Abstract
This randomized and longitudinal in vivo study aimed to assess different protocols for the treatment of dentin hypersensitivity with low-power laser (with different doses), high-power laser, and a desensitizing agent, for a period of 12 and 18 months. The lesions from 32 patients (117 lesions), who were submitted to the inclusion and exclusion criteria, were divided into nine groups (n = 13): G1: Gluma Desensitizer (Heraeus Kulzer), G2: low-power laser with low dose (three points of irradiation in vestibular portion and an apical point 30 mW, 10 J/cm2, 9 s per point with the wavelength of 810 nm, with three sessions with an interval of 72 h), G3: low-power laser with high dose (one point in the cervical area, and one apical point 100 mW, 40 J/cm2, 11 s per point with the wavelength of 810 nm in three sessions with an interval of 72 h), G4: low-power laser with low dose + Gluma Desensitizer, G5: low-power laser with high dose + Gluma Desensitizer, G6: Nd:YAG laser (Power Laser™ ST6, Research® in contact 1.0 W, 10 Hz and 100 mJ, ≈85 J/cm2, with the wavelength of 1064 nm), G7: Nd:YAG laser + Gluma Desensitizer, G8: low-power laser with low dose + Nd:YAG laser, and G9: low-power laser with high dose + Nd:YAG laser. The level of sensitivity of each volunteer was assessed by visual analog scale of pain (VAS) with the aid of air from the triple syringe and exploration probe, 12 and 18 months after treatment. All analyses were performed separately for air and probe stimulus. The level of significance was considered for values of p < 0.05. After statistical analysis, all treatments were shown to be effective in reducing dentinal hypersensitivity, and the results were considered not statistically different from those at 12 months. Therefore, until the 18-month evaluation, it could be said that no statistical differences were observed in the sensitivity levels for all treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dentin hypersensitivity (DH) is clinically described as a non-spontaneous, localized, intense pain of short duration that ceases when stimuli (thermal, chemical, mechanical, evaporative, or osmotic) are removed, and cannot be attributed to any other form of dental defect or pathology [1–9].
DH prevalence has ranged between 4 and 74% in the various populations studied [10–15]. This wide variation in the numbers can be explained by the difference in populations and methods used for verifying pain [16].
There are many etiologic and predisposing factors related to DH [17]. Enamel loss and consequent dentin exposure may result from the combined processes of abfraction, abrasion, and erosion. Denuding of the root surface is also a result of loss of cement and periodontal tissue [18]. The origin of this dentin exposure may be multifactorial, resulting from chronic brushing problems with oral hygiene, occlusal trauma, periodontal diseases, periodontal surgeries, parafunctional habits, abrasion by tooth brushing force, erosion due to dietary factors, poor positioning of the teeth in the arch, age, or a combination of these factors [19–21].
Concerning the treatment, a number of possibilities have been described and lasers are known for their easy reproducibility and fast responses. However, in 2003, the Canadian Advisory Board for Dentin Hypersensitivity [22], in their report on recommendations for the diagnosis and treatment of DH, concluded that irrespective of studies conducted with low- or high-power laser, further long-term clinical follow-up, randomized, double-blind, and placebo studies should be conducted.
In 2012, Jokstad [23] analyzed the study of Sgolastra et al. [24] in a special supplement of the Journal of Evidence-Based Dental Practice, and concluded that the treatment of DH with lasers appeared to reduce the pain; however, evidence of this effectiveness was still weak due to the wide variation in the methodologies used.
High-power lasers are effective for the treatment of DH due to the melting that occurs as a result of heat transmission. This interaction results in fusion and resolidification of dentin, with the consequent effect of sealing and reducing the diameter of the dentinal tubules [6, 25].
Unlike high-power lasers, low-power lasers do not emit heat and stimulate the normality of cell functions [26, 27]. This is because they lead to the occurrence of change in the electrical potential of the cell membrane, activating the Na+/K+ ATPase pumps, providing an increase in adenosine triphosphate (ATP) synthesis, and bringing about analgesic, potential anti-inflammatory and biomodulation benefits to the cells [19, 26]. Up to now, studies have not yet been conclusive, but have affirmed that these lasers blocked the depolarization of afferent C fibers, so that the neural transmission of pulp pain stimulus did not reach the central nervous system, thus providing immediate analgesia [28, 29]. In addition to the temporarily effect on hyper-polarizing C afferents fibers, the current literature has also supported the theory that low-power laser effects also stimulated healing by increasing PGF2, COX2, and growth factors [30].
There is a possibility that low-power lasers may obliterate the dentinal tubules by the effect of photobiomodulation on dental pulp, due to an increase in cellular metabolic activity of the odontoblasts, so that they intensify the production of tertiary dentin [26, 27, 31, 32].
Ideally, the most appropriate treatment for DH would be the type that has a long-lasting effect, is resistant to oral challenges, has immediate action, and brings comfort to patients with DH. Based on these concepts, a variety of treatments have been described, such as occlusal adjustment, diet advice, brushing instruction, use of specific toothpastes with desensitizers, application of adhesive systems and/or restoration, application of desensitizing products, and more currently, the use of low- and high-power laser irradiations; however, due to the wide variety of treatments and protocols existent, doubts remain about which strategy to use and individualizing the treatment for each patient [22].
Therefore, the aim of the present in vivo study was to evaluate different protocols for the treatment of DH with desensitizing agent, low-power and high-power lasers, and associations for a period of up to 18 months.
Materials and methods
The research protocol was initially submitted and approved by the Ethics Committee from the School of Dentistry of University of São Paulo (Protocol 62/07). This study was a continuation of two previous published studies [33, 34].
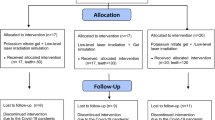
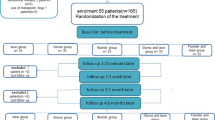
After clinical examination and inclusion and exclusion criteria, 32 patients between 22 and 53 years were selected, and 117 teeth (n = 13) received treatment. Patients who gave their oral and voluntary written informed consent, and were aware of the study inclusion and exclusion criteria, were examined before participating in the study. A detailed medical and dental history was recorded. Patients were considered suitable for the study if they had sensitive teeth showing tooth wear or gingival recession with exposure of cervical dentin. Patients with teeth showing evidence of irreversible pulpitis or necrosis, carious lesions, defective restorations, facets of attrition, premature contact, cracked enamel, active periodontal disease, use of daily doses of medications, or any factor that could be responsible for sensitivity were excluded. Also excluded were patients who had undergone professional desensitizing therapy during the previous 3 months and women who were pregnant or breast-feeding. Differential diagnosis was performed to exclude the possibility of other pathologies, and the vitality of all teeth was verified before, after the treatment and in the periods of evaluation through vertical and horizontal percussion tests and Rx.
After this, all patients were standardized and the lesions were randomly assigned to the groups. The degree of DH was determined by a visual analog scale (VAS). All patients were asked to define their level of DH by using the VAS on a scale from 0 to 10, where 0 represented “no pain” and 10 “the worst pain.” Each patient was asked to rate the perception of discomfort after the application of air using a dental syringe for 3 s, 2 mm away from and perpendicular to the root surface. The adjacent teeth were isolated with cotton rolls to prevent false positive results. In addition, a dental probe was used to scratch the tooth surface in a mesial to distal direction, and the VAS scale was used to determine the level of pain.
One trained operator applied all stimuli, with the patient seated in the same dental chair, with the same equipment yielding similar air pressure and probe pressure. This first measurement was considered the baseline (pre 01). The level of sensitivity was measured by VAS scale at time intervals of 5 min (post 1), 12 months (post 2) and 18 months (post 3) after the treatments. The cervical lesions and recessions were randomly divided into the nine experimental groups using a Microsoft Office Excel program. No negative control group (placebo group) was allowed by the Ethics Committee.
G1—desensitizer agent (Gluma Desensitizer)
A few drops of Gluma Desensitizer (Heraeus Kulzer, Armonk, NY, USA) were applied with a cotton pellet using a gentle but firm rubbing motion. After 30–60 s, the area was dried thoroughly until the fluid disappeared and the surface was not shiny, and then the surface was rinsed with water.
G2 - low power laser with low dose (LPLD)
Irradiation was performed with Photon Lase (DMC, São Carlos, SP, Brazil) in four points of the sensitive tooth: three vestibular cervical points (mesial, central, and distal) and one apical point. Irradiation was performed perpendicular to the surface and in contact with the tooth, in the following parameters: spot size of 0.028 cm2, 30 mW of power, energy density of ≅10 J/cm2, and 9 s on each point (dose of 0.27 J per point). The treatment was performed in three different sessions, and an interval of 72 h between every session was respected.
G3 - low power laser with high dose (LPHD)
Irradiation was performed with Photon Lase (DMC, São Carlos, SP, Brazil) on one cervical and one apical point. Irradiation was performed perpendicular to the surface and in contact with the tooth in the following parameters: 100 mW power, energy density of ≅40 J/cm2, and 11 s on each point (dose of 1.1 J per point). The treatment was performed in three sessions with an interval of 72 h between irradiations.
G4—LPLD + Gluma Desensitizer
In this group low-power laser irradiation was performed as described for group 2, and after the third laser irradiation session (end of the three laser irradiations), the Gluma Desensitizer agent was applied, as described in group 1.
G5—LPHD + Gluma Desensitizer
In the same way as in the previous group, in group 5, the patients received the three sessions of low-power laser irradiation at a high dose, as described in group 3; however, in the third irradiation session (72 h after the initial irradiation), the Gluma Desensitizer agent was applied after laser irradiation.
G6—Nd:YAG laser irradiation
The irradiation with Nd:YAG laser (Power LaserTM ST6, Lares Research, San Clemente, CA, USA) was performed in pulsed form, contact mode, perpendicular to the surface, pulse duration of 120 μs, energy per pulse of 100 mJ, energy density of ≈85 J/cm2, in contact, power of 1 W, and repetition rate of 10 Hz. A 400-μm quartz fiber was used, with pre-established movements in the occluso-apical and mesiodistal directions, and vice versa. Four irradiations of 15 s each were performed, totaling 60 s of irradiation. An interval of 10 s between the irradiations was necessary for thermal relaxation of the tissue.
G7—Nd:YAG laser + Gluma Desensitizer
The association of Nd:YAG laser irradiation and application of desensitizing agent Gluma Desensitizer was used, the irradiation protocol was initially performed, and then the product was applied according to the manufacturer’s instructions.
G8—LPLD + Nd:YAG laser
In this protocol, the association of low-power laser irradiation (low dosage) and a high-power laser (Nd:YAG) were associated, with parameters described in groups G2 and G6, respectively. The three sessions of low-power laser irradiation (low dosage) were conducted, but in the third session, the LPHD laser was applied followed by Nd:YAG irradiation.
G9—LPHD + Nd:YAG laser
In this protocol, the association of irradiation with low- and high-power lasers (Nd:YAG) was also associated, as described in G3 and G6, respectively. In the same way as in G8, three sessions of low-power laser irradiation (high dose) were conducted, but in the third session, the LPHD laser application was performed, followed by Nd:YAG irradiation.
Statistical analysis
Initially, a comparison of pain was made at baseline (pre). As there was no statistical significance, that is, the pain of patients, measured by the visual analog scale before treatment began was homogeneous between the groups, it was not necessary to transform the data so that the groups would be compatible over the course of time.
The Kolmogorov-Smirnov test, used to verify the distribution of data, showed that data were not normally distributed; therefore, comparisons between the treatment groups and periods analyzed were made by non-parametric tests (Kruskal-Wallis and Friedman tests, respectively).
In the case of statistical significance in either the comparison between groups or the comparison between the evaluations, the methodology of multiple comparisons was used to identify which groups (or periods) differed between them.
All analyses were performed separately for air and probe stimuli. The level of significance was considered for values of p < 0.05. The statistical software Minitab version 16.1 was used for data analysis and construction of the graphs.
Results
Air stimulus
Since the data did not present normal distribution, they were analyzed with non-parametric tests. Table 1 shows the pain (median) obtained according to treatment used and period of evaluation, and Table 2 shows the standard deviation.
Comparison between the groups showed no significant difference in pain in the pre-treatment period (p = 0.097); therefore, it was unnecessary to transform the data to compare them over the course of time. In each time interval also, no significant differences were found between treatments (post 1 p = 0.365; post 2 p = 0.964; post 3 p = 0.620).
For the evaluation of pain over the course of time, each group was analyzed separately. The multiple comparisons test indicated that there were significant differences in pain in all the groups (p < 0.001 for each group).
Probe stimulus
The probe stimulation data evaluated were also not normally distributed; thus, they were analyzed with non-parametrical tests. Table 3 shows the pain (median) obtained according to treatment used and period of evaluation, and Table 4 shows the standard deviation.
As occurred with the results obtained with air stimulus, comparisons between the groups using probe stimulus showed no significant difference in pain in the pre-treatment period (p = 0.321); therefore, it was unnecessary to transform the data to compare them over the course of time. In each time interval also, no significant differences were found between treatments (post 1 p = 0131; post 2 p = 0.770; post 3 p = 0.754).
For the evaluation of pain over the course of time, each group was analyzed separately. All groups presented significant differences in pain (p < 0.001 for each group), except for group 8 (LPLD + Nd:YAG) p = 0.002 that showed a decrease in pain values since the post 1 evaluation.
The multiple comparison test showed that only in groups 4 (LPLD + Gluma), 5 (LPHD + Gluma), 6 (Nd:YAG), 1 (Gluma), and 7 (Nd:YAG + Gluma) that this difference became significant as from the period post 1.
Discussion
Over the last 20 years, contemporary dentistry has led to a significant reduction in tooth loss caused by caries. However, population aging in developed and developing countries has led to increased life expectancy and behavioral changes, and these factors have resulted in increasing occurrence of non-carious cervical lesions. Therefore, it would not be unreasonable to suggest that the incidence of DH, a consequence of non-carious cervical lesions, will increase in the future [5, 22, 35–38]. Epidemiological studies have suggested that the prevalence of DH has increased along with the rising incidence in non-carious lesions (erosion, abrasion, abfraction) increases [15, 39–41].
DH affects the daily life of patients and may lead to reduction in their quality of life [42]. Thus, DH, which is not a recent or rare problem, must be considered an important ongoing clinical condition that has, up to now, continued without any effective, commercially available treatment [5, 22, 35].
In the present study, some of the patients reported a pain so severe that this pathology became a physical and emotional problem, as was mentioned in a previous article of the group that evaluated the patients for 6 months [33, 34]. Many patients reported that they were unable to ingest hot or cold foods or liquids, sweets, acid foods, or drinks and even had difficulty with brushing their teeth due to the pain reported. In view of these reports, it has become necessary to seek safe and long-lasting DH treatment protocols.
The desensitizing agent Gluma® Desensitizer, a treatment widely used in dental offices, has a formulation containing 5% glutaraldehyde and 35% hydroxyethylmethacrylate (HEMA). The mechanism of action of this product, based on the formation of precipitations arising from the reaction of glutaraldehyde with the proteins present in dentinal tubules, leads to reducing tubule diameters. These precipitations may also lead to the polymerization of HEMA, thus obliterating or occluding dentinal tubules [43, 44], by means of tags capable of reaching a depth of 200 μm [45].
In the present study, the group treated with Gluma Desensitizer showed significantly reduced pain levels, both for air and probe stimulations. Immediately after application, the pain levels were reduced, and remained the same until the evaluation at 18-month post-treatment. This was the only group that presented no increase in pain over the course of time, an effect that may be considered as effective and long lasting. Further research may be useful considering Gluma as a non-invasive treatment option for DH.
The introduction of laser technology in dentistry has opened new possibilities of therapy for DH. Treatment with low- and high-power lasers has been shown to have the characteristics proposed by Grossman [46], and their use has been extensively investigated in the literature.
Among the high-power lasers, Nd:YAG laser is considered as a gold standard for the treatment of DH [4, 47–54] because it has been shown to have the capacity to obliterate the dentinal tubules, by melting and resolidifying dentin, without pulp injuries or cracks in irradiated dentin, when used with an adequate protocol [48, 55]. Moreover, a depth of sealing of 4 μm within the dentinal tubules has been shown [50], so that there was immediate improvement of DH. However, little is known about its in vivo effects in the long term and associations with other treatments.
In addition, according to the literature, apparently only the Nd:YAG laser had an additional analgesic effect when compared with the other high-power lasers. This probably occurred because the irradiation might have temporarily altered the final part of the sensory axons [56] and blocked both the C and Aβ fibers [57], preventing the patient from feeling pain.
In spite of melting occurring after irradiation with Nd:YAG laser, the authors could not be clinically certain that there was occlusion of all the dentinal tubules with the protocol applied in this study; however, increasing the power of the equipment could also increase the energy density and temperature. Zapletalová et al. [58] showed that power over 1.5 W might cause microcracks or even carbonization of the tooth surface and consequent increase in intrapulp temperature, causing irreversible injuries. The guarantee of not having occluded of all the dentinal tubules may explain why some patients, even after irradiation with Nd:YAG laser, still demonstrated pain.
Another possible explanation for the reports of pain after treatment with Nd:YAG laser was the one given by Niemz [59]. The absorption and consequent efficiency of irradiation with this laser depended on the presence of water molecules, proteins, and pigments in the dental structure. Since each tooth presented a different dentin structure as regards chemical composition and color, a tooth with more transparent dentin would absorb less irradiation by Nd:YAG laser than dentin with pigmentation. This led the authors to thinking that the treatment could be more efficient in older patients who had a higher degree of pigmentation than young patients.
Another explanation for the immediate effect of the reduction in DH by irradiation with high-power lasers, in addition to melting, would be their secondary action on dental pulp, that is, analgesic action [60]. Thus, high-power lasers would act as low-intensity lasers.
In addition to high-power lasers, treatments for DH with low-power lasers have revealed that their interaction with dental pulp caused a photo biomodulating effect, causing an increase in the metabolic activity of odontoblast cells, and possibly resulting in obliteration of the dentinal tubules with intensification in tertiary dentin production [31, 61, 62]. Moreover, the low-power laser wavelength (660 to 900 nm) is believed to stimulate local cellular microcirculation and activity, bringing about anti-inflammatory effects, analgesia, and a state of normality to the tissues [26, 63].
The immediate reduction in DH when a low-power laser with a wavelength in the infrared band was used could be explained based on physiological experiments that demonstrated that when light acted on the cell membrane, it allowed greater passage, and consequent increase in Ca2+, Na2+, and K+ ions. Consequently, the endorphin system and the action potential of neural cells increased, and at the same time, the depolarization of C fiber afferents was blocked, not allowing the pain information to reach the central nervous system [28].
Whereas, a possible explanation for the lasting effect of low-power lasers in the treatment of DH might be the formation of a layer of tertiary dentin, proved by Tate et al. [32]; however, this is still at the stage of studies by many research groups.
There is a reciprocal action and reaction between the physiological and psychological interactions of DH treatment [64]. Kimura et al. [19] reported that both the patient and the dentist must believe in the success of the method of treatment. This affirmation was proved when the power/force of placebo treatments was observed in studies that have investigated the effect of desensitizing products. Their effectiveness has been evaluated at 20 to 60% of pain reduction in patients with DH [64] or in comparison with groups of treatment with laser compared with placebo groups [52, 65, 66]. For the present clinical study, it would have been interesting and of great importance to have had a placebo group, but this was not possible, because it was not approved by the Ethics Committee of the local institution.
All the treatments used had advantages and disadvantages, but if they were performed concomitantly with instructions to the patient (instructions on diet or tooth brushing), they would have been more effective. The present study demonstrated that all the treatments were efficient for reducing DH; in spite of DH presenting a numerical increase after the evaluation at 12 months [33, 34], with exception of the Gluma group that remained stable at zero, there were no statistical differences between the time intervals of analysis.
When the authors compared the two protocols for low-power laser (at a low and a high dose), it was possible to observe the distinct modes of action; however, in the long term, the results of pain were similar for the two groups. Previous clinical evaluation [34] showed different effects for dosage, with the low dose presenting immediate effects, whereas the groups of low-power laser at a high dose showed results only 1 week after the treatment. However, in the long-term evaluation, the results of pain were similar for both protocols.
It is necessary to report that it was difficult to qualify the pain caused by DH. The visual analog scale (VAS) was chosen to measure the pain of the patients in this study, because of being considered adequate for the measurement of pain in studies, according to the literature, with the advantage of being a continuous scale, easy for patients to understand, and with the capacity to discriminate different types of pain in different studies, such as the pain in DH [26, 31, 33, 34, 52, 65, 67, 68].
In spite of the wide variety of materials and techniques existent, DH is still considered a chronic problem in dentistry, due to the difficulty of measuring pain and choice of the most suitable material and technique, and due to the uncertain prognosis [29].
Until now, no method has been developed for satisfactorily resolving DH, and recurrences are common. The patients with DH must be carefully evaluated for differential diagnosis and control of the etiological factors, because treatment should bring fast and permanent pain relief. Traumatic brushing techniques or daily habits are risk factors that must be diagnosed during anamnesis and changed during therapy.
Conclusions
In view of the results of this in vivo study, some conclusions could be reached:
All treatments performed were efficient in the reduction of cervical dentin hypersensitivity.
When all the treatments performed in vivo, in the time intervals of evaluation performed, all were efficient in the same manner.
After pain reduction was achieved, there was no statistically significant difference in the levels of pain until the last evaluation was performed (18 months).
The treatments performed with low-power laser at a low dose and low-power laser at a high dose were both equally effective in decreasing pain in the long-term evaluation.
The desensitizing agent Gluma Desensitizer was the only group that presented no increase in pain over the course of time, being considered as an effective and non-invasive treatment option.
References
Holland GR, Narhi MN, Addy M, Gangarosa L, Orchardson R (1997) Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J Clin Periodontol 24:808–813
Addy M (2002) Dentine hypersensitivity: new perspectives on an old problem. Int Dent J 52:367–375
Rees JS, Addy M (2002) A cross-sectional study of dentine hypersensitivity. J Clin Periodontol 29:997–1003
Ciaramicoli MT, Carvalho RCR, Eduardo CP (2003) Treatment of cervical dentin hypersensitivity using neodymium: yttrium-aluminum-garnet laser. Clinical evaluation. Lasers Surg Med 33:358–362
Banfield N, Addy M (2004) Dentine hypersensitivity: development and evaluation of a model in situ to study tubule patency. J Clin Periodontol 31:325–335
Lan WH, Lee BS, Liu HC, Lin CP (2004) Morphologic study of Nd:YAG laser in treatment of dentinal hypersensitivity. J Endod 30:131–134
Que K, Ruan J, Fan X, Liang X, Hu D (2010) A multi-centre and cross-sectional study of dentine hypersensitivity in China. J Clin Periodontol 37:631–637
Winston AE, Charig AJ, Thong S (2010) Mechanism of action of a desensitizing fluoride toothpaste delivering calcium and phosphate ingredients in the treatment of dental hypersensitivity. Part III: prevention of dye penetration through dentin vs a calcium- and phosphate-free control. Compend Contin Educ Dent 31(46–48):50–52
Ali S, Farooq I (2013) Dentin hypersensitivity: a review of its etiology, mechanism, prevention strategies and recent advancements in its management. World J Dent 4:188–192
Taani DQ, Awartani F (2001) Prevalence and distribution of dentin hypersensitivity and plaque in a dental hospital population. Quintessence Int 32:372–376
Rees JS, Jin LJ, Lam S, Kudanowska I, Vowles R (2003) The prevalence of dentine hypersensitivity in a hospital clinic population in Hong Kong. J Dent 31:453–461
Bartold PM (2006) Dentinal hypersensitivity: a review. Aust Dent J 51:212–218
Gillam DG (2013) Current diagnosis of dentin hypersensitivity in the dental office: an overview. Clin Oral Investig 17:21–29
Christian HS, Tachou A (2013) Epidemiology of dentin hypersensitivity. Clin Oral Invest 17:S3–S8
Scaramucci T, Anfe TEA, Ferreira SS, Sobral MAP (2014) Investigation of the prevalence, clinical features, and risk factors of dentin hypersensitivity in a selected Brazilian population. Clin Oral Invest 18:651–657
Lochaiwatana Y, Poolthong S, Hirata I, Okazaki M, Swasdison S, Vongsavan N (2015) The synthesis and characterization of a novel potassium chloride-fluoridated hydroxyapatite varnish for treating dentin hypersensitivity. Dent Mater J 34:31–40
Pradeep AR, Sharma A (2010) Comparison of clinical efficacy of a dentifrice containing calcium sodium phosphosilicate to a dentifrice containing potassium nitrate and to a placebo on dentinal hypersensitivity: a randomized clinical trial. J Periodontol 81:1167–1173
Walters PA (2005) Dentinal hypersensitivity: a review. J Contemp Dent Pract 6:107–117
Kimura Y, Wider-Smith P, Yonaga K, Matsumoto K (2000) Treatment of dentine hypersensitivity by lasers: a review. J Clin Periodontol 27:715–721
Miller GD, Jarvis JK, McBean LD (2001) The importance of meeting calcium needs with foods. J Am Coll Nutr 20:168S–185S
Orchardson R, Gillam DG (2006) Managing dentin hypersensitivity. J Am Dent Assoc 137:990–998
Canadian Advisory Board on Dentin Hypersensitivity (2003) Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc 69:221–226
Jokstad A (2012) The effectiveness of lasers to reduce dentinal hypersensitivity remains unclear. J Evid Based Dent Pract 12:231–232
Sgolastra F, Petrucci A, Gatto R, Monaco A (2011) Effectiveness of laser in dentinal hypersensitivity treatment: a systematic review. J Endod 37:297–303
Aranha AC, Pimenta LA, Marchi GM (2009) Clinical evaluation of desensitizing treatments for cervical dentin hypersensitivity. Braz Oral Res 23:333–339
Gerschman JA, Ruben J, Gebart-Eaglemont J (1994) Low level laser therapy for dentinal tooth hypersensitivity. Aust Dent J 39:353–357
Corona SA, Nascimento TN, Catirse AB, Lizarelli RF, Dinelli W, Palma-Dibb RG (2003) Clinical evaluation of low-level laser therapy and fluoride varnish for treating cervical dentinal hypersensitivity. J Oral Rehabil 30:1183–1189
Wakabayashi H, Hamba M, Matsumoto K, Tachibana H (1993) Effect of irradiation by semiconductor laser on responses evoked in trigeminal caudal neurons by tooth pulp stimulation. Lasers Surg Med 13:605–610
Yilmaz HG, Cengiz E, Kurtulmus-Yilmaz S, Leblebicioglu B (2011) Effectiveness of Er,Cr:YSGG laser on dentine hypersensitivity: a controlled clinical trial. J Clin Periodontol 38:341–346
Sakurai Y, Yamaguchi M, Abiko Y (2000) Inhibitory effect of low-level laser irradiation on LPS-stimulated prostaglandin E2 production and cyclooxygenase-2 in human gingival fibroblasts. Eur J Oral Sci 108:29–34
Ladalardo TC, Pinheiro A, Campos RA, Brugnera Júnior A, Zanin F, Albernaz PL, Weckx LL (2004) Laser therapy in the treatment of dentine hypersensitivity. Braz Dent J 15:144–150
Tate Y, Yoshiba K, Yoshiba N, Iwaku M, Okiji T, Ohshima H (2006) Odontoblast responses to GaAlAs laser irradiation in rat molars: an experimental study using heat-shock protein-25 immunohistochemistry. Eur J Oral Sci 114:50–57
Lopes AO, Aranha AC (2013) Comparative evaluation of the effects of Nd:YAG laser and a desensitizer agent on the treatment of dentin hypersensitivity: a clinical study. Photomed Laser Surg 31:132–138
Lopes AO, Eduardo CP, Aranha AC (2015) Clinical evaluation of low-power laser and a desensitizing agent on dentin hypersensitivity. Lasers Med Sci 30:823–829
Rösing CK, Fiorini T, Liberman DN, Cavagni J (2009) Dentine hypersensitivity: analysis of self-care products. Braz Oral Res 23:56–63
Aranha AC, Eduardo CP (2012) Effects of Er:YAG and Er,Cr:YSGG lasers on dentine hypersensitivity. Short-term clinical evaluation. Lasers Med Sci 27:813–818
Aranha AC, Eduardo CP (2012) In vitro effects of Er,Cr:YSGG laser on dentine hypersensitivity. Dentine permeability and scanning electron microscopy analysis. Lasers Med Sci 27:827–834
Dong Z, Chang J, Deng Y, Joiner A (2011) Tricalcium silicate induced mineralization for occlusion of dentinal tubules. Aust Dent J 56:175–180
Ahmed TR, Mordan NJ, Gilthorpe MS, Gillam DG (2005) In vitro quantification of changes in human dentine tubule parameters using SEM and digital analysis. J Oral Rehabil 32:589–597
Ritter AV, de L Dias W, Miguez P, Caplan DJ, Swift EJ (2006) Treating cervical dentin hypersensitivity with fluoride varnish: a randomized clinical study. J Am Dent Assoc 137:1013–1020
Oderinu OH, Savage KO, Uti OG, Adegbulugbe IC (2011) Prevalence of self-reported hypersensitive teeth among a group of Nigerian undergraduate students. Niger Postgrad Med J 18:205–209
Bekes K, John MT, Schaller HG, Hirsch C (2009) Oral health-related quality of life in patients seeking care for dentin hypersensitivity. J Oral Rehabil 36:45–51
Qin C, Xu J, Zhang Y (2006) Spectroscopic investigation of the function of aqueous 2-hydroxyethylmethacrylate/glutaraldehyde solution as a dentin desensitizer. Eur J Oral Sci 114:354–359
Ishihata H, Finger WJ, Kanehira M, Shimauchi H, Komatsu M (2011) In vitro dentin permeability after application of Gluma® desensitizer as aqueous solution or aqueous fumed silica dispersion. J Appl Oral Sci 19:147–153
Schüpbach P, Lutz F, Finger W (1997) Closing of dentinal tubules by Gluma desensitizer. Eur J Oral Sci 105:414–421
Grossman LI (1935) A systematic method for the treatment of hypersensitive dentine. J Am Dent Assoc 22:592–598
Gelskey S, White JM, Pruthi VK (1993) The effectiveness of the Nd:YAG laser in the treatment of dental sensitivity. J Can Dent Assoc 59:377–386
Lan WH, Liu HC (1996) Treatment of dentin hypersensitivity by Nd:YAG laser. J Clin Laser Med Surg 14:89–92
Gutknecht N, Moritz A, Dercks HW, Lampert F (1997) Treatment of hypersensitive teeth using neodymium: yttrium-aluminum-garnet lasers: a comparison of the use of various settings in an in vivo study. J Clin Laser Med Surg 15:171–174
Liu HC, Lin CP, Lan WH (1997) Sealing depth of Nd:YAG laser on human dentinal tubules. J Endod 23(11):691–693
Aranha AC, Domingues FB, Franco VO, Gutknecht N, Eduardo CP (2005) Effects of Er:YAG and Nd:YAG lasers on dentin permeability in root surfaces: a preliminary in vitro study. Photomed Laser Surg 23:504–508
Birang R, Poursamimi J, Gutknecht N, Lamper F, Mir M (2007) Comparative evaluation of the effects of Nd:YAG and Er:YAG laser in dentin hypersensitivity treatment. Lasers Med Sci 22:21–24
Dilsiz A, Aydn T, Emrem G (2010) Effects of the combined desensitizing dentifrice and diode laser therapy in the treatment of desensitization of teeth with gingival recession. Photomed Laser Surg 28:69–74
Gholami GA, Fekrazad R, Esmaiel-Nejad A, Kalhori KAM (2011) An evaluation of the occluding effects of Er;Cr:YSGG, Nd:YAG, CO2 and diode lasers on dentinal tubules: a scanning electron microscope in vitro study. Photomed Laser Surg 29:115–121
Lan WH, Liu HC (1995) Sealing oh human dentinal tubules by Nd:YAG laser. J Clin Laser Med Surg 13:329–333
Myers TD, McDaniel JD (1991) The pulsed Nd:YAG dental laser: review of clinical applications. J Calif Dent Assoc 19:25–30
Orchardson R, Gillam DG (2000) The efficacy of potassium salts as agents for treating dentin hypersensitivity. J Orofac Pain 14:9–19
Zapletalová Z, Peřina J, Novotvý R, Chmeličková H (2007) Suitable conditions for sealing of open dentinal tubules using a pulsed Nd:YAG laser. Photomed Laser Surg 25:495–499
Niemz MH (2002) Laser tissue interactions. Springer, Berlin
Zeredo JL, Sasaki KM, Fujiyama R, Okada Y, Toda K (2003) Effects of low power Er:YAG laser on the tooth pulp-evoked jaw-opening reflex. Lasers Surg Med 33:169–172
Marsilio AL, Rodrigues JR, Borges AB (2003) Effect of the clinical application of the GaAlAs laser in the treatment of dentine hypersensitivity. J Clin Laser Med Surg 21:291–296
Tengrungsun T, Sangkla W (2008) Comparative study in desensitizing efficacy using the GaAlAs laser and dentin bonding agent. J Dent 36:392–395
Midda M, Renton-Harper P (1991) Lasers in dentistry. Br Dent J 120:343–346
Gentile LC, Greghi SLA (2004) Clinical evaluation of dentin hypersensitivity treatment with the low intensity gallium-aluminum-arsenide laser—AsGaAl. J Appl Oral Sci 12:267–272
Lier BB, Rosing CK, Aass AM, Gjermo P (2002) Treatment of dentin hypersensitivity by Nd:YAG laser. J Clin Periodontol 29:501–506
Vieira AHM, Passos VF, De Assis JS, Mendonça JS, Santiago SL (2009) Clinical evaluation of a 3% potassium oxalate gel and a GaAlAs laser for the treatment of dentinal hypersensitivity. Photomed Laser Surg 27(5):807–812
Ide M, Wilson RF, Ashley (2001) The reproducibility of methods of assessment for cervical dentine hypersensitivity. J Clin Periodontol 28:16–22
Merika K, Hefti AF, Preshaw PM (2006) Comparison of two topical treatments for dentine sensitivity. Eur J Prosthodont Restor Dent 14:38–41
Acknowledgments
The authors would like to thank the Special Laboratory of Lasers in Dentistry (LELO) from the School of Dentistry of University of São Paulo. The authors would also like to thank FAPESP (São Paulo Research Foundation) for supporting the research (grant no. FAPESP 2012/20423-0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The research protocol was initially submitted and approved by the Ethics Committee from the School of Dentistry of University of São Paulo (Protocol 62/07).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Role of funding source
This study was funded by FAPESP—State of São Paulo Research Foundation (grant no. 2012/20423-0).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lopes, A.O., de Paula Eduardo, C. & Aranha, A.C.C. Evaluation of different treatment protocols for dentin hypersensitivity: an 18-month randomized clinical trial. Lasers Med Sci 32, 1023–1030 (2017). https://doi.org/10.1007/s10103-017-2203-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-017-2203-0