Abstract
This clinical trial aimed to compare the effects of low-level laser therapy (LLLT), Er,Cr;YSGG laser, and fluoride varnish, as compared to the placebo laser on decreasing dentin hypersensitivity (DH). This randomized, double-blinded clinical trial included 60 jaw quadrants in 24 patients who underwent periodontal surgery. The quadrants were randomly assigned to 4 groups and received treatments as follows. Group 1: LLLT with a combination of red and infrared wavelengths, group 2: Er,Cr:YSGG laser (0.25 W and 0.5 W), group 3: fluoride varnish, and group 4: placebo laser. The sensitivity response to the cold spray was recorded using visual analogue scale (VAS) at baseline, immediately, and 1 week post-treatment. The data were analyzed by repeated measures analysis at the significance level of P<0.05. There was a significant reduction in DH after treatment by low-level lasers, Er,Cr:YSGG laser, or fluoride varnish compared to the baseline data (P<0.05), but the placebo group displayed no significant alteration in DH (P=0.069). At 1 week, the VAS score in the Er,Cr:YSGG laser group was significantly lower than that of the LLLT (P= 0.043) and placebo (P<0.001) groups. Furthermore, the subjects who received fluoride varnish exhibited significantly lower DH compared with the placebo group (P = 0.023). Er,Cr:YSGG laser was the most effective strategy in dealing with DH, as it caused the greatest pain reduction over the study period and showed a significant superiority over LLLT and placebo groups. Alternatively, the application of fluoride varnish could be recommended for attenuating DH following periodontal surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dentin hypersensitivity (DH) is an acute, immediate, and short- or long-lasting pain that occurs in dentin exposed to the oral environment, and cannot be ascribed to any other form of dental pathology [1, 2]. Under normal situations, dentin is covered by enamel or cementum. The loss of this protective cover may occur due to several factors including attrition from occlusal wear or parafunctional habits, acid erosion, abrasive tooth brushing, coronal fracture, gingival recession, and periodontal diseases [3,4,5]. The most accepted mechanism for DH is the hydrodynamic theory proposed by Brännström [6]. Accordingly, pain is caused by sudden displacement of fluid inwardly or outwardly across the dentinal tubules as a result of thermal, evaporative, chemical, mechanical, or osmotic stimuli. The fluid movement inside dentinal tubules activates nerve endings at the pulp/dentin interface and is transmitted as a painful sensation [4].
Periodontal problems are a major etiologic factor for DH. The thin layer of cementum (approximately 20–50 μm in depth) is easily lost during the root surface cleaning procedures [4, 7]. The loss of cementum and the overlying periodontal tissues leads to denuding of numerous dentinal tubules, making them accessible to a great sort of irritants in the oral environment. Patients who undergo periodontal surgery usually complain of intolerable and long-lasting tooth sensitivity that does not resolve even within a few weeks after treatment. The resulting pain and discomfort may prevent oral hygiene maintenance and thus leading to plaque accumulation and the relapse of periodontal problems [3]. The long-lasting DH could also negatively affect the quality of life of the patients [3, 8].
It has been demonstrated that the frequency and diameter of dentinal tubules are greater in hypersensitive than normal dentin areas [3, 4, 7]. Therefore, a great variety of desensitizing agents are based on occluding dentinal tubules or decreasing their diameter to reduce dentin permeability or fluid movement [4, 9]. Other treatment options for DH rely on blocking the conduction of noxious stimuli through desensitizing the nerve terminals [9]. Among the therapeutic products to attenuate DH are special dentifrices (containing calcium phosphate, potassium nitrate, or oxalate), dentin adhesives, casein phosphopeptide-amorphous calcium phosphate, restorative procedures, fluoride containing agents, and laser irradiation [8].
Lasers have been employed for various purposes in dentistry. Both high-power and low-power lasers could be considered as modern treatment options for DH, although their mechanism of action would be different. Low-power laser therapy (also called as low-level laser therapy or LLLT) is well-known for its proven biologic effects in modulating inflammation, accelerating wound healing, promoting angiogenesis and cellular metabolism, and minimizing pain and discomfort in different clinical conditions [2, 10, 11]. It is believed that low-power lasers can reduce DH by depressing nerve transmission or stimulating dentin formation [2, 4, 7, 12], whereas the effects of high power lasers are mainly based on obstructing the dentinal tubules by melting of dentin or evaporation of the dentinal fluid [1, 13,14,15,16]. The erbium, chromium:yttrium-scandium-gallium-garnet (Er,Cr:YSGG) laser has gained a lot of popularity for treatment of DH. The wavelength of 2780 nm emitted by this laser is mainly absorbed in water and also OH ions of hydroxyapatite, and can lead to physical and chemical alterations such as melting and recrystallization in dentin structure [16, 17].
Fluoride varnish has been commonly used as an effective approach for the relief of DH following periodontal surgery. Varnishes can seal dentinal tubules and prevent fluid flow by producing a mechanical barrier which gradually releases various desensitizing and anti-cavity ingredients [7, 8]. However, this modality may create short-lived effects and require repeated applications, as the varnish may be removed during tooth brushing and before achieving the desired effects [4, 7, 18, 19].
There are limited studies on the desensitizing effects of Er,Cr:YSGG laser and some controversial reports [2, 20, 21] about the efficacy of low-power lasers in the management of DH. It is of interest to compare the clinical performance of high- and low-power lasers with a commonly used modality in the treatment of sensitive teeth. This in vivo study was conducted to compare the effectiveness of several modalities including low-level laser therapy, Er,Cr;YSGG laser radiation, and fluoride varnish application on decreasing tooth sensitivity after periodontal surgery and compare the results with that of the placebo laser application.
Methods and materials
Patient selection
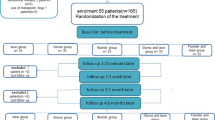
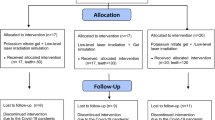
This randomized, double-blinded, placebo-controlled, clinical trial included 60 jaw quadrants in 24 patients who underwent periodontal surgery in at least two quadrants of the upper/lower jaw due to increased pocket depth (> 5 mm). The patients were 18 females and 6 males with age range of 20 to 65 years (mean age: 44 ± 9.7 years), and the treatments were performed at the Department of Periodontics, School of Dentistry, Mashhad University of Medical Sciences, Mashhad, Iran between March 2019 and October 2019. The subjects who had carious lesions, crown fractures, defective restorations, or teeth with evidence of pulpitis, as well as pregnant and feeding women, were excluded from the sample. The exclusion criteria also involved subjects who were under medication with analgesic/anti-inflammatory drugs over the last 72 h, as well as those who received any professional desensitizing treatment during the last 6 months or used anti-sensitive toothpaste over the last 3 months prior to the surgical periodontal therapy. The study was approved by the ethics committee of Mashhad University of Medical sciences, and it was registered in the Iranian Registry of Clinical Trials (IRCT) website under the identification number IRCT20091118002736N5. The study purpose and procedures were explained thoroughly to the patients, and informed consent forms were signed by all the participants before the study commencement. The study was conducted in accordance with the principles stated in the Declaration of Helsinki.
Sensitivity assessment
The periodontal surgery was performed at one quadrant of the upper/lower jaw at each time point, and an interval of at least 3 weeks was planned between the surgical procedures. The patients referred 1 to 2 weeks after the operation. At this appointment, the periodontal pack was removed and tooth sensitivity was measured by a cold spray (1,1,1,2-tetrafluoroethane; Luber, Iran). The teeth were dried and isolated with cotton rolls before the assessment. The cold spray was deposited on a small cotton pallet and placed over the tooth surface. The patient was asked to record the severity of pain using a visual analogue scale (VAS). This scale was a 10-cm horizontal line with the left side (0) indicating no pain and the right side (10) representing the worst possible pain. The patient was instructed to mark a point on this scale according to the degree of perceived discomfort. DH was measured on the canine and first and second premolars and the tooth with highest pain score was selected for further DH measurements.
Treatment strategies
After measuring DH, the jaw quadrant was assigned to one of the 4 treatment groups using a table of random sequence. The allocation was concealed in sealed envelopes and kept by another person who was not involved in the study process. All the teeth in the selected quadrant were treated in the same manner, but follow-up assessments were performed on the tooth with the highest sensitivity at baseline. The treatments applied in the study groups were as follows:
Group 1 (Low-level laser therapy [LLLT]): The patients in this group underwent low-level laser therapy with indium-gallium-aluminum-phosphide (InGaAlP; Thor DD2 Control Unit, Thor, London, UK) and gallium-aluminum-arsenide (GaAIAS; Thor DD2 Control Unit) diode lasers, both operating at the power of 200 mW, and continuous wave mode. The InGaAlP laser emitted a red light at the wavelength of 660 nm and was held in contact with four points located at the cervical part of the root (mesial, center, distal, and apical to the cementoenamel margin) for 10 s each. The infrared GAAlAs laser (810 nm) was then irradiated on the same points, similar to that described previously. Each point received the energy of 2 J, and the total energy delivered to the tooth by each laser was 8 J. Since the surface areas of the laser probes were different, the energy densities per point were calculated as 28 J/cm2 and 7 J/cm2 for red and infrared wavelengths, respectively.
Group 2 (Er,Cr:YSGG laser): In this group, Er,Cr:YSGG laser (Waterlase MD, Biolase Technology, Irvine, CA, USA) was employed. The laser irradiated the wavelength of 2780 nm and was used in non-contact mode (at the distance of about 1 mm from the tooth surface) with the MZ6 sapphire tip (600 μm in diameter and 6 mm in length). The pulse duration of the device was 140 μs and the radiation was performed without air and water spray. Initially, the beam was emitted at the power of 0.25 W and repetition rate of 25 Hz to scan the cervical part of the buccal surface for 10 s. Following 15 min of lasing the teeth in the entire quadrant, the irradiation was repeated for 10 s per tooth at the power setting of 0.5 W and frequency of 25 Hz. The local anesthesia was not performed before laser irradiation.
Group 3 (fluoride varnish): The 15 jaw quadrants in group 3 received topical fluoride treatment. Following tooth drying and isolation with cotton rolls, two coats of a 5% sodium fluoride varnish (Aria Dent Preventa; Asia Chemi Teb Co., Tehran, Iran) was painted over the cervical region of the buccal and lingual tooth surfaces by a disposable applicator. The patient was instructed not to eat/drink for 1 h and not to brush the teeth on the day of varnish application, thereby reinforcing fluoride interaction with dentin tubules.
Group 4 (placebo laser): The jaw quadrants in group 4 underwent low-level laser therapy, as explained in group 1, but the lasers were turned off.
The treatments were rendered at one visit and by the same operator. The patients were asked to brush twice per day by a very soft toothbrush and toothpaste and not use any desensitizing or fluoride agent following therapy. Furthermore, the patients were requested to avoid the use of analgesics as much as possible, but if they perceived severe pain, they should take gelofen 400 mg and record the number and frequency of consumption. The study was considered double-blinded, as the patient and the subject who performed sensitivity assessments were blinded to the kind of therapy applied.
Follow-up evaluations
The baseline sensitivity evaluation (T0) was accomplished after removing the periodontal pack and before the desensitizing treatment, as explained previously. Follow-up measurements of pain severity were performed on the selected teeth immediately after treatment (T1) and at 1 week post-treatment (T2), and the sensitivity response to the cold spray was recorded using VAS.
Statistical analysis
For comparing the effectiveness of different desensitizing treatments, each jaw quadrant was used as a statistical unit rather than the patient. The normal distribution of the data was confirmed by the Shapiro-Wilk test (P> 0.05). The repeated measures analysis was run to detect any significant difference in tooth sensitivity between the different groups and time intervals. The statistical analysis was performed through SPSS software (version 16; SPSS Inc., Chicago, IL, USA) and the statistical significance was set at P<0.05.
Results
All the patients completed the period of the experiment. Only two subjects reported the use of analgesic, one after the placebo application and one after the LLLT treatment. No adverse effect such as pulp necrosis was noticed during the study. The repeated measures analysis revealed an interaction between the time interval and treatment group (P< 0.001). Therefore, the two factors were analyzed separately.
Comparison of pain scores between the different intervals
Table 1 presents the mean and standard deviation (SD) of pain scores at different time points in the study groups. The severity of pain decreased from baseline (T0) to immediately after treatment (T1) in all groups. After that, the VAS scores in subjects treated by Er,Cr:YSGG laser or fluoride varnish continued reduction. However, the LLLT and placebo groups experienced an increase in pain level between the immediately after treatment and 1 week later (T2). In the placebo group, the VAS score at T2 even reached a higher score than that of the baseline examination. Figure 1 illustrates the variations in pain scores throughout the experiment.
Intragroup comparisons revealed significant differences in DH between the measurement intervals in subjects treated by LLLT, Er,Cr:YSGG laser, or fluoride varnish (P<0.05; Table 1), but the placebo group displayed no significant variation in tooth sensitivity over the study period (P=0.069; Table 1). The least significant difference (LSD) test indicated that both LLLT and fluoride varnish caused statistically significant reduction in DH between baseline and immediately after treatment and between baseline and 1 week later (P<0.05; Table 1). In the Er,Cr:YSGG laser group, the decrease in VAS scores was significant among all assessment intervals (P<0.001; Table 1).
Comparison of pain scores between the different study groups
One-way analysis of variance (ANOVA) revealed no significant difference in the severity of pain between the four groups at either baseline or immediately after treatment (P>0.05; Table 1). However, a significant between-group difference was found 1 week after therapy (P<0.001). Pairwise comparisons by Games Howell test revealed that the mean VAS score in the Er,Cr:YSGG laser group was significantly lower than that of the LLLT (P= 0.043) and placebo (P<0.001) groups. Furthermore, the subjects received fluoride varnish exhibited a significantly lower tooth sensitivity level compared with the placebo group (P = 0.023).
Discussion
This study evaluated the clinical outcomes of LLLT, Er,Cr:YSGG laser, and fluoride varnish as compared to placebo laser in reducing DH over 1 week after therapy. A thermal test was carried out for measuring DH, using a cold spray. Cold testing a tooth is a common, accurate, and easily applicable approach that irritates Aδ fibers in the pulpal tissue, leading to the perception of sharp pain. The overall outcomes of this study revealed that the three experimental modalities (LLLT, Er,Cr:YSGG laser, and fluoride varnish) were effective in reducing pain immediately and 1 week after therapy compared to the baseline scores, but in the placebo group, the severity of pain did not display a significant alteration over the study period. At the 1-week interval, the subjects treated by Er,Cr:YSGG laser or fluoride varnish demonstrated significantly lower VAS scores than the placebo group.
The mean severity of pain in the LLLT group was 6.43 ± 1.90 at baseline. After irradiation, a 23% reduction in pain level occurred, which was followed by a 6% reversal, so that VAS score at 1-week interval was 17% lower compared to the baseline value. The results of the present study revealed that the combined treatment with red and infrared lasers was effective in reducing DH, as it caused significant pain suppression immediately after treatment, which was still significant 1 week later. However, the effect of LLLT was not so strong to cause a significant superiority over the placebo application during the study period. The immediate analgesic effect of laser therapy in alleviating DH can be ascribed to the depression of neural transmission networks through blocking the depolarization of afferent C-fibers [2, 4, 7, 12]. Another mechanism that may cause a prolonged desensitizing effect is the promotion of tertiary dentin production at the pulp/dentin interface by stimulating the proliferation and differentiation of odontoblasts [2, 4, 7]. It is also possible that LLLT provides anti-inflammatory and healing effects on the injured pulp tissues and thus reducing DH [4]. It should be noted that dentin formation occurs over a longer interval and may require repeated laser applications, but we applied LLLT once and followed the results for only 1 week. Therefore, any significant effect at the cellular pulp level should not be expected within the conditions of this study.
In laser therapy, the selection of appropriate laser parameters is critical to achieve the satisfactory results. Several parameters such as wavelength, energy, energy density (dose), mode of application (stationary versus scanning movement), frequency of irradiation, and the interval between treatment sessions should be considered for therapeutic purposes. In this study, the combination of low-power red and infrared lasers was employed. The reason was the possible synergistic effect between the red and infrared lasers that may increase the treatment efficacy [22]. The laser was applied stationary in contact with four points at the cervical part of the root in just one session. The energy and energy density are generally considered the critical parameters to induce biologic effects [10, 23]. In this study, laser therapy was contemplated with energy of 2 J per point (8 J per tooth) and energy density of 28 J/cm2 for red and 7 J/cm2 for infrared wavelengths. Although the dose was relatively high due to the small probe size, the delivered energy seems suitable.
In the Er,Cr:YSGG laser group, the pain degree was 7.83 at baseline, and it declined to 5.47 and 3.50 immediately and 1 week after irradiation, corresponding to 30% and 55% reduction in the severity of pain, respectively. The considerable alleviation of sensitivity was not only significant between baseline and immediately after treatment but also between immediate and 1-week intervals. This shows that the structural and chemical reactions that occur after radiation of Er,Cr:YSGG laser continue after intervention. At the 1-week interval, the patients treated by Er,Cr:YSGG laser perceived significantly lower pain level than the LLLT and placebo groups, implying the strong superiority of this modality in the management of DH. The desensitizing effects of Er,Cr:YSGG laser could be attributed to its high absorption in water that leads to dentin fluid evaporation and deposition of insoluble salts, thus obliterating the dentinal tubules [1, 5]. In a SEM analysis, Gholami et al. [17] observed that Er,Cr:YSGG laser at 0.25 W was capable to melt peritubular dentin, so that the diameter of tubular entrance was reduced more than 50% after radiation. Others [16] also observed morphologic alterations including portions of melting and recrystallization following the irradiation of Er,Cr:YSGG laser.
In the current study, the Er,Cr:YSGG laser was used at the subablative threshold and without air and water spray to avoid the ablating effect. The irradiation was performed in one session and two phases (0.25 W and 0.5 W) with a latency of 15 min between them, and the duration of irradiation was 10 s per phase (20 s in total). Other studies used the power of 0.25 W or 0.5 W and exposure duration of 10 s to 30 s [1, 3, 5]. It has been assumed that the ablative threshold of Er,Cr:YSGG laser on dentin is 0.5 W, when it is applied without water mist [3, 16]. Yilmaz and Bayindir [16] compared the desensitizing and tubule occluding effects of Er,Cr:YSGG laser with 0.25 and 0.5 W power settings. The results showed that both active laser groups provided a desensitizing effect immediately after treatment as compared to the baseline data and control group. However, VAS scores were significantly lower and the tubule diameters were significantly smaller in subjects emitted by 0.5 W than those irradiated by 0.25 W.
The severity of pain in the fluoride varnish group was 7.20 ± 1.84 at baseline, and it showed 25% reduction immediately after treatment. A gradual but insignificant reduction in DH occurred between immediately and 1 week after therapy, so that the mean percentage of pain reduction in this group was 29% at the end of the experiment. This indicates that the efficacy of fluoride varnish appears instantly and is maintained over 1 week later. Between-group comparisons at 1-week interval indicated that the mean sensitivity score in subjects treated by the fluoride varnish was significantly lower than that of the placebo group. The efficacy of fluoride varnish in alleviating DH may be related to the narrowing of tubule openings as a result of precipitating calcium fluoride crystals [4, 7]. As the crystal size is small (less than 0.05 μm), repeated applications of fluoride varnish are required to fill the diameter of dentinal tubules [4, 7]. Furthermore, by evaporation of varnish solvents, a thin layer of material remains that covers the tooth surface and provides a temporary barrier against different stimuli [4, 8]. The application of fluoride varnish is an easy, practical, inexpensive, and accessible method for the practitioners and could provide superior results compared to the placebo laser according to the outcomes of the present study.
It has been proposed that laser therapy can provide a placebo effect on pain perception, which may be due to receiving treatment from a high technology apparatus combined with a good relationship between the patient and clinician [2, 23,24,25,26]. In the present study, the placebo group experienced a small and insignificant reduction in DH immediately after treatment, but at 1 week later, pain degree increased to the extent that it was even greater than that of the baseline value. Overall, the alterations in DH were not significant in the placebo group over the period of this study, implying that the placebo effect of laser is negligible and cannot alleviate the severe pain usually perceived following the periodontal surgery. In contrast, several studies reported a strong placebo effect for different approaches of DH treatment, especially lasers [27,28,29,30].
The outcomes of this study are in agreement with the results of several investigations that exhibited the superiority of Er,Cr:YSGG laser as a desensitizing agent compared to the baseline data and placebo application [1, 3, 5, 16]. Pourshahidi et al. [3] observed a reduction in DH immediately, 1 week and 1 month following the application of both Er,Cr:YSGG and diode (940 nm) lasers, although the result of Er,Cr:YSGG laser was significantly better than that of the diode laser at the 1-month interval. Similar to the results of this study, Gojkov-Vukelic et al. demonstrated significant analgesic effects of low-power lasers on sensitive teeth as compared to baseline data, but they did not use a control group [31]. Some systematic reviews concluded that although laser therapy decreases pain of DH, the evidence for its effectiveness is still weak and inconclusive [30, 32]. A recent meta-analysis [33] indicated that there is low-quality evidence to draw any conclusions concerning the superiority of lasers versus topical desensitizing agents for DH treatment. The results of this study are also consistent with numerous investigations that demonstrated the effectiveness of fluoride agents in dealing with DH [8, 34,35,36,37]. Osmari et al. [38] and Yilmaz et al. [18] concluded that fluoride varnish is an ideal choice when an immediate desensitizing effect is desired with just a single application.
The outcomes of this investigation contradict the results of several studies that indicated the clinical advantages of laser therapy over other treatment modalities for managing DH [4, 7, 12, 20, 29, 39,40,41]. Doshi et al. [40] included 60 sites that underwent periodontal flap surgery, and observed significant decreases in DH and pain in the laser irradiated sites (660 nm, 25 mW, 4.5 J) compared to the control sites on the seventh day. Naghsh et al. [12] employed 660 nm (30 mW) and 810 nm (100 mW) diode lasers for 2 min on sensitive teeth and found that both lasers were effective in reducing pain compared to the control group, although the effect of 810-nm laser was better than the 660-nm laser. Sgolastra et al. [42] assessed the efficacy of different lasers in reducing DH and revealed no differences for Er,Cr:YSGG laser vs. placebo, whereas Er:YAG, Nd:YAG, and GaAlAs lasers were found to be efficacious in DH reduction. Soares et al. [41] reported that laser therapy (40 mW, 4 J/cm2, 15 s per point on 4 points) resulted in significantly greater reduction in pain intensity than 2% neutral fluoride gel on hypersensitive teeth. The controversies regarding the desensitizing effects of lasers between the studies may be related to the differences in laser parameters and radiation protocols as well as to the discrepancies in study designs [2]. For example, most of the studies that reported superior analgesic effects of low-power lasers applied treatment for 3 or 4 sessions [12, 19, 29, 31, 40], but in this study, all treatments were performed only once. Some studies [43, 44] did not have a placebo or control group and just compared the results with baseline data or other treatment modalities.
The limitations of this study were the small sample size and the short follow-up period. Further randomized controlled trials with longer follow-ups and larger sample sizes are warranted to compare the efficacy of different modalities in attenuating DH. The LLLT should also be assessed with different irradiation parameters at more frequent appointments.
Conclusions
-
1-
All treatment strategies including LLLT, Er,Cr:YSGG laser and fluoride varnish caused a significant reduction in DH compared to baseline over the period of this experiment, but the alteration in pain degree was not significant in the placebo group.
-
2-
The Er,Cr:YSGG laser was the most effective strategy in dealing with DH after periodontal surgery, as it showed the greatest reduction over the 1-week period of this study. Furthermore, the degree of pain was significantly lower in the Er,Cr:YSGG laser group than the placebo and LLLT groups at the end of the experiment.
-
3-
The application of fluoride varnish exhibited a significant superiority over the placebo laser in relieving DH at the 1-week interval.
-
4-
Both Er,Cr:YSGG laser radiation and fluoride varnish application could be recommended for rapid DH reduction in subjects who underwent periodontal surgery.
References
Yilmaz HG, Kurtulmus-Yilmaz S, Cengiz E, Bayindir H, Aykac Y (2011) Clinical evaluation of Er,Cr:YSGG and GaAlAs laser therapy for treating dentine hypersensitivity: a randomized controlled clinical trial. J Dent 39(3):249–254. https://doi.org/10.1016/j.jdent.2011.01.003
Machado AC, EL Viana Í, Farias-Neto AM, Braga MM, de Paula EC, de Freitas PM, Aranha ACC (2018) Is photobiomodulation (PBM) effective for the treatment of dentin hypersensitivity? A systematic review. Lasers Med Sci 33(4):745–753. https://doi.org/10.1007/s10103-017-2403-7
Pourshahidi S, Ebrahimi H, Mansourian A, Mousavi Y (2019) Comparison of Er,Cr:YSGG and diode laser effects on dentin hypersensitivity: a split-mouth randomized clinical trial. Clin Oral Investig 23(11):4051–4058. https://doi.org/10.1007/s00784-019-02841-z
Corona SA, Nascimento TN, Catirse AB, Lizarelli RF, Dinelli W, Palma-Dibb RG (2003) Clinical evaluation of low-level laser therapy and fluoride varnish for treating cervical dentinal hypersensitivity. J Oral Rehabil 30(12):1183–1189. https://doi.org/10.1111/j.1365-2842.2003.01185.x
Yilmaz HG, Cengiz E, Kurtulmus-Yilmaz S, Leblebicioglu B (2011) Effectiveness of Er,Cr:YSGG laser on dentine hypersensitivity: a controlled clinical trial. J Clin Periodontol 38(4):341–346. https://doi.org/10.1111/j.1600-051X.2010.01694.x
Brännström M (1966) Sensitivity of dentine. Oral Surg Oral Med Oral Pathol 21(4):517–526. https://doi.org/10.1016/0030-4220(66)90411-7
Pesevska S, Nakova M, Ivanovski K, Angelov N, Kesic L, Obradovic R, Mindova S, Nares S (2010) Dentinal hypersensitivity following scaling and root planing: comparison of low-level laser and topical fluoride treatment. Lasers Med Sci 25(5):647–650. https://doi.org/10.1007/s10103-009-0685-0
Sivaramakrishnan G, Sridharan K (2019) Fluoride varnish versus glutaraldehyde for hypersensitive teeth: a randomized controlled trial, meta-analysis and trial sequential analysis. Clin Oral Investig 23(1):209–220. https://doi.org/10.1007/s00784-018-2428-8
Moraschini V, da Costa LS, Dos Santos GO (2018) Effectiveness for dentin hypersensitivity treatment of non-carious cervical lesions: a meta-analysis. Clin Oral Investig 22(2):617–631. https://doi.org/10.1007/s00784-017-2330-9
Ahrari F, Madani AS, Ghafouri ZS, Tunér J (2014) The efficacy of low-level laser therapy for the treatment of myogenous temporomandibular joint disorder. Lasers Med Sci 29(2):551–557. https://doi.org/10.1007/s10103-012-1253-6
Moosavi H, Arjmand N, Ahrari F, Zakeri M, Maleknejad F (2016) Effect of low-level laser therapy on tooth sensitivity induced by in-office bleaching. Lasers Med Sci 31(4):713–719. https://doi.org/10.1007/s10103-016-1913-z
Naghsh N, Kachuie M, Kachuie M, Birang R (2020) Evaluation of the effects of 660-nm and 810-nm low-level diode lasers on the treatment of dentin hypersensitivity. J Lasers Med Sci 11(2):126–134. https://doi.org/10.34172/jlms.2020.22
Rezazadeh F, Dehghanian P, Jafarpour D (2019) Laser effects on the prevention and treatment of dentinal hypersensitivity: a systematic review. J Lasers Med Sci 10(1):1–11. https://doi.org/10.15171/jlms.2019.01
Umana M, Heysselaer D, Tielemans M, Compere P, Zeinoun T, Nammour S (2013) Dentinal tubules sealing by means of diode lasers (810 and 980 nm): a preliminary in vitro study. Photomed Laser Surg 31(7):307–314. https://doi.org/10.1089/pho.2012.3443
Belal MH, Yassin A (2014) A comparative evaluation of CO2 and erbium-doped yttrium aluminium garnet laser therapy in the management of dentin hypersensitivity and assessment of mineral content. J Periodontal Implant Sci 44(5):227–234. https://doi.org/10.5051/jpis.2014.44.5.227
Yilmaz HG, Bayindir H (2014) Clinical and scanning electron microscopy evaluation of the Er,Cr:YSGG laser therapy for treating dentine hypersensitivity: short-term, randomised, controlled study. J Oral Rehabil 41(5):392–398. https://doi.org/10.1111/joor.12156
Gholami GA, Fekrazad R, Esmaiel-Nejad A, Kalhori KA (2011) An evaluation of the occluding effects of Er;Cr:YSGG, Nd:YAG, CO2 and diode lasers on dentinal tubules: a scanning electron microscope in vitro study. Photomed Laser Surg 29(2):115–121. https://doi.org/10.1089/pho.2009.2628
Yilmaz HG, Kurtulmus-Yilmaz S, Cengiz E (2011) Long-term effect of diode laser irradiation compared to sodium fluoride varnish in the treatment of dentine hypersensitivity in periodontal maintenance patients: a randomized controlled clinical study. Photomed Laser Surg 29(11):721–725. https://doi.org/10.1089/pho.2010.2974
Moura GF, Zeola LF, Silva MB, Sousa SC, Guedes FR, Soares PV (2019) Four-session protocol effectiveness in reducing cervical dentin hypersensitivity: a 24-week randomized clinical trial. Photobiomodul Photomed Laser Surg 37(2):117–123. https://doi.org/10.1089/photob.2018.4477
He S, Wang Y, Li X, Hu D (2011) Effectiveness of laser therapy and topical desensitising agents in treating dentine hypersensitivity: a systematic review. J Oral Rehabil 38(5):348–358. https://doi.org/10.1111/j.1365-2842.2010.02193.x
Blatz MB (2012) Laser therapy may be better than topical desensitizing agents for treating dentin hypersensitivity. J Evid Based Dent Pract 12(3 Suppl):229–230. https://doi.org/10.1016/s1532-3382(12)70044-1
Heravi F, Ahrari F, Mahdavi M, Basafa S (2014) Comparative evaluation of the effect of Er:YAG laser and low level laser irradiation combined with CPP-ACPF cream on treatment of enamel caries. J Clin Exp Dent 6(2):e121–e126. https://doi.org/10.4317/jced.51309
Madani AS, Ahrari F, Nasiri F, Abtahi M, Tunér J (2014) Low-level laser therapy for management of TMJ osteoarthritis. CRANIO 32(1):38–44. https://doi.org/10.1179/0886963413Z.0000000004
Biagi R, Cossellu G, Sarcina M, Pizzamiglio IT, Farronato G (2015) Laser-assisted treatment of dentinal hypersensitivity: a literature review. Ann Stomatol (Roma) 6(3-4):75–80. https://doi.org/10.11138/ads/2015.6.3.075
Moosavi H, Maleknejad F, Sharifi M, Ahrari F (2015) A randomized clinical trial of the effect of low-level laser therapy before composite placement on postoperative sensitivity in class V restorations. Lasers Med Sci 30(4):1245–1249. https://doi.org/10.1007/s10103-014-1565-9
Madani A, Ahrari F, Fallahrastegar A, Daghestani N (2020) A randomized clinical trial comparing the efficacy of low-level laser therapy (LLLT) and laser acupuncture therapy (LAT) in patients with temporomandibular disorders. Lasers Med Sci 35(1):181–192. https://doi.org/10.1007/s10103-019-02837-x
Lund RG, Silva AF, Piva E, Da Rosa WL, Heckmann SS, Demarco FF (2013) Clinical evaluation of two desensitizing treatments in southern Brazil: a 3-month follow-up. Acta Odontol Scand 71(6):1469–1474. https://doi.org/10.3109/00016357.2013.770919
Birang R, Poursamimi J, Gutknecht N, Lampert F, Mir M (2007) Comparative evaluation of the effects of Nd:YAG and Er:YAG laser in dentin hypersensitivity treatment. Lasers Med Sci 22(1):21–24. https://doi.org/10.1007/s10103-006-0412-z
Vieira AH, Passos VF, de Assis JS, Mendonça JS, Santiago SL (2009) Clinical evaluation of a 3% potassium oxalate gel and a GaAlAs laser for the treatment of dentinal hypersensitivity. Photomed Laser Surg 27(5):807–812. https://doi.org/10.1089/pho.2008.2364
Sgolastra F, Petrucci A, Gatto R, Monaco A (2011) Effectiveness of laser in dentinal hypersensitivity treatment: a systematic review. J Endod 37(3):297–303. https://doi.org/10.1016/j.joen.2010.11.034
Gojkov-Vukelic M, Hadzic S, Zukanovic A, Pasic E, Pavlic V (2016) Application of diode laser in the treatment of dentine hypersensitivity. Med Arch 70(6):466–469. https://doi.org/10.5455/medarh.2016.70.466-469
Jokstad A (2012) The effectiveness of lasers to reduce dentinal hypersensitivity remains unclear. J Evid Based Dent Pract 12(3 Suppl):231–232. https://doi.org/10.1016/S1532-3382(12)70045-3
Zhou K, Liu Q, Yu X (2020) Zeng X (2020) Laser therapy versus topical desensitising agents in the management of dentine hypersensitivity: a meta-analysis. Oral Dis. https://doi.org/10.1111/odi.13309
Abdollahi A, Jalalian E (2019) Effectiveness of two desensitizer materials, potassium nitrate and fluoride varnish in relieving hypersensitivity after crown preparation. J Contemp Dent Pract 20(4):489–493
Nardi GM, Sabatini S, Lauritano D, Silvestre F, Petruzzi M (2016) Effectiveness of two different desensitizing varnishes in reducing tooth sensitivity: a randomized double-blind clinical trial. Oral Implantol (Rome) 9(4):185–189. https://doi.org/10.11138/orl/2016.9.4.185
Petersson LG (2013) The role of fluoride in the preventive management of dentin hypersensitivity and root caries. Clin Oral Investig 17(Suppl 1):S63–S71. https://doi.org/10.1007/s00784-012-0916-9
Camilotti V, Zilly J, Busato Pdo M, Nassar CA, Nassar PO (2012) Desensitizing treatments for dentin hypersensitivity: a randomized, split-mouth clinical trial. Braz Oral Res 26(3):263–268. https://doi.org/10.1590/s1806-83242012000300013
Osmari D, Fraga S, Ferreira ACO, Eduardo CP, Marquezan M, Silveira BLD (2018) In-office treatments for dentin hypersensitivity: a randomized split-mouth clinical trial. Oral Health Prev Dent 16(2):125–130. https://doi.org/10.3290/j.ohpd.a40299
Lin PY, Cheng YW, Chu CY, Chien KL, Lin CP, Tu YK (2013) In-office treatment for dentin hypersensitivity: a systematic review and network meta-analysis. J Clin Periodontol 40(1):53–64. https://doi.org/10.1111/jcpe.12011
Doshi S, Jain S, Hegde R (2014) Effect of low-level laser therapy in reducing dentinal hypersensitivity and pain following periodontal flap surgery. Photomed Laser Surg 32(12):700–706. https://doi.org/10.1089/pho.2014.3802
Soares ML, Porciúncula GB, Lucena MI, Gueiros LA, Leão JC, Carvalho AA (2016) Efficacy of Nd:YAG and GaAlAs lasers in comparison to 2% fluoride gel for the treatment of dentinal hypersensitivity. Gen Dent 64(6):66–70
Sgolastra F, Petrucci A, Severino M, Gatto R, Monaco A (2013) Lasers for the treatment of dentin hypersensitivity: a meta-analysis. J Dent Res 92(6):492–499. https://doi.org/10.1177/0022034513487212
Orhan K, Aksoy U, Can-Karabulut DC, Kalender A (2011) Low-level laser therapy of dentin hypersensitivity: a short-term clinical trial. Lasers Med Sci 26(5):591–598. https://doi.org/10.1007/s10103-010-0794-9
Lopes AO, de Paula EC, Aranha ACC (2017) Evaluation of different treatment protocols for dentin hypersensitivity: an 18-month randomized clinical trial. Lasers Med Sci 32(5):1023–1030. https://doi.org/10.1007/s10103-017-2203-0
Acknowledgements
The authors would like to thank the Vice-Chancellor for Research of Mashhad University of Medical Sciences for the financial support of this project (grant number 941408). The results presented in this study have been taken from a student thesis (thesis number 3120).
Funding
The Vice-Chancellor for Research of Mashhad University of Medical Sciences
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
The protocol of the study was reviewed and approved by the ethics committee of Mashhad University of Medical sciences and it was registered in the Iranian Registry of Clinical Trials (IRCT) website under the identification number IRCT20091118002736N5.
Informed consent
The procedure was explained in detail for all the patients and signed consents were taken before the study commencement.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moeintaghavi, A., Ahrari, F., Nasrabadi, N. et al. Low level laser therapy, Er,Cr:YSGG laser and fluoride varnish for treatment of dentin hypersensitivity after periodontal surgery: A randomized clinical trial. Lasers Med Sci 36, 1949–1956 (2021). https://doi.org/10.1007/s10103-021-03310-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-021-03310-4