospective, longitudinal, and observational cohort study was performed following approval by the Institutional Review Board with waived informed consent. Data of patients managed by the Trauma Team at our Level I Trauma Center between October 2002 and November 2015 was analyzed. Since the beginning of the activities of the Trauma Team, information concerning all trauma patients is recorded in a database designed in accordance with the guidelines of the Atlanta Trauma Registry Workshop [15, 16].
Patient population
Among 4730 trauma patients admitted to the Emergency Department (ED) during the study period, retrospective record review identified 364 (7.69%) patients with an injured spleen. They were mostly males (79.29%), with mean age 35.6 ± 17.6 years. Thirteen patients were excluded from the study: six who had penetrating trauma, one who arrived late to the Trauma Team care, three due to lacking data in the database, and three who died in the shock room. The resulting final population included 351 patients. Regarding mechanism of injury, most of blunt splenic traumas were due to motor vehicle accidents (80%), followed by self-harm acts (6.8%) and workplace (6.1%) and home accidents (4.3%).
CT technique
All patients underwent MDCT using either a 4-slice (Somatom Volume Zoom 4, from 2002 to 2007) or 64-slice (Somatom Definition, from 2007 to 2015) scanner, both from Siemens Medical Systems, Erlangen, Germany, using a multiphase protocol with coverage from the skull base to the pelvic floor. The majority of patients were investigated using a three-phase protocol including precontrast, arterial-phase (automatic bolus detection with trigger at 150 HU in the thoracic aorta and a delay of 12 s from trigger), and venous-phase (70 s from trigger) acquisitions were obtained before and during intravenous injection of a 350–400 mg I/mL iodinated contrast agent at 3–4 mL/s injection speed. In selected patients with clinical or radiological suspicion of urinary system damage, additional delayed acquisitions (160 s from trigger) and excretory-phase acquisitions were obtained after 5–7 min. The arterial phase acquisition was completed with maximum intensity projection (MIP) vascular reconstructions. No patient received intrarectal or oral contrast agent [17,18,19].
As calculated using the Nexo Dose software (Bracco, Milan, Italy), in our patient population, the median dose-length product (DLP) was approximately 2000 mGy cm. This figure compares favorably to the computed tomography dose index (CTDI) recommended by the AAPM (American Association of Physicists in Medicine) which is 15 mGy, multiplied by two phases. On our 64-detector scanner, the ionizing radiation dose delivered during each acquisition phase was approximately 10 mGy, which allowed us to obtain three phases with a cumulative dose similar to two acquisitions (more phases with lower dose for every single phase).
Image analysis
The institutional picture archiving and communication system (PACS, Impax; Agfa-Gevaert, Mortsel, Belgium) was used to review CT images acquired during management of ED patients and/or performed later for follow-up during NOM. Blinded to patients’ clinical information and outcomes, two radiologists (with, respectively, 5 and 15 years of experience in trauma imaging) independently reviewed all images in order to identify clinically significant findings for management of splenic injuries, including
-
Grade of the injury according to AAST scale (Table 1).
-
Signs of either intraparenchymal (type A, Fig. 1) or extraparenchymal (type B, Fig. 2) active bleeding.
-
Presence of contained vascular injuries (CVI, Fig. 3) [17,18,19].
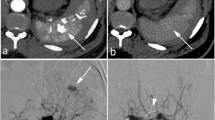
Contrast-enhanced MDCT in a 60-year-old woman hit by a motorcycle. Axial images show a focus of extravascular contrast enhancement within the splenic parenchyma (arrows) that progressively increases in size between arterial (a) and venous (b) phases, consistent with intraparenchymal active bleeding. The patient underwent splenectomy
Contrast-enhanced MDCT in a 65-year-old woman struck by a car. Axial images show an area of extravascular contrast enhancement adjacent to the splenic parenchyma (arrows) that progressively increases in size between arterial (a) and venous (b) phases, consistent with extraparenchymal active bleeding. The patient underwent splenectomy
Contrast-enhanced MDCT scan in a 53-year-old man involved in motorcycle accident. Axial images show a small-sized circumscribed area of extravascular contrast enhancement (arrows) with similar attenuation to the aorta in both arterial (a) and venous (b) phases, without increase in size, consistent with contained vascular injury (pseudoaneurysm or arteriovenous fistula). The patient underwent successful nonoperative management (NOM)
Foci of extravascular contrast-enhanced blood located in or adjacent to the splenic parenchyma that increased in size on portal venous and delayed phases were defined as active bleeding (type A or B, respectively). CVI were defined as circumscribed areas of extravascular contrast-enhanced blood, equal or similar in attenuation to the aorta or an adjacent major artery at all phases of image acquisition, showing washout aspect in delayed phases. The definition of CVI encompasses pseudoaneurysm and arteriovenous fistula, which are usually indistinguishable by means of CT [17,18,19]. In cases in which splenic CVI and active bleeding (type A or B) were found independently in distinct locations, the term “CVI plus bleeding” was used (Fig. 4).
Contrast-enhanced MDCT scan in a 28-year-old man involved in a car accident. Axial images show coexistent, independent vascular injuries in distinct locations: a small-sized area of extravascular contrast enhancement (thin white arrows) which is hyperattenuating in arterial phase (a) and less conspicuous in venous phase (b), consistent with contained vascular injury, and two areas of extravascular contrast enhancement within (thick white arrow) and adjacent (hollow arrow) to the splenic parenchyma that progressively increase in size between arterial (a) and venous (b) phases, consistent with intraparenchymal and extraparenchymal active bleeding, respectively
Associated extrasplenic lesions consistent with polytrauma were also identified and retrospectively classified according to their location, number, and degree as suggested by the Abbreviated Injury Scale (AIS) [20].
Management strategies
Regarding treatment of splenic lesions, two groups of patients were identified, according to the implemented strategy type: operative management (OM) and NOM. Within the latter group, a subset of cases experiencing failed NOM was selected, defined as the need for surgery to treat splenic injury, related complications, or other associated abdominal lesions. The definition of F-NOM encompassed the following two situations: (a) record of failure as a complication in the database or presence of explicit notice placed by the Trauma Team surgeon in the clinical diary and (b) abdominal operative procedure more than 2 h after admission the Emergency Department, in the case of hemodynamically normal patient or stabilized at admission. In the vast majority of patients, the timeframe for F-NOM was 48 h after ED admission.
Patients’ medical records were reviewed in order to analyze the following clinical features: injury severity score (ISS) [20], hemodynamic condition at prehospital phase and at ED arrival, angiographic procedures performed, need for blood transfusions (data available since 2009), days of hospitalization, and final outcomes.
Statistical analysis
Statistical analysis of the data was performed using IBM SPSS Statistics 21 software. To compare averages of continuous variables, the Student’s t test was used; to compare binary or categorical variables between two groups, the chi-squared test was applied. In situations where two groups were compared, the variables showing statistically significant difference were included in a logistic regression model. The odds ratio relating to categorical variables were referred to the first group of the same variable: for example, for the OIS grade lesion, the reference was OIS I, while for the vascular lesions, the reference was “absence of vascular injury.” Results with p value < 0.05 were considered statistically significant. The interobserver agreement was assessed using Cohen’s kappa coefficient (k).
Results
Imaging findings
Among the study population, CT scan was performed in 263 patients. The average ISS score was 32.3 ± 14.8 standard deviation (range 10.2 to 65.3). According to the AAST scale, splenic injuries were categorized as grade I in 37 of 263 patients (14%), grade II in 67 (25%), grade III in 107 (41%), grade IV in 45 (17%), and grade V in 7 (3%).
A total 156 vascular lesions (summarized in Table 2) were encountered in 59.3% of patients. An increased incidence of vascular lesions was noted along with the degree of splenic injury, with a statistically significant association for OIS grades III and IV (p value < 0.05). Interobserver agreement for both AAST grading of injuries and vascular lesions was excellent (k = 0.77).
Regarding associated extrasplenic lesions, brain and neurologic injuries were detected in 130 patients, chest injuries in 69, limbs injuries in 160, and other abdominal injuries in 123. Among the latter category, the liver and kidneys represented the most frequently involved organs.
Splenic injuries and management strategies
The management strategies of patients with splenic trauma are summarized in Fig. 5. Over the years, NOM increased from 42.2% (from 2002 to 2009) to 66.4% (from 2010 to 2015). The mean age of patients receiving OM and NOM was 37 and 33 years, respectively. Within the NOM group, 18 patients (12.5%) had age equal or higher to 55 years.
According to the results of bivariate logistic regression, statistically significant predictors of OM were hypotension (systolic blood pressure < 90 mmHg), OIS grades IV/V, type B bleeding, and bowel injuries (p value < 0.05). Furthermore, each increasing point of ISS score of trauma correlated with an increased probability of surgical treatment. Since 2009, the need for transfusion of blood components and the number of transfused blood units were also associated to operative management (p < 0.05).
The distribution of NOM over the years shows a general positive trend of success rate, from 75.6% from 2002 to 2009 to 95.7% afterwards. NOM failed in 23 of the 191 patients (12%), 19 males and 4 females with mean age of 34.4 ± 19.2 years (median 26 years). Among them, only four had more than 60 years. Age ≥ 55 years was not associated with an increased risk of F-NOM.
Regarding time of F-NOM presentation, the mean delay was 40.9 ± 9.1 h and the median 12 h. Three out of four (75%) cases of F-NOM occurred within 30 h. Only two failures occurred beyond 48 h, respectively, 208 and 408 h after nonoperative treatment decision.
When considered individually, the OIS degree of splenic injuries was not found to be associated with F-NOM. The majority of patients who experienced F-NOM showed grade III lesions; only one patient had injuries classified as grade 4 and none as grade 5 (Table 3).
The splenic vascular lesions of patients who experienced F-NOM are shown in Table 4. Eighty-nine patients with splenic vascular lesion were received NOM, which failed in 23 of them (25.8%). On the other hand, in the F-NOM group, nine patients (39.1%) had no vascular lesion. Active bleeding (either type A or B), found in 11 of 23 patients (47%), was associated with F-NOM (p value < 0.05). Conversely, the presence of a CVI had no statistical power to predict F-NOM.
As a supplement of NOM, 26 splenic angiographies were performed, 16 of which completed with proximal (9 cases) or distal (7 cases) embolization procedures. The 18 interventional procedures performed from 2010 to 2015 accounted for 10.2% of all patients subjected to NOM. Within the F-NOM group, only distal embolization was performed and none of the patients submitted to proximal embolization failed NOM. Also, the embolization strategy evolved: after 2009, the use of proximal embolization has doubled, and angiography without embolization decreased from 50 to 30% of all procedures.
Following F-NOM, splenectomy was required in 14 patients (60.9% of cases). In the remaining patients, a conservative surgical management was adopted, consisting in hemostasis in five patients (21.7%) and partial splenic resection in four patients (17.4%).
Within the F-NOM group, the average stay in the intensive care unit was 3.57 ± 1.7 days; the overall days of hospitalization were 15.7 ± 11.2 (median 7 days). Two patients deceased, both of which with OIS grade I splenic lesion but severe associated head injuries.
Final outcomes
The overall mortality related to blunt splenic trauma was 15%. The following binary and categorical variables showed a positive association with mortality (p value < 0.05): prehospital cardiac arrest, systolic blood pressure < 90 mmHg at the ED admission, need for transfusion of blood components, OM of splenic trauma, presence of high-grade OIS with associated head, and chest and hepatic lesions. F-NOM was not associated with an increased mortality.
Discussion
In agreement with the literature [3, 18], in our study, splenic trauma mostly affected the young adult population, predominantly males involved in road traffic accidents, resulting in an overall mortality rate of 15%.
In our study, the incidence of splenic vascular lesions identified with CT examinations (59.3%) was significantly higher than the data reported in the literature, which is approximately 20% [19, 21,22,23]. This can be attributed to the combined effect of a selection bias (our population mostly includes severely injured polytraumatized individuals) and other factors such as (a) the mechanism of trauma (penetrating injuries are uncommon at our institution compared to American reports, and have not been included in this study), (b) the extensive use of CT after successful resuscitation in the “shock room” of unstable and transient responder patients, (c) the 24-h in-house availability of attending skilled radiologists, and (d) the meticulous search for vascular and microvascular abnormalities on thin-section CT images in arterial and venous phases, with prompt referral for interventional radiology, which may result in more accurate diagnosis even in tricky CT patterns.
Among vascular injuries, only type B bleeding proved to be a predictive factor of OM. This reflects the intuitive fact that an extraparenchymal splenic bleeding has less probability to be self-limited compared to an intraparenchymal one. The presence of “arterial blush” has been consistently considered the main risk factor for F-NOM [23,24,25]. In our study, 47% of patients with MDCT evidence of active bleeding experienced F-NOM, and both types of bleeding (A and B) were found to be statistically significant predictors of failure (Fig. 6). Therefore, particular attention should be given to these MDCT findings and their implications for patient management.
Contrast-enhanced MDCT scan in a 24-year-old man involved in a car accident. Axial arterial phase images of the same patient performed at the initial presentation (a) and 20 h later (b). During this time interval, signs of active bleeding increased (thick arrows), the size of the splenic/perisplenic hematoma (hollow arrows) and the amount of the perihepatic effusion (thin arrow) also increased. This patient experienced failed NOM and required splenectomy
The other key, novel finding from our study was F-NOM occurred within 30 h from the trauma event in 75% of patients, albeit without significantly increased mortality: knowledge of this crucial timeframe may impact the trauma management strategies, particularly in the presence of actively bleeding lesions at CT.
In order to control the hemorrhagic focus, hemodynamically stable patients can undergo SAE but arterial blush at MDCT is not confirmed angiographically in up to 17% of patients. In the case of CT angiography discrepancy, the risk of rebleeding is over two times higher (25 versus 10%) compared with those with angiographic evidence of bleeding. However, some studies raised consideration for empiric SAE even in the absence of angiographic blush, particularly in the presence of high-grade splenic injuries. Our study seems to confirm the superiority of proximal embolization for treatment of vascular lesions. Although the number of embolization considered in this study is not enough to have statistical significance, the data may have a considerable clinical importance [6, 7, 21].
Conversely, the presence of a CVI did not show statistical significance in the prediction of F-NOM. Considering that pseudoaneurysms and arteriovenous fistulas may develop late after the traumatic event, it is possible that they may be not present at initial CT and detected only on follow-up examinations. In the present study, 54 follow-up CT studies were performed, in which 12 cases of vascular lesion were detected (only one patient experienced F-NOM), while patients with OIS grades I and II were followed using abdominal ultrasound. Although it is clear that latent CVIs develop predominantly in higher grade injuries, a study reported that such lesions can also develop in grade OIS I and II, suggesting that all patients could benefit from repeated CT, given the risk of delayed rupture and bleeding of these lesions [26].
The number of follow-up CT studies was particularly low in the F-NOM group (5 of 23 patients). Similar to previous reports [27], this figure probably results from the fact that 75% of F-NOM occurred within 30 h and that patients who develop hemodynamic instability during the course of monitoring are immediately treated surgically. Furthermore, this suggests that earlier follow-up imaging studies within 24–48 h should be considered compared to the usual repeated CT at 48–72 h at our institution. Future researches are needed to update the follow-up imaging approach of patients with blunt splenic injury.
The F-NOM group did not experience an increased mortality rate, a fact which can be attributed to two factors: (a) the selection of patients subjected to NOM was optimized and (b) clinical, laboratory, and diagnostic imaging monitoring allowed early detection of actively bleeding lesions associated with hemodynamic instability. Along the years, by acquiring theoretical and practical skills, all components of the Trauma Team are more likely to apply the NOM protocols, which can be demonstrated by the positive trend of the success rates. Therefore, in response to a correct selection and by performing a proper short-period follow-up, F-NOM does not pose a threat to patients’ survival.
In our series, the overall splenectomy rate (32.8%) over the entire study period is higher than those reported in most recent papers. However, owing to the progressively improving expertise of the Trauma Team in managing blunt splenic injuries and to the growing NOM success rate, splenectomy decreased from 45.1% (from 2002 to 2009) to 30.1% (from 2010 to 2015). Meanwhile, the use of SAE also increased from 2.9 to 10.2%. In a retrospective paper from Sweden, splenectomy dropped from 38.4% in 2007 to 10.5% in 2013, with an ample (over 42%) use of SAE [28]. In another study, splenectomies decreased to a lesser extent, from 20% in 2010 to 14.9% in 2014, with a corresponding increase in SAE use [29]. Conversely, a very large review reported stable splenectomy rate (24.3%) from 2008 to 2014 despite more than doubled SAE interventions (13.5% compared to 5.3%) [30].
There were a few limitations in our study. In first place, it is intrinsically retrospective. Secondly, since it is a long-term study, it is important to note that the management of patients with splenic trauma has significantly changed over the years, which can be attributed to four factors: (a) increasing clinical experience of the trauma team, (b) the introduction of massive transfusion protocol, (c) the increased use of splenic angiographic procedures, and (d) the decreasing number of head traumas. This may represent a clinical and statically significant fact, probably influencing the overall results. In third place, there are reports that trauma patients may return to hospitals different from the one of initial presentation; therefore, it is possible that single-center studies may not provide a complete depiction of the outcomes of these patients after discharge.
Conclusion
In conclusion, this study demonstrated that multiphase MDCT plays a pivotal role in the setting of splenic trauma, both in the initial assessment and in subsequent follow-up, since imaging findings are crucial to guide the most appropriate management of the patients. By consistently identifying both parenchymal and vascular lesions, MDCT contributes to the shift toward NOM in hemodynamically stable patients and further supports NOM as the current standard of care.
References
Soreide K (2009) Epidemiology of major trauma. Br J Surg 96:697–698
Richardson JD (2005) Changes in the management of injuries to the liver and spleen. J Am Coll Surg 200:648–669
Stassen NA, Bhullar I, Cheng JD, Crandall ML, Friese RS, Guillamondegui OD, Jawa RS, Maung AA, Rohs TJ Jr, Sangosanya A, Schuster KM, Seamon MJ, Tchorz KM, Zarzuar BL, Kerwin AJ, Eastern Association for the Surgery of Trauma (2012) Selective nonoperative management of blunt splenic injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg 73:S294–S300
Stein DM, Scalea TM (2006) Nonoperative management of spleen and liver injuries. J Intensive Care Med 21:296–304
Haan JM, Bochicchio GV, Kramer N, Scalea TM (2005) Nonoperative management of blunt splenic injury: a 5-year experience. J Trauma 58:492–498
Brillantino A, Iacobellis F, Robustelli U, Villamaina E, Maglione F, Colletti O, de Palma M, Paladino F, Noschese G (2016) Non operative management of blunt splenic trauma: a prospective evaluation of a standardized treatment protocol. Eur J Trauma Emerg Surg 42:593–598
Tugnoli G, Bianchi E, Biscardi A, Coniglio C, Isceri S, Simonetti L, Gordini G, di Saverio S (2015) Nonoperative management of blunt splenic injury in adults: there is (still) a long way to go. The results of the Bologna-Maggiore Hospital trauma center experience and development of a clinical algorithm. Surg Today 45:1210–1217
Raza M, Abbas Y, Devi V, Prasad KV, Rizk KN, Nair PP (2013) Non operative management of abdominal trauma—a 10 years review. World J Emerg Surg 8:14
Becker CD, Spring P, Glattli A et al (1994) Blunt splenic trauma in adults: can CT findings be used to determine the need for surgery? AJR Am J Roentgenol 162:343–347
Shanmuganathan K, Mirvis SE, Boyd-Kranis R, Takada T, Scalea TM (2000) Nonsurgical management of blunt splenic injury: use of CT criteria to select patients for splenic arteriography and potential endovascular therapy. Radiology 217:75–82
Shanmuganathan K, Mirvis SE, Sover ER (1993) Value of contrast-enhanced CT in detecting active hemorrhage in patients with blunt abdominal or pelvic trauma. AJR Am J Roentgenol 161:65–69
Thompson BE, Munera F, Cohn SM et al (2006) Novel computed tomography scan scoring system predicts the need for intervention after splenic injury. J Trauma 60:1083–1086
Willmann JK, Roos JE, Platz A, Pfammatter T, Hilfiker PR, Marincek B, Weishaupt D (2002) Multidetector CT: detection of active hemorrhage in patients with blunt abdominal trauma. AJR Am J Roentgenol 179:437–444
Anderson SW, Varghese JC, Lucey BC, Burke PA, Hirsch EF, Soto JA (2007) Blunt splenic trauma: delayed-phase CT for differentiation of active haemorrhage from contained vascular injury. Radiology 243:88–95
No Authors listed (1989) Report from the 1988 Trauma Registry Workshop, including recommendations for hospital-based trauma registries. J Trauma 29:827–834
Moore L, Clark DE (2008) The value of trauma registries. Injury 39:686–695
Graves JA, Hanna TN, Herr KD (2017) Pearls and pitfalls of hepatobiliary and splenic trauma: what every trauma radiologist needs to know. Emerg Radiol 24:557–568
Boscak AR, Shanmuganathan K, Mirvis SE, Fleiter TR, Miller LA, Sliker CW, Steenburg SD, Alexander M (2013) Optimizing trauma multidetector CT protocol for blunt splenic injury: need for arterial and portal venous phase scans. Radiology 268:79–88
Uyeda JW, LeBedis CA, Penn DR et al (2014) Active hemorrhage and vascular injuries in splenic trauma: utility of the arterial phase in multidetector CT. Radiology 270:99–106
Medicine AftAoA (1990) The abbreviated injury scale 1990 revision. AAAM, Des Plaines
Alarhayem AQ, Myers JG, Dent D, Lamus D, Lopera J, Liao L, Cestero R, Stewart R, Eastridge BJ (2015) “Blush at first sight”: significance of computed tomographic and angiographic discrepancy in patients with blunt abdominal trauma. Am J Surg 210:1104–1111
Federle MP, Courcoulas AP, Powell M, Ferris JV, Peitzman AB (1998) Blunt splenic injury in adults: clinical and CT criteria for management, with emphasis on active extravasation. Radiology 206:137–142
Marmery H, Shanmuganathan K, Mirvis SE, Richard H III, Sliker C, Miller LA, Haan JM, Witlus D, Scalea TM (2008) Correlation of multidetector CT findings with splenic arteriography and surgery: prospective study in 392 patients. J Am Coll Surg 206:685–693
Haan J, Ilahi ON, Kramer M, Scalea TM (2003) Protocol-driven nonoperative management in patients with blunt splenic trauma and minimal associated injury decreases length of stay. J Trauma 55:317–321 discussion 321-312
Schurr MJ, Fabian TC, Gavant M et al (1995) Management of blunt splenic trauma: computed tomographic contrast blush predicts failure of nonoperative management. J Trauma 39:507–512 discussion 512-503
Leeper WR, Leeper TJ, Ouellette D, Moffat B, Sivakumaran T, Charyk-Stewart T, Kribs S, Parry NG, Gray DK (2014) Delayed hemorrhagic complications in the nonoperative management of blunt splenic trauma: early screening leads to a decrease in failure rate. J Trauma Acute Care Surg 76:1349–1353
Peitzman AB, Harbrecht BG, Rivera L, Heil B, Eastern Association for the Surgery of Trauma Multiinstitutional Trials Workgroup (2005) Failure of observation of blunt splenic injury in adults: variability in practice and adverse consequences. J Am Coll Surg 201:179–187
Dehli T, Bagenholm A, Trasti NC et al (2015) The treatment of spleen injuries: a retrospective study. Scand J Trauma Resusc Emerg Med 23:85
Yiannoullou P, Hall C, Newton K, Pearce L, Bouamra O, Jenks T, Scrimshire AB, Hughes J, Lecky F, Macdonald ADH (2017) A review of the management of blunt splenic trauma in England and Wales: have regional trauma networks influenced management strategies and outcomes? Ann R Coll Surg Engl 99:63–69
Dolejs SC, Savage SA, Hartwell JL, Zarzaur BL (2017) Overall splenectomy rates stable despite increasing usage of angiography in the management of high-grade blunt splenic injury. Ann Surg 1. https://doi.org/10.1097/SLA.0000000000002246
Funding
Dr. Fernanda Garozzo Velloni received a scholarship from Bracco Foundation in order to attend to Progetto Diventerò, in partnership with the Diagnostic Imaging Society of Sao Paulo (Brazil).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Prof. Angelo Vanzulli was a paid speaker at ECR 2014 and 2016 for Bracco Imaging. Dr. Fernanda Garozzo Velloni is a Bracco Foundation alumna, in partnership with Radiological and Diagnostic Imaging Society of Sao Paulo (SPR, Brazil).
All other authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Margari, S., Garozzo Velloni, F., Tonolini, M. et al. Emergency CT for assessment and management of blunt traumatic splenic injuries at a Level 1 Trauma Center: 13-year study. Emerg Radiol 25, 489–497 (2018). https://doi.org/10.1007/s10140-018-1607-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-018-1607-x