Abstract
This study aimed to histologically and radiographically evaluate the effectiveness of low-intensity laser irradiation of different wavelengths (660 or 808 nm) as an adjunct to scaling and root planing in the treatment of experimental periodontitis in rats. Periodontitis was induced by placing a ligature around the mandibular first molar of the rats. In total, 40 Wistar rats were randomly divided into five groups (n = 8 each): control (CG), periodontal disease (PD), scaling and root planing (SRP), SRP + 660 nm laser (GL660) and SRP + 808 nm laser (GL808). Groups with laser use received radiation at 6 points in the first molar. The animals were euthanized at baseline and at 7 and 14 days after the interventions. Mandibles were surgically removed for histomorphometric and radiographic assessment of periodontal tissues. The GL660 group showed lesser bone loss than the PD group (P < 0.05) and greater alveolar bone margin after 14 days, indicating a better long-term treatment response (P < 0.05). These findings suggest that SRP with the 660 nm laser as an adjunct results in more favorable radiographic and histological responses than the 808 nm laser.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontal disease (PD) is an immunoinflammatory process triggered by the accumulation of the bacterial biofilm on the external surface of the tooth, which may affect gingival tissue, periodontal ligament, cementum, and alveolar bone in susceptible individuals [1]. Common clinical signs of periodontal disease include gingival bleeding, alveolar bone resorption, periodontal pocket formation, halitosis, and dental mobility; advanced cases can also include spontaneous tooth loss [2].
Periodontopathogenic bacteria are the primary causative agent that can cause destruction of the supporting periodontal tissues directly, through the action of their components, particularly the lipopolysaccharide (LPS that is present on the cell wall of gram-negative bacteria) and in secreted molecules [3]. In addition, indirectly, the activity of microorganisms can stimulate host cells to express certain genes and secrete mediators, resulting in an exacerbated inflammatory response [4]. The severity of PD is proportional to the extent of tissue destruction caused by the magnified response of the susceptible host, probably influenced by environmental, acquired, or genetic risk factors [5].
Treatment of PD should, therefore, involve the removal of dental biofilm and the control of inflammation through clinical scaling and root planing (SRP), which are considered the conventional and essential procedures in periodontal therapy [6]. Although SRP represent the standard procedure for periodontal treatment, they may fail to remove some pathogenic organisms, mainly due to the difficulty in accessing furcation regions or deep pockets [7]. Recently, laser therapy, either as photodynamic or photobiomodulation therapy, has been suggested as a promising alternative treatment for bacterial control or modulation of host response, respectively [8].
Response modulation in the host using laser may contribute to improving the patient’s inflammatory response through tissue photobiomodulation, although literature on PD models is scarce [9, 10]. The supporting use of dental laser in periodontitis treatment is based on benefits such as greater reduction of gingival bleeding in deep pockets, activation of the microcirculation, production of new capillaries, regeneration of tissues, control of alveolar bone loss, and regulation of bone metabolism [11].
Only a few studies have compared the degree of bone loss with the use of different laser types in periodontal treatment. In this study, we compared the effect of 660 and 808 nm diode laser as an adjunct to SRP on radiographic bone loss and histological inflammatory parameters, including tissue changes in the periodontal ligament, in a ligature-induced periodontitis rat model.
Materials and methods
Design study
This was an experimental study in an animal model, and it approached pathophysiological aspects from the histomorphometric and radiographic analysis. The experimental protocol was approved by the Ethics Committee on Animal Use of Federal University of Maranhão (CEUA/UFMA, protocol no. 32/15).
Animals
The study included 40 male rats (Rattus, norvergius, albinus, Wistar), aged approximately 11 weeks and weighing 300–350 g. The animals were randomly allocated to five groups (n = 8 each): control group (CG), periodontal disease group (PD), scaling and root planing (SRP), SRP + laser 660 group (GL660), and SRP + laser 808 group (GL808). The animals were conditioned in plastic boxes (dimensions, 37.5 × 32 × 16 cm3) in a controlled-environment room (temperature, 23 °C ± 3 °C; humidity, 25%; light/dark cycle, 12/12 h), with free access to rat food and water ad libitum.
Induction of periodontal disease
PD was induced at the beginning of the experiment by the accumulation of bacterial biofilm through the ligature-induced periodontitis in the first lower molar [12]. Ligature placement was performed under general anesthesia by an intraperitoneal injection with a combination of 10% ketamine hydrochloride (0.4 mL/kg) (Cetamin, Syntec, Brazil) and 2% xylazine hydrochloride (0.2 mL/kg) (Xilazin, Syntec, Brazil).
A black cotton thread no. 24 (Corrente, Brazil) was introduced into the interproximal space between the first and second lower molars and maintained in the gingival sulcus around the first molar, in the most cervical position possible, by two nodes on the buccal face. The ligature remained in the PD group until euthanasia, and in the SRP, GL660, and GL808 groups until the day of intervention. PD was not induced in the CG.
Periodontal treatment
Periodontal treatment was performed under general anesthesia by an intraperitoneal injection with a combination of 10% ketamine hydrochloride (0.4 mL/kg) (Cetamin, Syntec, Brazil) and 2% xylazine hydrochloride (0.2 mL/kg) (Xilazin, Syntec, Brazil). After 2 weeks of PD induction, the cotton thread was removed. Molars were submitted to SRP with manual curettes (no. 13–14/mini-five, Hu-FriedyCo. Inc., Chicago, IL, USA) from distal to mesial traction movements. After the procedure, the region was irrigated with 1 mL of saline solution.
Photobiomodulation therapy
Photobiomodulation therapy was performed under general anesthesia by an intraperitoneal injection with a combination of 10% ketamine hydrochloride (0.4 mL/kg) (Cetamin, Syntec, Brazil) and 2% xylazine hydrochloride (0.2 mL/kg) (Xilazin, Syntec, Brazil). The laser subgroup was assigned to one of the two treatments: SRP and irrigation with 1 mL of saline solution, followed by the low-intensity laser application at 660 nm for 1 min, or SRP and irrigation with 1 mL of saline solution, followed by the application of low-intensity laser at 808 nm for 1 min.
The low-intensity lasers used in this study were a gallium-aluminum-arsenide (GaALAs) (laser MMO Premier Twin Flex; MMO Optics, São Carlos, SP, Brazil) with a wavelength of 808 nm and a point size of 0.03 cm2, and an InGaALP (laser MMO Premier Twin Flex, MMO Optics, São Carlos, SP, Brazil) with a wavelength of 660 nm and point size of 0.03 cm2. After 1 min of saline irrigation, the low-intensity laser was applied at three equidistant points on the buccal and lingual surface (at the following points: mesial, central, and distal of each face) of the lower first molar in contact with the gingival tissue. The laser was launched with a power of 0.03 W for 60 s per point, dose of 60 J/cm2, and energy of 1.8 J per point. The treated area received a total energy of 10.8 J.
Euthanasia
At the end of the experiment, the rats were euthanized with an overdose of Tipentax (sodium thiopental, 100 mg/kg) and sacrificed by aortic puncture and exsanguination. After 7 and 14 days of the periodontal treatment with or without laser, the animals were euthanized to verify the tissue response to treatment. Thus, SRP, GL660, and GL808 groups were subdivided into two subgroups (n = 4 each). The CG animals were euthanized at baseline, and the PD group animals were euthanized 2 weeks after PD induction (Fig. 1).
Radiographic evaluation
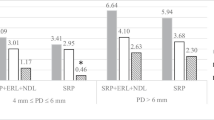
The hemi-mandibles were removed to determine the degree of alveolar bone loss using standard radiographs obtained by a digital radiography system (New IDA Dabi Atlante, Gnatus, São Paulo, Brazil). Electronic sensors were exposed at 70 kV and 8 mA with an exposure time of 0.4 s, and the distance from the source to the film was 30 cm, according to the manufacturer’s recommendations. Radiographic images were analyzed using the ImageJ software (version 1.37; National Institutes of Health, Bethesda, MD). The fixed points calibrated for the measures of alveolar bone loss were the tip of the distal cusp of the tooth (C), the point of the bony crest or bone defect (B), and the apex of the root (A) on the distal side of the first mandibular molar. Linear measurements from points A to B and A to C were measured (in mm), and the periodontal bone support was calculated using the formula AB/AC × 100 (Fig. 2) [13,14,15].
Histological evaluation
After euthanasia, the hemi-mandibles were removed in block and fixed in 4% paraformaldehyde solution for 48 h. The pieces were then placed in 7% EDTA solution for decalcification for 3 months. The EDTA solution was changed every 2 days. Subsequently, the specimens were included in paraffin and subjected to serial cuts of 5 μm in the mesio-distal direction, followed by hematoxylin and eosin staining.
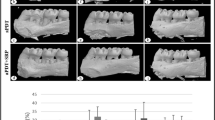
In the descriptive histological analysis, alterations in the periodontal ligament, alveolar bone in the region of the bone crest, and inter-radicular bone, in addition to the presence of inflammatory infiltrates, osteoblasts, and osteoclasts, were evaluated (Fig. 3). Images of four equidistant sections of each hemi-mandible were selected for histometric analysis using the Axio Vision Rel. 4.8 software s(Carl Zeiss, Germany).
Histological analysis. (a) Photomicrograph of the first molar. (b) Photomicrograph of the periodontal ligament. Arrows indicate blood vessels. ge, gingival epithelium; d, dentin; c, cementum; pl, periodontal ligament, where shows collagen fibers in pale pink filaments and purple elliptic nucleus of fibroblasts; ab. alveolar bone
Statistical analysis
The data were analyzed using SPSS 17.0 (IBM, Chicago, IL, USA). Continuous variables were submitted to the Shapiro–Wilk statistical test to verify their normality. All the groups showed a normal distribution (P > 0.05). After this procedure, analysis of variance (ANOVA), followed by the Tukey test, was used in the comparative analysis of the bone remaining between the groups. The difference between the times in the same intervention group was analyzed through independent sample t test. The level of significance was set at 5%.
Results
Radiographic evaluation
A total of 80 hemi-mandibles were analyzed for the measurement of the ratio between bone remaining and height of the first lower molar. Radiographic analysis revealed that there was a statistically significant reduction between the CG (healthy) and the PD group (P < 0.05, Tukey test), showing that the ligature insertion caused reabsorption of the periodontal bone support. In both times, the 660-nm laser group presented significant differences compared with the PD group (P < 0.05, Tukey test), especially after 14 days. At 14 days, the SRP group showed an increase in periodontal bone support compared with the PD group (P < 0.05, Tukey test). The laser 808 nm groups did not differ from either the CG or the PD group (Table 1).
Histological evaluation
Longitudinal histological sections of the lower first molar were prepared for visualization of the mesial periodontal tissue, furcation, and distal region. The representative laminas of each study group are shown in Fig. 4. The CG (Fig. 4a) had a uniform periodontal ligament width in the cervical furcation region, blood vessels of reduced diameter, clear collagen fibers inserted in both cemented and bone margins, and fusiform fibroblasts arranged in fascicles. The PD group (Fig. 4b) presented with distended and hyperemic blood vessels, as well as disorganization of collagen fiber bundles. The SRP groups without laser (Fig. 4c, d) showed well-distended and hyperemic blood vessels, lower fibroblastic density than the CG, and stretching of collagen fibers. The laser groups 660 nm (Fig. 4e, f) also had broad blood vessels, but they were narrower than the other treated groups, especially the 660-nm group at 14 days, with no signs of osteoclastic activity. The 808 nm groups (Fig. 4g, h) had distended blood vessels, which were less in number and diameter at 14 days.
Figure 5 is the photomicrographs of periodontal ligament details. In the CG (Fig. 5a), we observed a large density of fibroblasts, few blood vessels, and a well-defined bone and cement margin. The PD group (Fig. 5b) pictomicrograph revealed zones of bone resorption, evidenced by the presence of Howship gaps, where osteoclasts are housed in concavities in the alveolar bone margin. The Howship gap is highlighted in Fig. 6. In the treated groups (Fig. 5c–h), we noted a periodontal ligament with large blood vessels, with slight stretching of the Sharpey fibers, but without the presence of an active bone resorption zone.
Discussion
This study evaluated the effects of low-intensity laser as an adjuvant to mechanical periodontal treatment in a rat PD model. The main findings of this study suggest that mechanical periodontal treatment with the adjunctive use of laser 660 nm can optimize the effects of non-surgical periodontal therapy, by reducing alveolar bone loss, as evidenced in the radiographic analysis. In the histomorphometric analysis, we observed a re-establishment of the periodontal ligament and reduction of the local inflammatory disease.
We noticed that non-surgical periodontal therapy with SRP did not show significant differences in the control of alveolar bone loss when compared with the PD group. SRP is recognized in the literature as the most effective therapy for PD [9, 16], but it presents limitations by the use of hand instruments due to difficultly in accessing the furcation region, concavities, distal molars, and deep pockets. To overcome this limitation, adjuvants to conventional treatment have been proposed [17].
In the radiographic analyses of alveolar bone loss after SRP, restoration of bone support was better in the laser groups, especially the laser 660-nm group, compared with the SRP group without the laser. The results suggest that the mechanical periodontal treatment with the adjunctive use of laser 660 nm can optimize the effects of non-surgical periodontal therapy, with the restoration of the periodontal ligament and reduction of local inflammatory condition. The results of the study are similar to the radiographic analysis performed by Swerts et al. [18], in which rats treated with low-intensity SRP and laser treatment had lower alveolar bone loss than the group that received no treatment and the group that received only SRP.
The absorption of the laser light by the tissues is necessary to produce a clinical effect; therefore, only absorbed photons in cells may affect cellular metabolism. For photon absorption in a tissue, chromophores (hemoglobin and melanin) are required. These molecules promote signaling pathways that initiate a cascade of events that stimulate increase in ATP production, modulation of reactive oxygen species, and induction of several gene transcript products responsible for the beneficial effects [19, 20]. This absorption depends on the matching between the laser wavelength and the target tissue composition. Therefore, the presence of tissue chromophores capable of absorbing the incident wavelength must be considered to achieve clinical success.
The depth degree of the laser energy range depends on the absorption and dispersion, the energy dispersion being inversely proportional to the wavelength, that is, the smaller the wavelength is, the smaller the depth of the laser will be and, therefore, greater will be the degree of dispersion on the surface tissue. Thus, the visible-red emission (660 nm) acts on superficial tissues and accelerates cicatrization and the infra-red (808 nm) acts on deep tissues and treats chronic pain [21]. This healing activity may have contributed to the best results in the 660-nm laser groups in our study.
Lee et al. [22], using 660-nm GaAlAs diode laser, and Uslu et al. [16], using 810-nm GaAlAs diode laser, studied the effects of low-intensity laser as an adjuvant treatment for periodontitis in human periodontal ligament cells and rat periodontal tissues, respectively. They reported that low-level laser therapy (LLLT) has an anti-inflammatory effect by inhibiting pro-inflammatory effects through the positive regulation of cyclic adenosine monophosphate (cAMP) levels and the negative regulation of kappa nuclear factor B (NF-kB) in the intracellular medium. It also has the effect of reducing oxidative stress in the periodontal tissues, responsible for bone loss. These findings may explain the reduction of bone loss and the inflammatory process observed in our study.
Choi et al. [10] studied the effects of LLLT on human periodontal ligament fibroblast cells by using the 810-nm GaAlAs diode laser. They concluded that the low-intensity laser can stimulate the proliferation and differentiation of periodontal ligament fibroblasts, meaning the laser can optimize the wound healing process. Demirturk-Gocgun et al. [23] evaluated the role of LLLT using 808-nm GaAlAs diode laser as an adjunct to SRP in patients with chronic PD and type 2 diabetes mellitus. They reported improvement in bleeding on probing the deep periodontal pockets 1 month after treatment. This demonstrates that LLLT optimizes the healing time of periodontal pockets.
In this study, histological analysis of the PD group revealed periodontal changes in the furcation region, characterized by increased alveolar bone loss, disorganized collagen fiber bundles, distended and hyperemic blood vessels, and presence of osteoclast cell activities, implying periodontal tissue inflammation and bone resorption. Thus, the method used to induce periodontitis in our study, by adapting a cotton thread around the lower first molar in one of the hemiarcades [12], was effective, as evidenced by radiographic and histomorphometric analyses between the CG and PD groups. In addition, this method aided the comparative analysis between the experimental groups.
Favorable results were observed in the animal PD model proposed in this study. However, clinical studies are warranted to test the effects of laser 660 nm as an adjunct to periodontal therapy in reducing bone loss and modulating inflammatory processes in humans. These findings will help researchers to propose more effective therapeutic protocols for managing PD.
Conclusion
Photobiomodulation therapy as an adjuvant to non-surgical periodontal treatment in experimental periodontitis induced in rats was effective in reducing bone loss, modulating the inflammatory processes, and restoring the periodontal ligament. The 660 nm laser obtained better results than the 808 nm laser.
References
Kinane DF, Stathopoulou PG, Papapanou PN (2017) Periodontal diseases. Nat Rev Dis Prim 3:17038. https://doi.org/10.1038/nrdp.2017.38
Kulkarni C (2000) Kinane DF (2014) Host response in aggressive periodontitis. Periodontology 65(1):79–91. https://doi.org/10.1111/prd.12017
Kebschull M, Guarnieri P, Demmer RT, Boulesteix AL, Pavlidis P, Papapanou PN (2013) Molecular differences between chronic and aggressive periodontitis. J Dent Res 92(12):1081–1088. https://doi.org/10.1177/0022034513506011
Arora A, Sumanth KN, Prashanti E, Bhat KG, Kusum CK, Johnson TM, Lodi G (2017) Adjunctive systemic antimicrobials for the non-surgical treatment of chronic and aggressive periodontitis. Cochrane Database Syst Rev 2017(2):CD012568. https://doi.org/10.1002/14651858.CD012568
Borba TT, Molz P, Schlickmann DS, Santos C, Oliveira CF, Prá D, Kreather-Neto L, Franke SLR (2019) Periodontitis: genomic instability implications and associated risk factors. Mutat Res Genet Toxicol Environ Mutagen 840:20–23. https://doi.org/10.1016/j.mrgentox.2019.01.005
Jiao J, Shi D, Cao ZQ, Meng HX, Lu RF, Zhang L, Song Y, Zhao JR (2017) Effectiveness of no-surgical periodontal therapy in a large Chinese population with chronic periodontitis. J Clin Periodontol 44(1):42–50. https://doi.org/10.1111/jcpe.12637
Belinello-Souza EL, Alvarenga LH, Lima-Leal C et al (2017) Antimicrobial photodynamic therapy combined to periodontal treatment: experimental model. Photodiagn Photodyn Ther 18:275–278. https://doi.org/10.1016/j.pdpdt.2017.03.008
Mizutani K, Aoki A, Coluzzi D, Yukna R, Wang CY, Pavlic V, Izumi Y (2016) Lasers in minimally invasive periodontal and peri-implant therapy. Periodontol 2000 71(1):185–212. https://doi.org/10.1111/prd.12123
Pesevska S, Gjorgoski I, Ivanovski K, Soldatos NK, Angelov N (2017) The effect of low-level diode laser on COX-2 gene expression in chronic periodontitis patients. Lasers Med Sci 32(7):1463–1468. https://doi.org/10.1007/s10103-017-2231-9
Choi EJ, Yim JY, Koo KT et al (2010) Biological effects of a semiconductor diode laser on human periodontal ligament fibroblasts. J Periodontal Implant Sci 40(3):105–110. https://doi.org/10.5051/jpis.2010.40.3.105
Slot DE, Kranendonk AA, Paraskevas S, Van der Weijden F (2009) The effect of a pulsed Nd: YAG laser in non-surgical periodontal therapy. J Periodontol 80(7):1041–1056. https://doi.org/10.1902/jop.2009.080571
Johnson IH (1975) Effects of local irritation and dextran sulphate administration on the periodontium of the rat. J Periodontal Res 10(6):332–345. https://doi.org/10.1111/j.1600-0765.1975.tb00042.x
de Souza DM, Silva AA, Pereira KA, Gonsalves VS, Rodrigues VA, de Carvalho CC (2017) Alveolar bone loss induced by high alcohol consumption in rats. Braz Dent Sci 20(4):42–48. https://doi.org/10.14295/bds.2017.v20i4.1456
Peralta FS, Pallos D, Queiroz CS, Ricardo LH (2015) Previous exposure to Cyclosporine A and periodontal breakdown in rats. Arch Oral Biol 60(4):566–573. https://doi.org/10.1016/j.archoralbio.2015.01.004
Klausen B, Evans RT, Sfintescu C (1989) Two complementary methods of assessing periodontal bone level in rats. Eur J Oral Sci 97(6):494–499. https://doi.org/10.1111/j.1600-0722.1989.tb00922.x
Uslu MÖ, Eltas A, Marakoglu İ, Dundar S, Şahin K, Özercan İH (2018) Effects of diode laser application on inflammation and mpo in periodontal tissues in a rat model. J Appl Oral Sci 26:e20170266. https://doi.org/10.1590/1678-7757-2017-0266
Garcia VG, Fernandes LA, de Almeida JM et al (2010) Comparison between laser therapy and non-surgical therapy for periodontitis in rats treated with dexamethasone. Lasers Med Sci 25(2):197–206. https://doi.org/10.1007/s10103-009-0678-z
Swerts AA, Santos BFE, Bruzadelli SR, Brigagão MRPL, Fernandes LA (2017) Treatment of experimental periodontal disease by laser therapy in simvastatin-modified rats. J Appl Oral Sci 25(4):387–395. https://doi.org/10.1590/1678-7757-2016-0467
Marques L, Holgado LA, Francischone LA, Ximenez JP, Okamoto R, Kinoshita A (2015) New LLLT protocol to speed up the bone healing process - histometric and immunohistochemical analysis in rat calvarial bone defect. Lasers Med Sci 30(4):1225–1230. https://doi.org/10.1007/s10103-014-1580-x
Farivar S, Malekshahabi T, Shiari R (2014) Biological effects of low level laser therapy. J Lasers Med Sci 5(2):58–62
Herranz-Aparicio J, Vázquez-Delgado E, Arnabat-Domínguez J, España-Tost A, Gay-Escoda C (2013) The use of low level laser therapy in the treatment of temporomandibular joint disorders. Review of the literature. Med Oral Patol Oral Cir Bucal 18(4):e603. https://doi.org/10.4317/medoral.18794
Lee JH, Chiang MH, Chen PH, Ho ML, Lee HE, Wang YH (2018) Anti-inflammatory effects of low-level laser therapy on human periodontal ligament cells: in vitro study. Lasers Med Sci 33(3):469–477. https://doi.org/10.1007/s10103-017-2376-6
Demirturk-Gocgun O, Baser U, Aykol-Sahin G, Dinccag N, Issever H, Yalcin F (2017) Role of low-level laser therapy as an adjunct to initial periodontal treatment in type 2 diabetic patients: a split-mouth, randomized, controlled clinical trial. Photomed Laser Surg 35(2):111–115. https://doi.org/10.1089/pho.2016.4117
Funding
This study was supported by the Research Support Foundation of Maranhão State (FAPEMA grant number 009155/2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experimental protocol was approved by the Ethics Committee on Animal Use of Federal University of Maranhão (CEUA/UFMA, protocol no. 32/15).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pereira, S.R.A., de Oliveira, I.C.V., Vieira, R.C. et al. Effect of photobiomodulation therapy as an adjunct to scaling and root planing in a rat model of ligature-induced periodontitis: a histological and radiographic study. Lasers Med Sci 35, 991–998 (2020). https://doi.org/10.1007/s10103-020-02952-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-02952-0