Abstract
The present study aims to evaluate the current scientific data regarding the effectiveness of photobiomodulation (PBM) in the treatment of dentin hypersensitivity (DH) as an alternative method for pain control. A systematic review was conducted to assess the effectiveness of PBM as treatment for DH. A complete literature search was performed up to October 2016. Searches were conducted using Boolean operators and MeSH terms. References of all selected full-text articles and related reviews were scanned. A total of 280 articles were identified (241 articles were excluded by the title and abstract). Of the 39 articles selected for analysis, 36 were excluded because they presented one or more exclusion criteria. Therefore, three articles were qualified for inclusion in this systematic review. PBM may not lead to adverse effects provided that adequately controlled parameters are followed when treating DH. More consistent studies should be conducted in order to adequately observe the advantageous therapeutic effect of PBM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dentin hypersensitivity (DH) can be defined as short, sharp pain in response to stimuli, by exposed dentin with open dentinal tubules. This pain that results from applying thermal, evaporative, tactile, osmotic, or chemical stimuli to this exposed surface cannot be ascribed to any other form of dental defect or pathology [1,2,3]. DH is a common problem among the population, being one of the main reasons for patients seeking dental treatment [3,4,5,6].
The combination of various factors such as inappropriate or poor oral hygiene, periodontal therapy, non-bacterial acid exposure, excessive occlusal force, or premature occlusion can induce loss of enamel leading to coronal or root dentin exposure with opened dentin tubules that induce DH [7].
The hydrodynamic theory of pain proposed by Brännström is the most acceptable theory to explain the mechanisms of DH. It explains that dentin exposure with subsequent opening of dentinal tubules allows the fluid flows to enter or leave the tubules, while the incidence of the abovementioned stimuli activates baroreceptors in the pulp, resulting in the generation of impulses and perception of sensorineural pain [8].
DH treatment can be conservatively managed by two strategies. The first is related to the use of agents to physically occlude the dentinal tubules, isolating the tubule contents from the oral environment and preventing the flow and movement of tubular fluid. This strategy goes directly to the more accepted theory of pain, the hydrodynamic theory. According to this theory, pain receptors are stimulated by the dentinal fluid movement. So, if the dentinal fluid stops due to a physical occluding agent, such as high-power lasers, desensitizers based on glutaraldehyde, oxalates, strontium, varnishes, and bonding systems, no stimulation of pain receptor will occur and pain sensation is hindered. The second strategy is the use of chemical agents (potassium nitrate, low-power laser) to desensitize sensory nerves, blocking the transmission of noxious stimuli from the dentinal tubules to the central nervous system [2, 9].
According to the literature, the initial approach to the treatment of DH should be to use homemade products such as dentifrices containing desensitizing agents [2, 4]. Those desensitizing agents can be ions like sodium fluoride and strontium acetate arginine or calcium-carbonate products that could block the entrance of the dentinal tubule. If there are no medium-term and long-term effects, treatment should be done in office [2, 10, 11]. In-office agents may show better effects, since professionals offer a wide choice of more complex and powerful desensitizing agents, with immediate and long-term effects. However, the frequency of application is low and may present short longevity due to the oral conditions and daily habits of the patient showing that there is not a “gold standard” protocol or material for the treatment of DH [2, 3, 9]. Also, acidic conditions and overload of mastication forces may compromise the results.
In the mid-1980s, when Matsumoto et al. first used a high-power laser for the treatment of HD, a potential method for the treatment of DH was introduced [12]. The high-power lasers such as the Nd:YAG (neodymium-doped yttrium aluminum garnet), Er:YAG (erbium-doped yttrium aluminum garnet laser), Er,Cr:YSGG (erbium chromium doped yttrium scandium gallium garnet), and CO2 (carbon dioxide) have been tested with the main purpose of obliterating the dentinal tubules [13,14,15,16,17]. On the other hand, photobiomodulation (PBM) with low-power lasers such as the GaAlAs (gallium aluminum arsenide) and He-Ne (helium-neon) does not cause temperature rise that would cause irreversible damages to the pulp or on dentine and can promote therapeutic effects for the treatment of DH if correctly applied [18,19,20].
PBM using low-power lasers offers an alternative method for pain control, which induces changes in the nerve transmission of the dental pulp instead of changing the exposed dentin, unlike high-power irradiation [21] that promotes temperature rise and melting of the dentin surface. The mechanism by which low-power lasers exert its effects in reducing pain symptoms (desensitization) is based on the stimulation of nerve cells, more specifically the Na+/K+ pump in the cell membrane interfering with the polarity of the cell membrane by increasing the amplitude of the action potential of the membrane, blocking the transmission of painful stimuli [21]. Low-power lasers transmit low frequency energy through the enamel and dentin to react with the pulp tissue, promoting biomodulatory effects such as increasing blood flow, minimizing the pain, and reducing inflammation [22]. Energy is applied on cellular level where best results are achieved. The regenerative effect of PBM is also capable of increasing the metabolic activity of odontoblast-like cells and promoting enhanced production of tertiary dentin thus obliterating the dentinal tubules with protocols that can vary from 3 to 10 J/cm2 [23,24,25,26]. Despite the reported effectiveness by many in vitro and in vivo studies, there are still many controversies related to the PBM protocols, application methods, and clinical effectiveness [27].
In view of the above, the aim of this study was to conduct a systematic review to assess the effectiveness of PBM as treatment for DH.
Materials and methods
This systematic review was conducted in accordance with the recommendation of Cochrane Collaboration [28] and the principles of PRISMA statement [29].
Search strategy
A complete literature search was conducted in the MEDLINE (via PubMed) database up to October 2016. Two blinded researchers (ACM and IELV) performed the study selection process. The inter-evaluator reliability was determined by the Cohen k test, with an acceptable assumed limit value of 0.8 [30, 31]. Discrepancies found in the inclusion or exclusion of studies were resolved by discussion between the reviewers who selected them (ACM, IE.LV, AMFN). Searches were conducted using Boolean operators and MeSH terms.
The database was searched by using the following strategy and key words: (laser low level OR laser low power OR diode laser OR 660 nm OR 790 nm OR 780 nm) AND (dentin hypersensitivity OR dentin sensitivity OR dental pain OR dental sensitivity). In addition, some journals without complete abstracts were searched manually. Initially, no language restriction was applied. Finally, the references of all selected full-text articles and related reviews were scanned.
Selection criteria
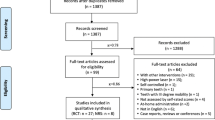
The study selection process was conducted by two blinded reviewers (AMC and IELV) in two stages. In the first stage, the studies were selected according to the following inclusion criteria (A): (A1) controlled randomized clinical trials; (A2) studies comparing treatment of DH with low-power laser with other in-office treatments, placebo, or no treatment; and (A3) studies conducted with adults (age > 18 years). Only studies that fulfilled all the inclusion criteria were admitted in the second stage (39), which consisted of analyzing the pre-selected studied in accordance with the exclusion criteria (B): (B1) studies including patients with systemic diseases or those who underwent treatment, or were taking medications that could change the perception of pain; (B2) studies that did not evaluate DH by means of a scale or score; (B3) studies that did not present numerical data; (B4) studies whose laser was coupled to electric brushes; (B5) studies that used high-power diode laser at low dosage (high-power laser equipments with low energy); (B6) studies that were not in the English language; and (B7) studies that did not present well-detailed and well-presented irradiation protocols.
Data collection
Data was extracted from each study by one reviewer (ACM): authors/year, place (university, private clinic, or hospital), study design (split-mouth, parallel, randomized-controlled trial, or not reported), number of participants, mean age, intervention (laser settings), follow-up, stimulus and evaluation, adverse effects, and results/conclusions.
Outcome variables
Based on the results of the search, two comparisons were possible: low-power laser vs. placebo and low-power laser vs. other in-office treatments for HD. The primary outcome of interest was change in the level of pain from the beginning to the end of the study. The secondary outcome was the cost-efficacy analysis.
Quality evaluation
The quality of the methodology of all the studies included was independently evaluated by two blinded reviewers (ACM and IELV) in accordance with the recommendation of the CONSORT Statement checklist. The level of inter-reviewer agreement was calculated as described above. After the scores were determined, an overall estimate of the plausible risk of bias (low, moderate, or high) was made for each study selected. Low risk of bias was estimated when all the criteria were fulfilled; a moderate risk was estimated when one or more criteria were partially fulfilled, and a high risk of bias was estimated when one or more criteria were not fulfilled [26].
Results
Search/selection of studies
By means of electronic search in the MEDLINE databases (via PubMed), a total of 280 articles were identified. Of these, 241 articles were excluded by the title and abstract because they included high-power laser or because they mentioned any other type of treatment, without the use of low-power laser.
Of the 39 articles selected for analysis, 36 were excluded because they presented one or more exclusion criteria. Therefore, three articles were qualified for inclusion in this systematic review (with inter-reviewer agreement k = 1) as shown in Table 1. The PRISMA flow sheet of the complete process of the study is illustrated in Fig. 1.
Description of studies included
Table 1 presents the data of the three studies selected at the end of the evaluation, by the inclusion and exclusion criteria of the studies.
All the articles included were randomized studies that compared low-power laser with placebo treatments or some other in-office treatment for DH, of which only one study presented placebo groups [33]. All the publications were in the English language. All the studies were conducted in universities.
The diagnostic method varied among the studies. Two studies used both thermal-evaporative and tactile stimulus [32, 33]; one single study used only thermal stimulus [26].
All the studies used a triple syringe for applying the thermal-evaporative stimulus; however, the time and intensity of the jet of air varied among them. The studies that used tactile stimulus used different methods of evaluation, the most common being the use of an exploratory probe. Whereas, the study that performed thermal stimulus used ice spray.
To measure the DH, the large majority of the studies (2) used the visual analog scale (VAS) and one study [26] used numerical scales specifically for measuring DH.
The equipment used, as well as the low-power laser parameters used, varied according to each study (Table 1). The authors of this review observed that not only the wavelength but also dose, energy density, power, and irradiation time varied among the studies selected. Table 2 describes in detailed the irradiation protocols used in each study. The interval of evaluation and follow-up of treatments varied between the immediate times of application up to 6 months of evaluation.
None of the studies reported adverse effects of the treatments used. None of the articles affirmed that the laser equipment could show higher costs than those of the other in-office treatments.
Due to the high heterogeneity in terms of the different types of laser, wavelengths, energy settings, number of sessions, and variety of follow-up periods used, a meta-analysis among the studies selected was considered inappropriate. Table 2 shows the detailed information concerning laser protocols used in the three selected studies.
Discussion
The present study attempted to evaluate the actual evidence of low-power laser for the treatment of DH, observe the real effectiveness of this contemporary therapy, and show evidence-based results found in the data collected through a systematic review process, as here described. An overview of the studies that related the treatment of DH with low-power lasers showed a wide variety of types of equipment and irradiation parameters. Taking into account the subjectivity of DH and the difficulty in objectively assessing its symptoms, contradictory results were observed in the literature and these conflicting outcomes resulted in inaccurate articles and erroneous use of lasers by professionals. Most of the systematic reviews published until now have attempted to evaluate low-power and high-power lasers compared with each other or with other desensitizing agents, so that the report of this study is the first manuscript to analyze the effects and results of photobiomodulation and its contribution to the treatment of DH.
In 2013, Lin et al. [34] conducted a systematic review and meta-analysis study with the main objective of analyzing the clinical trials related to in-office treatments for DH. The authors reported that the choice of an effective treatment was a challenge because of the large number of options available. The authors divided the treatments into two distinct groups, and among the comparisons of treatments. Meta-analysis showed that the laser therapy group achieved better results than the physical occlusion group. Likewise, He et al. [35] performed a systematic analysis that included only eight studies. The authors emphasized the conflicting results, but indicated that PBM showed a clinical advantage over topical treatments. The authors concluded that irradiation with lasers in controlled protocols did not induce adverse effects, as was also observed in the present systematic review.
The review of literature performed by Cunha-Cruz et al. [36] reported that irradiation with high and low-power lasers in the treatment of DH had a small clinical advantage over other topical treatments. However, the authors concluded that larger samples in controlled clinical trials with long-term analysis were needed before definitive conclusions could be made. As shown by Sgolastra et al. [37], these authors pointed out that only three publications out of 18,661 articles published on the subject met the inclusion criteria for a systematic review. However, the authors reported that a meta-analysis was not possible due to wide variation of information in the three papers, as also happened in the case of the present systematic review. The authors showed that in spite of PBM decreasing the pain of DH, the evidence was not yet conclusive. Jokstad in 2012 [38] reviewed the work of Sgolastra et al. [37] and concluded that the treatment of DH with laser seemed to reduce pain, but evidence for this effectiveness was still weak due to the large variation in the methods used.
Unfortunately, this uncertainty has led to disbelief in the use of low-power laser among the scientific community [27]. Due to a wide variety of laser types, mode of pain evaluation, stimulus applied, duration of clinical follow-up, and mainly the protocols used, the present systematic study would like to emphasize the need for conducting research with consistent evidence to validate the benefits of PBM in the treatment of DH. This consistency was not observed in the majority of the papers analyzed, and it was sometimes quite difficult for the dental professional to select which parameter and protocol to use.
In the present systematic review, only three trials met the rigorous inclusion criteria. Only low-power lasers were selected, and protocols were compared with desensitizer agents such as primers from adhesive systems and salts such as potassium oxalates. Within the three selected studies, all of them not only reported positive effects for low-power laser irradiation but also reported that application of other desensitizer agents was also effective, as shown in the placebo group of the studied conducted by Vieira et al. [33]. The authors reported statistically significant differences, also presenting a relative positive result in terms of decreasing pain [33]. This is a very important issue to discuss, since PBM presents a strong correlation to the placebo effect. Sgolastra et al. [37], in a systematic review, showed that there was a high level of evidence of the placebo effect in DH laser therapy. This has been described as a complex physiological and psychological interaction that depended on the relationship between the patients and the professional [14, 39]. In addition, patients’ response to sensorial stimulation was a subjective issue, largely dependent on the individual’s pain threshold, which could influence the results obtained in clinical trials. Possibly, longer periods of follow-up could enhance the ability of researchers to detect differences between active and placebo groups. According to West et al. [39], there was a need for further investigation of a wash-in period and examination of the placebo effect when evaluating DH trials. On the other hand, one study has shown that local ethics committee did not accept the inclusion of a placebo group for long periods of follow-up [32]. Thus, the decision of including a placebo treatment will depend on the country and region in which the clinical trial will be conducted.
Concerning the region where the studies reported in the articles selected for this systematic review were conducted, all of them were conducted in different parts of Brazil, where a large number of low-power laser companies are concentrated.
The effect of PBM, which relies upon immediate analgesic effect, by changing the neural transmission network and possibly obliterating dentinal tubules by tertiary dentin due to an increase in metabolic activity of odontoblasts [24,25,26], could easily be observed in most of the selected articles, in the VAS evaluation after the application of hydrodynamic stimuli. It has been suggested that at least two hydrodynamic stimuli should be used for assessing dentinal pain in clinical trials and the least severe stimuli should be applied first. According to Holland et al. [1], the tactile and air blast stimulation (thermal-evaporative) to elicit pain were recommended for quantifying dentinal pain in clinical trials, because they are both physiological and controllable. The authors of this review observed that two of the studies used the thermal-evaporative stimuli and also the tactile stimuli with a probe [32, 33]. Only one study used the thermal stimuli [24]. According to Sgolastra et al. [40], differences in DH assessment methods could have led to discrepancies in the levels of reproducibility among the studies, contributing to the high level of heterogeneity.
All the studies used a triple syringe for the application of the thermal-evaporative stimulus; however, the time and intensity of the air jet varied among them. The study that carried out thermal stimulation used ice spray [26].
In order to measure DH, the majority of the studies (2) used the VAS and the other study used numerical scales specifically for measuring HD [26]. The visual analog scale seemed to be the most appropriate method for evaluating DH. It is considered as an objective method for assessing dentinal pain in which each tooth can act as its own control [1]. It also offers the advantage of being a continuous scale, and it is being widely used in DH clinical studies. However, even if VAS was the most used pain assessment method, it has well-known shortcomings that must be considered when interpreting the results [36].
One point that was discussed in all systematic reviews cited was the discrepancy between laser protocols, and more problematically the unfeasibility of reproducing the parameters used. Most of the selected articles in the first stage did not describe the exact laser parameters so that readers could not extrapolate the protocol to their laser equipment, making it difficult not only to reproduce the parameters but also to enable discussion among protocols, impairing the real measurement of laser effectiveness and placebo effect. So, exclusion criteria that would select the studies that present well-documented and well-described protocols were added. The addition of this exclusion factor further reduced the number of studies selected in this systematic review to only three studies.
To begin with, the wavelength differed from one study to another. One study was observed to use the red wavelength (660 nm) [33] and one the infrared wavelength (810 nm) [32]. One selected study used both wavelengths with the objective of making a comparison between the 660 and 830-nm wavelengths [26]. The authors showed that the 660-nm red diode laser was more effective than the 830-nm infrared laser and a higher level of desensitization was observed at the 15 and 30-min post-irradiation examinations. The immediate and late therapeutic effects of the 660-nm red diode laser were more evident in 25–35-year-old patients compared with those of the 830-nm infrared diode laser, in terms of the different age groups. Even after the study published in 2004 and the favorable results for the red wavelength, one study used the infrared laser for DH [32]. The figures that both wavelengths presented satisfactory results were not surprising, although physicists considered the infrared more penetrable than red the wavelength; both wavelengths can be used for the treatment of DH.
The number of sessions was also a concern. Within the included studies in the first stage of this systematic analysis, a mean of 3.8 sessions was performed. While most of the articles showed that at least three sessions were needed to treat DH, others reported the performance of only one session of irradiation, which could hinder the exact mechanism of low-power laser or provide a tangible result. Concerning the three selected studies, they are consonant that more than one session can provide satisfactory results, as shown by Ladalardo et al. [26] with four sessions, Vieira et al. [33] with four sessions, and Lopes et al. [32] with three sessions. All of them reported intervals between sessions.
With regard to adverse effects, none of the included studies reported any side effects or pulp damage during the study period, consistent with the majority of the studies that used low-power laser in the treatment of DH. On the other hand, it is relevant to mention the importance of using a power meter to ensure that the adequate and appropriate parameters are being used. Only one study reported the use of a power meter to consistently measure the real power and energy delivered [32].
According to the results of the present systematic review, there was evidence to support that PBM using low-power laser was adequate and effective for reducing DH; however, longer observational periods could enhance the ability of studies to detect differences between active and placebo groups. When developing research projects, authors should be aware of the need to completely describe the methodology used. A consensus among the researchers of the area would be of great importance and value, regarding the study design (parallel vs. split-mount), time of clinical follow-up, methods of DH assessment (stimuli), and especially the complete description of protocols and settings of the low-power laser used (wavelength, diameter of the laser beam, output power, energy density, dose, number of points, energy per point, time of irradiation, mode of irradiation—contact/non-contact, number of sessions, described in Table 2). With standard study procedures, laser therapy will be considered a well-accepted, reliable, immediate, and reproducible analgesic therapy for the treatment of DH for different categories of patients.
To conclude, DH must only be identified and diagnosed after a detailed clinical/radiographic examination and medical history of patients has been obtained. It is recommended that each and every patient should be provided with oral care; dietary instructions and occlusal adjustment, if needed, before any treatment plan can be made. Uninstructed patients may be very difficult to treat if their biological, behavioral, and chemical factors were not changed or modified. In the present systematic review, only one study mentioned patient education [32], and this fact leads us to drawing the attention of both the scientific and clinical community to the correct and efficient treatment of DH.
Conclusions
Based on the available evidence, PBM is an effective and contemporary therapy for the treatment of DH. It may not lead to adverse effects provided that adequately controlled parameters are followed. More consistent studies should be conducted in order to adequately observe the advantageous therapeutic effect of PBM.
References
Holland GR, Narhi MN, Addy M, Gangarosa L, Orchardson R (1997) Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J Clin Periodontol 24:808–813
Canadian Advisory Board on Dentin Hypersensitivity (2003) Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc 69:221–228
Chen CL, Parolia A, Pau A, de Moraes Porto ICC (2015) Comparative evaluation of the effectiveness of desensitizing agents in dentine tubule occlusion using scanning electron microscopy. Aust Dent J 60:650–672
West NX, Sanz M, Lussi A, Bartlett D, Bouchard P, Bourgeois D (2013) Prevalence of dentine hypersensitivity and study of associated factors: a European population-based cross-sectional study. J Dent 41:841–851
Scaramucci T, de Almeida Anfe TE, da Silva Ferreira S, Frias AC, Sobral MA (2014) Investigation of the prevalence, clinical features, and risk factors of dentin hypersensitivity in a selected Brazilian population. Clin Oral Investig 18:651–657
Yoshizaki KT, Francisconi-Dos-Rios LF, Sobral MA, Aranha AC, Mendes FM, Scaramucci T (2017) Clinical features and factors associated with non-carious cervical lesions and dentin hypersensitivity. J Oral Rehabil 44:112–118
Addy M, Urquhat E (1992) Dentine hypersensitivity: its prevalence, aetiology and clinical management. Dent Update 19:410–412
Brannstrom M, Jonhson G, Nordenvall KJ (1979) Transmission and control of dentinal pain: resin impregnation of dentin. J Am Dent Assoc 19:692–698
Ling TY, Gillam DG (1996) The effectiveness of desensitizing agents for the treatment of cervical dentine sensitivity (CDS)—a review. J West Soc Periodontol Periodontal Abstr 44:5–12
Porto I, Andrade A, Montes M (2009) Diagnosis and treatment of dentinal hypersensitivity. J Oral Sci 51:323–332
Mohammad A, Masoumeh M (2013) Effectiveness of lasers in the treatment of dentin hypersensitivity. J Lasers Med Sci 4:1–7
Matsumoto K, Funai H, Shirasuka T, Wakabayashi H (1985) Effects of Nd:YAG caser in treatmemt of cervical hypersensitive dentine. Jpn J Conserv Dent 28:760–765
Palazon MT, Scaramucci T, Aranha AC, Prates RA, Lachowski KM, Hanashiro FS, Youssef MN (2013) Immediate and short-term effects of in-office desensitizing treatments for dentinal tubule occlusion. Photomed Laser Surg 31:274–282
Kimura Y, Wilder-Smith P, Yonaga K, Matsumoto K (2000) Treatment of dentine hypersensitivity by lasers: a review. J Clin Periodontol 27:715–721
Naylor F, Aranha AC, Eduardo CP, Arana-Chavez VE, Sobral MA (2006) Micromorphological analysis of dentinal structure after irradiation with Nd:YAG laser and immersion in acidic beverages. Photomed Laser Surg 24:745–752
Gholami GA, Fekrazad R, Esmaiel-Nejad A, Kalhori KA (2011) An evaluation of the occluding effects of Er;Cr:YSGG, Nd:YAG, CO2 and diode lasers on dentineal tubules: a scanning electron microscope in vitro study. Photomed Laser Surg 29:115–121
Aranha A, Eduardo CP (2012) Effects of Er:YAG and Er,Cr:YSGG lasers on dentine hypersensitivity. Short-term clinical evaluation. Lasers Med Sci 27:813–818
Aranha AC, Pimenta LA, Marchi GM (2009) Clinical evaluation of desensitizing treatments for cervical dentin hypersensitivity. Braz Oral Res 23:333–339
Corona SA, Nascimento TN, Catirse AB, Lizarelli RF, Dinelli W, Palma-Dibb RG (2003) Clinical evaluation of low-level laser therapy and fluoride varnish for treating cervical dentinal hypersensitivity. J Oral Rehabil 30:1183–1189
Dantas EM, Amorim FK, Nóbrega FJ, Dantas PM, Vasconcelos RG, Queiroz LM (2016) Clinical efficacy of fluoride varnish and low-level laser radiation in treating dentin hypersensitivity. Braz Dent J 27:79–82
Wakabayashi H, Hamba M, Matsumoto K, Tachibana H (1993) Effect of irradiation by semiconductor laser on responses evoked in trigeminal caudal neurons by tooth pulp stimulation. Lasers Surg Med 13:605–610
Karu T (1989) Photobiology of low-power laser effects. Health Phys 56:691–704
Ferreira AN, Silveira L, Genovese WJ, de Araújo VC, Frigo L, de Mesquita RA, Guedes E (2006) Effect of GaAIAs laser on reactional dentinogenesis induction in human teeth. Photomed Laser Surg 24:358–365
Tengrungsun T, Sangkla W (2008) Comparative study in desensitizing efficacy using the GaAlAs laser and dentin bonding agent. J Dent 36:392–395
Orhan K, Aksoy U, Can-Karabulut DC, Kalender A (2011) Low-level laser therapy of dentin hypersensitivity: a short-term clinical trial. Lasers Med Sci 26:591–598
Ladalardo TC, Pinheiro A, Campos RA, Brugnera Júnior A, Zanin F, Albernaz PL, Weckx LL (2004) Laser therapy in the treatment of dentine hypersensitivity. Braz Dent J 15:144–150
Benetti AR, Franco EB, Franco EJ, Pereira JC (2004) Laser therapy for dentin hypersensitivity: a critical appraisal. J Oral Laser Appl 4:271–278
Higgins JP, Green S eds (2011) Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Accessed on 4/1/2013 at: http://www.cochrane-handbook.org
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264–269
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Landis JR, Koch GG (1977) An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 33:363–374
Lopes AO, Eduardo CP, Aranha AC (2015) Clinical evaluation of low-power laser and a desensitizing agent on dentin hypersensitivity. Lasers Med Sci 30:823–829
Vieira AH, Passos VF, de Assis JS, Mendonça JS, Santiago SL (2009) Clinical evaluation of a 3% potassium oxalate gel and a GaAlAs laser for the treatment of dentinal hypersensitivity. Photomed Laser Surg 27:807–812
Lin PY, Cheng YW, Chu CY, Chien KL, Lin CP, Tu YK (2013) In-office treatment for dentin hypersensitivity: a systematic review and network meta-analysis. J Clin Periodontol 40:53–64
He S, Wang Y, Li X, Hu D (2011) Effectiveness of laser therapy and topical desensitising agents in treating dentine hypersensitivity: a systematic review. J Oral Rehabil 38:348–358
Cunha-Cruz J, Wataha JC, Zhou L, Manning W, Trantow M, Bettendorf MM, Heaton LJ, Berg J (2010) Treating dentin hypersensitivity: therapeutic choices made by dentists of the northwest PRECEDENT network. J Am Dent Assoc 141:1097–1105
Sgolastra F, Petrucci A, Gatto R, Monaco A (2011) Effectiveness of laser in dentinal hypersensitivity treatment: a systematic review. J Endod 37:297–303
Jokstad A (2012) The effectiveness of lasers to reduce dentinal hypersensitivity remains unclear. J Evid Based Dent Pract 3(Suppl):231–232
West NX, Addy M, Jackson RJ, Ridge DB (1997) Dentine hypersensitivity and the placebo response. A comparison of the effect of strontium acetate, potassium nitrate and fluoride toothpastes. J Clin Periodontol 24:209–215
Sgolastra F, Petrucci A, Severino M, Gatto R, Monaco A (2013) Lasers for the treatment of dentin hypersensitivity: a meta-analysis. J Dent Res 92:492–499
Acknowledgments
The authors would like to express their gratitude to the Special Laboratory of Lasers in Dentistry (LELO) from the Department of Restorative Dentistry, School of Dentistry of USP and FAPESP (São Paulo Research Foundation).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding information
This manuscript was not financed by any development agency.
Ethical approval
This research does not involve human participants and/or animals that would necessarily demand informed consent documents or approval of the Local Ethics Committee.
Rights and permissions
About this article
Cite this article
Machado, A.C., Viana, Í.E.L., Farias-Neto, A.M. et al. Is photobiomodulation (PBM) effective for the treatment of dentin hypersensitivity? A systematic review. Lasers Med Sci 33, 745–753 (2018). https://doi.org/10.1007/s10103-017-2403-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-017-2403-7