Abstract
The aim of this study was to compare low-level laser therapy (LLLT) as adjuvant treatment for induced periodontitis with scaling and root planing (SRP) in dexamethasone-treated rats. One-hundred twenty rats were divided into groups: D group (n = 60), treated with dexamethasone; ND group (n = 60) treated with saline solution. In both groups, periodontal disease was induced by ligature at the left first mandibular molar. After 7 days, the ligature was removed and all animals were subjected to SRP and were divided according to the following treatments: SRP, irrigation with saline solution (SS); SRP + LLLT, SS and laser irradiation (660 nm; 24 J; 0.428 W/cm2). Ten animals in each treatment were killed after 7 days, 15 days and 30 days. The radiographic and histometric values were statistically analyzed. In all groups radiographic and histometric analysis showed less bone loss (P < 0.05) in animals treated with SRP + LLLT in all experimental periods. SRP + LLLT was an effective adjuvant conventional treatment for periodontitis in rats treated with dexamethasone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontal disease is the result of collapse of the structures supporting the teeth, caused by the local action of periodontopathogenic microorganisms. These microorganisms release substances that injury periodontal tissues and induce tissue destruction by inflammatory and immunologic responses of the host. Systemic factors such as diabetes, tobacco and stress have been found to be associated with severe and/or rapidly progressive periodontitis [1]. Furthermore, some medications have an impact on the periodontium and its response to bacterial plaque [2].
In the past decades, organ transplantation has become an accepted treatment for a range of acquired and congenital disorders. Corticoids are commonly used to treat many different diseases because of their anti-inflammatory effect and immunosuppressant properties. Glucocorticoids link to receptors inside the cell and cause redistribution of the lymphocytes. They also reduce T-cell proliferation, with a decrease in interleukin-2, and they also downregulate interleukin-1 and interleukin-6, thereby curtailing inflammation [3].
Prolonged therapy with corticoids may cause osteoporosis, which is now regarded as a risk factor for periodontal disease [2]. The systemic use of drugs such as non-steroidal anti-inflammatory substances and their possible effects upon periodontal disease have been studied [1, 4–6]. Experimental studies have demonstrated that the use of corticoids can provoke many conditions, from gingival ulceration to the downward migration of the epithelium, attachment loss and disruption of transeptal fibers [4, 6]. In addition, the systemic use of high doses of glucocorticoids leads to inhibition of fibroblast activity, loss of collagen and connective tissue, with decreased re-epithelization and angiogenesis [7], reduction in the number and activity of the osteoblasts, and increased osteoclast function [8]. However, clinical studies are somewhat equivocal with respect to the effect of systemic glucocorticoids on periodontal tissues [5, 9].
The treatment of periodontal disease is based on the reduction of pathogenic microbiota by scaling and root planing [10]. However, mechanical therapy used on its own can fail to eliminate pathogenic bacteria that are located in the soft tissue and in areas inaccessible to the periodontal instrument, such as furcation areas and root depressions [11].
Systemic disease and adverse drug reactions are strategic challenges to the conventional periodontal treatment plan, leading to the use of complementary therapies to compensate for the intrinsic changes related to the periodontal repair process. Because of these limitations, adjuvant methods that promote the elimination of periodontal pathogens have called the attention of many researchers, who consider antibiotic and antiseptic use to be effective in periodontal treatment [12]. On the other hand, the literature also evidences uncountable research studies that demonstrate the selection and resistance of bacteria promoted by the overuse of antimicrobial drugs in periodontal therapeutics [13].
Utilization of low-level laser therapy (LLLT) as a biomodulation therapy has the advantages of promoting biomodulation in the tissue to be repaired, since laser therapy may increase mitochondrial respiration and adenosine triphosphate (ATP) synthesis [14], favor the repair process [15, 16], induce cell proliferation [17, 18], promote production of nucleic acid [19], and increase cell division [20] and collagen synthesis [15]. Additionally, it has a low bactericidal effect [21]. In bone tissue it can accelerate new bone formation, promoting increased osteoblastic activity [22], increased vascularization [23], and organization of collagen fibers [24].
Recent studies in animals [25–27] have examined the effects of LLLT on the evolution of periodontal disease. However, some studies have analyzed LLLT effects plus scaling and root planing (SRP) for periodontal treatment [28]. On the other hand, few studies have evaluated the effects of LLLT on immunosuppression [7]. In this context, the aim of our study was to compare the efficacy of low-level laser plus conventional mechanical therapy with scaling and root planing on alveolar bone loss in periodontitis induced in rats either treated or not treated with dexamethasone.
Materials and methods
Animals
This study was conducted on 120 adult male Wistar rats (120–140 g). The animals were kept in plastic cages with access to food and water ad libitum. Prior to surgical procedures, all animals were allowed to acclimatize to the laboratory environment for a period of 5 days. All protocols described below were approved by the Institutional Review Board of Araçatuba Dental School, São Paulo State University, Brazil (no. 22/06).
Experimental design
Drug administration
The animals (n = 120) were numbered and divided randomly into two groups, with 60 rats in each: D group was given injections of 2 mg/kg [7] body weight of dexamethasone (Decadron® 2 mg, Prodome) (Aché Pharmaceutical Laboratories SA, Campinas, São Paulo, Brazil); ND group (non-dexamethasone) was subjected to injections of 2 mg/kg [7] body weight of saline solution. The subcutaneous injections were initiated 24 h before the induction of periodontal disease and maintained every 3 days [6], during all the periods of killing.
The administration site was on the backs of the animals, next to the cephalic region, and the injections were always scheduled for the morning. The animals were weighed weekly with regard to dose maintenance throughout the experimental period.
Experimental periodontal disease
General anesthesia was obtained by ketamine (0.4 ml/kg) with xylazine (0.2 ml/kg), via intramuscular injection. One mandibular first left molar of each animal in the ND and D groups was selected to be given the cotton ligature (2-0) in a submarginal position to induce experimental periodontitis [26]. Seven days after the induction of periodontal disease, the ligature was removed from all animals in both groups. The left molars were submitted to scaling and SRP with manual curettes (n.13-14/mini-five, Hu-Friedy Co. Inc., Chicago, IL, USA) through ten distal-to-mesial traction movements. This was done by the same experienced operator. The animals in each group were randomly assigned to one of the two treatments: for SRP (n = 30/group), the mandibular left molars were submitted to SRP and irrigation with 1 ml of saline solution; for SRP + LLLT (n = 30/group), the mandibular left molars were submitted to SRP and irrigation with 1 ml of saline solution, followed by the application of low-intensity laser for 1 min afterwards.
SRP + LLLT treatment
The low-intensity laser used in this study was a gallium–aluminum–Arsenide (GaAlAs) (Laser Bio Wave LLLT; Kondortech Equipment, São Carlos, SP, Brazil) with a wavelength of 660 nm and a spot size of 0.07 cm2. After 1 min of saline solution application, low-intensity laser was applied to three equidistant points at each buccal and lingual aspect (mesial, central and distal) of the first mandibular molar in contact with the gingival tissue. The laser was released with power of 0.03 W for 133 s per point, power density of 0.428 W/cm2, and energy of 4 J per point (57.14 J/cm2 per point). The treated area received total energy of 24 J. Saline solution was deposited into the periodontal pocket slowly, with a syringe (1 ml) and an insulin needle (13 mm × 0.45 mm) (Becton Dickinson Ind. Ltd, Curitiba, PR, Brazil) without a bevel.
Experimental periods
Twenty animals from the ND and D groups (SRP = 10; SRP + LLLT = 10) were killed 7 days, 15 days and 30 days after the periodontal disease treatment by administration of a lethal dose of thiopental (150 mg/kg) (Cristália, Ltd, Itapira, SP, Brazil). Their jaws were removed and fixed in 10% neutral formalin for 48 h.
Laboratory procedures
The specimens were demineralized for 15 days in a solution of equal parts of 50% formic acid and 20% sodium citrate. Serial sections (6 µm) were obtained in a mesiodistal direction, embedded in paraffin, and stained with hematoxylin and eosin (HE) or Masson’s trichrome (MT).
Radiographic analysis
We removed the left mandibles to determine the degree of bone loss. Standardized radiographs were obtained with the use of digital radiographic images provided by the computerized imaging system Digora (Soredex, Orion Corporation, Helsinki, Finland), which uses a sensor instead of an X-ray film. Electronic sensors were exposed at 70 kV and 8 mA, with an exposure time of 0.4 s. The source-to-film distance was 50 cm. The distance between the cementum–enamel junction and the alveolar bone was determined for the mesial root surface of the mandibular left first molars [26]. Millimeters of bone loss on each radiograph were measured in a blind fashion three times by the same examiner.
Histological and histometric analysis
We examined the HE-stained sections by light microscopy to establish the bone loss and characteristics of the periodontal ligament in the furcation region of the first molars. Collagen fibers were examined in the sections stained with MT.
The area of bone loss in the furcation region was histometrically determined with an image analysis system (Image Tool, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA). After we had excluded the first and last sections where the furcation region was evident, we selected five equidistant sections of each specimen block and captured them with a digital camera connected to a light microscope. The mean values were averaged and compared statistically. In a blind fashion, one trained examiner selected the sections for histometric and histological analysis. Another qualified examiner conducted the histometric analysis, also in a blind fashion. The amounts of bone loss in each section were measured three times by the same examiner, on different days, to reduce variation in the data [27].
Statistical analysis
The hypothesis that there were no differences in bone loss rate in the furcation region between treatment groups was tested by Bioestat 3.0 software (Bioestat, Windows 1995, Sonopress Brazilian Industry, Manaus, AM, Brazil).
After the normality test, radiographic and histometric data were analyzed by Shapiro–Wilk test, and the intra-group and inter-group analysis by two-way analysis of variance (ANOVA; P < 0.05). When the ANOVA detected a statistical difference, multiple comparisons were performed with a Tukey test (P < 0.05).
Results
Clinical analysis
All the non-dexamethasone (ND) group of animals, regardless of their treatment, showed no clinical differences in general health, and weight gain was within the predicted range for healthy rats (Table 1).
The dexamethasone-treated group (D group) showed progressive weight loss, at a significant level, when compared with the animals not treated with dexamethasone (Table 1), which showed trends of immunosuppression and systemic changes.
Radiographic analysis
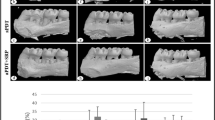
Intra-group radiographic analysis showed in both groups that there was significantly less bone loss in the animals treated by SRP + LLLT than by SRP in all experimental periods than (Fig. 1). In the inter-group radiographic analysis the comparison between the SRP treatment data showed that the animals in the D group presented greater bone loss than those in the ND group, but there was no significant difference in bone loss of SRP + LLLT treatment between the ND and D groups. In the ND group, treated with SRP, there was greater bone loss than in the D group, treated with SRP + LLLT, in all experimental periods; and in the ND group, treated with SRP + LLLT, there was less bone loss than in the D group, treated with SRP, in all experimental periods (Fig. 1).
Mean and standard deviationn = 10) of the distance between the cemento-enamel junction and the alveolar bone crest (in millimeters) on the mesial surface of the mandibular first molars for each group, treatment and period. * Significant difference with SRP + LLLT treatment in the same period and the same group (P < 0.05). ANOVA and Tukey tests, & significant difference between groups in the same treatment period(P < 0.05). ANOVA and Tukey tests, † significant difference inter-group and between treatment in the same period (P < 0.05). ANOVA and Tukey tests
Histological analysis
SRP treatment
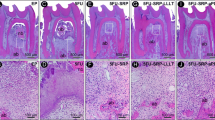
After 7 days, 15 days and 30 days, most specimens from the ND group that received the SRP treatment showed connective tissue with a high number of degenerating neutrophils and bone tissue with thin bone trabeculae and resorption areas (Fig. 2a, b). After 7 days, 15 days and 30 days, most specimens in the D group that received SRP treatment showed disorganized connective tissue, with a slight number of fibroblasts. There were areas of bone resorption with thin bone trabeculae and intense inflammatory infiltrate (Fig. 2c and d). The cementum surface in most specimens showed resorption areas.
Photomicrograph illustrating area of bone loss in the furcation region of the mandibular first molars in the two groups (ND and D) treated with SRP. a ND group, treatment with SRP for 30 days. Middle third into the furcation region. ×12.5. b Note the thin bone trabeculae with resorption activity. ×50. c D group, treatment with SRP for 30 days. Apical third into the furcation region. ×12.5. d Areas of bone resorption with thin bone trabeculae. ×50. All sections stained with HE
SRP + LLLT treatment
At 7 days, 15 days and 30 days, in most specimens from the ND group (Fig. 3a, b) and the D group (Fig. 3c, d) that received the laser treatment, the periodontal ligament was found to be intact, and organized, with parallel collagen fibers and lack of inflammatory infiltrate. The bone tissue showed organization, with thick bone trabeculae and no signs of resorption. The cementum surface did not show resorption areas.
Photomicrograph illustrating bone loss area in the furcation region of the mandibular first molars in the twp groups (ND and D) treated with SRP + LLLT. a ND group, treatment, treatment with SRP + LLLT for 30 days. Coronary third into the furcation region. ×12.5. b Thick bone trabeculae with no signs of resorption. ×50). c D group, treatment with SRP + LLLT for 30 days. Coronary third into the furcation region. ×12.5. d Thick bone trabeculae with no signs of resorption. ×50. All sections stained with HE
Histometric analysis
The histometric data are showed in Table 2. In the ND group, statistical analysis revealed greater bone loss in the rats treated with SRP (1.12 ± 0.13 mm2; 0.90 ± 0.27 mm2; 1.00 ± 0.16 mm2) than in those treated with SRP + LLLT (0.59 ± 0.04 mm2; 0.60 ± 0.04 mm2; 0.61 ± 0.03 mm2) for all experimental periods (Fig. 4a and c).
Photomicrograph illustrating bone tissue in the furcation region of the mandibular first molars in the two groups (ND and D) for different treatments a ND group, treatment with SRP for 30 days. b D group, treatment with SRP for 30 days. c ND group, treatment with SRP + LLLT for 30 days. d D group, treatment with SRP + LLLT for 30 days. All Masson’s trichrome, ×12.5
In the D group, statistical analysis of histometric data showed a greater bone loss in rats subjected to SRP treatment after 7 days, 15 days and 30 days (Fig. 4b) (1.65 ± 0.15 mm2; 1.71 ± 0.11 mm2 1,5 ± 0.25 mm2) than those subjected to SRP + LLLT treatment (0.64 ± 0.01 mm2; 0.63 ± 0.04 mm2; 0.65 ± 0.04 mm2) (Fig. 4d).
Histometrically, intergroup analysis demonstrated in ND group, treated with SRP (1.12 ± 0.13 mm2; 0.90 ± 0.27 mm2; 1.00 ± 0.16 mm2), a greater bone loss than in the D group, treated with SRP + LLLT, in all experimental periods (0.64 ± 0.01 mm2; 0.63 ± 0.04 mm2; 0.65 ± 0.04 mm2).
Discussion
In our study we reproduced a previously reported model of periodontitis in rats [26, 29] caused by a ligature around a tooth. This experimental model is characterized by accumulation of plaque, flattening and displacement of the gingival crest, increased proliferation of epithelium into underlying connective tissue, and infiltration of mononuclear inflammatory cells [29].
The aim of this study was to compare the influence of low-level laser therapy (LLLT) as an adjuvant treatment on induced periodontitis in rats treated with dexamethasone. In the study the induced periodontal disease was characterized by clinical signs of gingival inflammation, such as edema, redness and attachment loss. In the dexamethasone-treated animals (D), the clinical signs of gingival inflammation were more exacerbated, characterized as greater bone loss in the furcation region, disorganization of connective tissue, discreet fibroblasts and intense inflammatory infiltrate in all experimental periods, than in the non-treated rats (ND).
The animals treated with this drug presented lethargy, hematoma and alopecia at the time they were killed. Furthermore, there was a significant weight reduction during the study; this was probably because the drug decreases the gastrointestinal absorption of nutrients [30]. These changes have already been described by other authors [4], showing a trend towards immunosuppression and systemic changes.
The results of the study also demonstrated that group D animals had greater bone loss in the furcation area and more disorganized connective tissue than the group ND animals. These changes have been described in other studies that have also evaluated corticoid effects upon periodontal tissues [4, 6].
On the other hand, a clinical study has not demonstrated an influence of corticosteroid therapy on clinical parameters of periodontal disease in patients suffering from neurological disease [5]. The use of high doses of corticoid leads to a reduction in the number and activity of the osteoblasts and an increase in osteoclast function [8]. It also reduces gastrointestinal absorption of calcium, which, in turn, results in lower calcium blood levels and triggers parathyroid hormone (PTH) secretion that leads to systemic bone resorption [31].
In the analysis of the histometric results the group ND and D animals, which were subjected to low-level laser treatment, exhibited less significant bone loss than the animals treated with only SRP, in all experimental periods.
The scaling and root planing was not effective in controlling bone loss in the furcation areas in either animal group. Clinically, it is evident that SRP with hand instruments provides the best results for the treatment of periodontal disease [32]. However, several anatomic variations may limit the success of conventional SRP, such as root concavities, dental crowding, and deep pockets and furcation areas, which may hinder the access of hand instruments to the periodontal pocket. In this context, adjuvant periodontal therapies have been proposed to compensate for these limitations [33].
It was also evident in our study that group D animals, which received SRP + LLLT treatment, presented less bone loss than the group ND animals, which received only SRP treatment, in all experimental periods. Further, significant bone loss was observed in the animals treated with dexamethasone and subjected to SRP when they were compared with non-treated animals that had also received the same local treatment. There were no significant differences in alveolar bone loss in the SRP + LLLT treated areas between the animals not treated with dexamethasone and those treated with dexamethasone. In both groups (ND and D), radiographic examination showed that there was significant bone loss in the animals treated with SRP + LLLT in all experimental periods than in those undergoing SRP only.
In a previous study by our group [27] LLLT did not control bone loss in furcation regions for all periods, only after 15 days. The failure of LLLT to control bone loss in the previous study could be explained by the low action of the isolated use of LLLT on the viability of different bacteria. In this case the low action of LLLT can be explained by its discrete interaction with the bacteria on the tooth’s biofilm, because, in this research, the areas examined were not treated with mechanical instruments. However, in a clinical study on humans, Yilmaz et al. [28] did not observed beneficial effect of LLLT associated or not associated with conventional periodontal therapy. These controversial results must have been due to the differences in the parameters of irradiation utilized in the studies.
The action of LLLT on either the viability or activity of bacterial cells is species dependent [34] and directly related to absorption of laser wavelength by endogenous porphyrins, which are present in several bacteria [35]. Many bacteria do not have components able to absorb visible or infrared light, except for Porphyromonas gingivalis and Prevotella intermedia, which have protohemin and protoporphyrin, respectively [36]. Sigusch et al. [25] showed, in vivo, that the isolated application of laser represented no significant influence on the frequency of Porphyromonas gingivalis and Fusobacterium nucleatum. On the other hand, Karu [37] contraindicated the isolated use of laser in infected areas. However, several studies in the literature demonstrated the efficacy of LLLT to accelerate tissue repair in either normal or infected areas [38].
However, in our study, SRP + LLLT was more effective in the treatment of periodontal disease than was SRP alone. This was probably related to the ability of LLLT to promote angiogenesis, cell proliferation, control of the inflammatory process and acceleration of events involved in tissue repair [39]
Corticoids can delay the healing process by decreased angiogenesis and capillary proliferation [7], which reduce blood flow [40]. They also interfere in phagocytosis and antigen digestion, inhibiting macrophage migration and stabilizing lysosomes, avoiding the liberation of proteolytic enzymes. In addition, they modify fibroblast function, delaying their migration, and damaging type I and type II pro-collagen synthesis by modifying mRNA and mitotic activity [41]. Recent research by our group [7] in animals systemically treated with the same corticoid doses as used in this study has demonstrated a reduction in inflammatory infiltrate, higher epithelial differentiation, and a more intense differentiation repair process, with more collagen deposition in cutaneous wounds irradiated with low-intensity laser, but with distinct parameters from those in this study.
A possible explanation for the results of our study could be the biomodulatory action of the isolated low intensity laser. Studies have reported that this light source inhibits inflammatory mediator production by periodontal ligament cells, favors cellular chemotaxis, and promotes local vasodilatation and angiogenesis [39]. Thus, LLLT must promote increased oxygen diffusion through the tissue, favoring the repair process, because collagen secretion by fibroblasts in the extracellular space occurs only in the presence of high oxygen pressure [42].
Susceptibility to the irradiation and activation depends on the physiologic state of the irradiated cells. Cells with reduced redox potential (as in some pathologic states) are more sensitive to irradiation [37].
It should be mentioned that the biological effects of LLLT may be modified if some variables are considered: energy density, wavelength, power intensity, exposure time, and type of application [21, 43]. Also, the light intensity of laser should be enough to allow significant penetration into the tooth–gingiva interface [43].
Laser irradiation is able to modify cell behavior, dependent on wavelength, besides presenting complex light dispersion by the tissue components [44]. The laser’s power, the exposure time (liberated energy) and the spot beam diameter can have an influence on biologic tissues. Despite this, the factors inherent in irradiated tissue, such as pH, tissue color, thermal conductivity, water content, organic material and cell density, can also have an influence [21].
The systemic use of corticoids has been indicated in low and high doses for many treatments, such as mucocutaneous and respiratory diseases, tendinitis, bursitis, arthritis and cysts in general [45]. It is also used in all levels of immunotherapy, based on the patient’s need and the regimen prescribed by the individual practitioner [3]. One of the side effects of this drug is the increased risk of infection, because of the inhibitory effects of cell immunity, which could cause more severe periodontal damages [4, 6], as demonstrated in this study.
From these facts, the systemic use of antibiotics has been indicated as an adjuvant periodontal treatment to conventional SRP, in spite of the development of bacterial drug resistance [13]. Although the isolated use of low-intensity laser is not very effective in reducing the numbers of biofilm bacteria, it has a local action and does not promote development of the resistant bacterial strains.
Within the limits of this study, it can be concluded that low-intensity laser improved the results, as an adjuvant therapy to conventional treatment by SRP on experimental periodontal disease induced by bacterial plaque and modified by high doses of corticoid. Further, it led to reduced bone loss , to levels identical to those in the animals that suffered the same local treatment and that did not receive systemic applications of this drug. These encouraging results suggest that further experimental and clinical studies be carried out to determine effective parameters of irradiation for clinical applicability to the periodontal treatment of immunosuppressed patients.
References
Breivik T, Gundersen Y, Osmundsen H, Fonnum F, Opstad PK (2006) Neonatal dexamethasone and chronic tianeptine treatment inhibit ligature-induced periodontitis in adult rats. J Periodontal Res 41:23–32. doi:10.1111/j.1600-0765.2005.00833.x
Seymour RA (2006) Effects of medications on the periodontal tissues in health and disease. Periodontol 2000:120–129. doi:10.1111/j.1600-0757.2005.00137.x
Vasanthan A, Dallal N (2007) Periodontal treatment considerations for cell transplant and organ transplant patients. Periodontol 2000:82–102. doi:10.1111/j.1600-0757.2006.00198.x
Lipari WA, Blake LC, Zipkin I (1974) Preferential response of the periodontal apparatus and the epiphyseal plate to hydrocortisone and fluoride in the rat. J Periodontol 45:879–889
Safkan B, Knuuttila M (1984) Corticosteroid therapy and periodontal disease. J Clin Periodontol 11:515–522. doi:10.1111/j.1600-051X.1984.tb00903.x
Cavagni J, Soletti AC, Gaio EG, Rosing CK (2005) The effect of dexamethasone in the pathogenesis of ligature-induced periodontal disease in Wistar rats. Braz Oral Res 19:290–294. doi:10.1590/S1806-83242005000400010
Pessoa ES, Melhado RM, Theodoro LH, Garcia VG (2004) A histological assessment of the influence of low-intensity laser therapy on wound healing in steroid-treated animals. Photomed Laser Surg 22:199–204. doi:10.1089/1549541041438533
Sattler AM, Schoppet M, Schaefer JR, LC Hofbauer (2004) Novel aspects on RANK ligand and osteoprotegrin in osteoporosis and vascular disease. Calcif Tissue Int 74:103–106. doi:10.1007/s00223-003-0011-y
Oettinger-Barak O, Segal E, Machtei EE, Barak S, Baruch Y, Ish-Shalom S (2007) Alveolar bone loss in liver transplantation patients: relationship with prolonged steroid treatment and parathyroid hormone levels. J Clin Periodontol 34:1039–1045. doi:10.1111/j.1600-051X.2007.01153.x
Kaldahl WB, Kalkwarf KL, Patil KD (1993) A review of longitudinal studies that compared periodontal therapies. J Periodontol 64:243–253
Adriaens PA, Edwards CA, DeBoever JA, Loesche WJ (1988) Ultrastructural observations on bacterial invasion in cementum and radicular dentin of periodontally diseased human teeth. J Periodontol 59:493–503
Rams TE, Slots J (1996) Local delivery of antimicrobial agents in the periodontal pocket. Periodontol 2000:139–159. doi:10.1111/j.1600-0757.1996.tb00072.x
Van Winkelhoff AJ, Rams TE, Slots J (1996) Systemic antibiotic therapy in periodontics. Periodontol 2000:45–78. doi:10.1111/j.1600-0757.1996.tb00068.x
Yu W, Naim JO, McGowan M, Ippolito K, Lanzafame RJ (1997) Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria. Photochem Photobiol 66:866–871. doi:10.1111/j.1751-1097.1997.tb03239.x
Enwemeka CS (1990) Laser photostimulation. Clin Manage 10:24–29
Fahey TJ 3rd, Sadaty A, Jones WG 2nd, Barber A, Smoller B, Shires GT (1991) Diabetes impairs the late inflammatory response to wound healing. J Surg Res 50:308–313. doi:10.1016/0022-4804(91)90196-S
Haas AF, Isseroff RR, Wheeland RG, Rood PA, Graves PJ (1990) Low-energy helium-neon laser irradiation increases the motility of cultured human keratinocytes. J Invest Dermatol 94:822–826. doi:10.1111/1523-1747.ep12874679
Almeida-Lopes L, Rigau J, Zangaro RA, Guidugli-Neto J, Jaeger MM (2001) Comparison of the low level laser therapy effects on cultured human gingival fibroblasts proliferation using different irradiance and same fluence. Lasers Surg Med 29:179–184. doi:10.1002/lsm.1107
Saperia D, Glassberg E, Lyons RF, Abergel RP, Baneux P, Castel JC, Dwyer RM, Uitto J (1986) Demonstration of elevated type I and type III procollagen mRNA levels in cutaneous wounds treated with helium-neon laser. Proposed mechanism for enhanced wound healing. Biochem Biophys Res Commun 138:1123–1128. doi:10.1016/S0006-291X(86)80399-0
Passarella S, Casamassima E, Molinari S, Pastore D, Quagliariello E, Catalano IM, Cingolani A (1984) Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser. FEBS Lett 175:95–99. doi:10.1016/0014-5793(84)80577-3
Wilson M (1994) Bactericidal effect of laser light and its potential use in the treatment of plaque-related diseases. Int Dent J 44:181–189
Freitas LGF, Baranauskas V, Cruz-Höfling MA (2000) Laser effects on osteogenesis. Appl Surf Sci 154–155:548–554. doi:10.1016/S0169-4332(99)00431-6
Trelles MA, Mayayo E (1987) Bone fracture consolidates faster with low-power laser. Lasers Surg Med 7:36–45. doi:10.1002/lsm.1900070107
Garavello-Freitas I, Baranauskas V, Joazeiro PP, Padovani CR, Dal Pai-Silva M, da Cruz-Höfling MA (2003) Low-power laser irradiation improves histomorphometrical parameters and bone matrix organization during tibia wound healing in rats. J Photochem Photobiol B 70:81–89. doi:10.1016/S1011-1344(03)00058-7
Sigusch BW, Pfitzner A, Albrecht V, Glockmann E (2005) Efficacy of photodynamic therapy on inflammatory signs and two selected periodontopathogenic species in a beagle dog model. J Periodontol 76:1100–1105. doi:10.1902/jop. 2005.76.7.1100
Almeida JM, Theodoro LH, Bosco AF, Nagata MJ, Oshiiwa M, Garcia VG (2007) Influence of photodynamic therapy on the development of ligature-induced periodontitis in rats. J Periodontol 78:566–575. doi:10.1902/jop. 2007.060214
Almeida JM, Theodoro LH, Bosco AF, Nagata MJ, Oshiiwa M, Garcia VG (2008) In vivo effect of photodynamic therapy on periodontal bone loss in dental furcations. J Periodontol 79:1081–1088. doi:10.1902/jop. 2008.070456
Yilmaz S, Kuru B, Kuru L, Noyan U, Argun D, Kadir T (2002) Effect of gallium arsenide diode laser on human periodontal disease: a microbiological and clinical study. Lasers Surg Med 30:60–66. doi:10.1002/lsm.10010
Bezerra MM, de Lima V, Alencar VB, Vieira IB, Brito GA, Ribeiro RA, Rocha FA (2000) Selective cyclooxygenase-2 inhibition prevents alveolar bone loss in experimental periodontitis in rats. J Periodontol 71:1009–1014. doi:10.1902/jop. 2000.71.6.1009
Metzger Z, Klein H, Klein A, Tagger M (2002) Periapical lesion development in rats inhibited by dexamethasone. J Endod 28:643–645. doi:10.1097/00004770-200209000-00004
Suzuki Y, Ichikawa Y, Saito E, Homma M (1983) Importance of increased urinary calcium excretion in the development of secondary hyperparathyroidism of patients under glucocorticoid therapy. Metabolism 32:151–156. doi:10.1016/0026-0495(83)90221-4
Lindhe J, Westfelt E, Nyman S, Socransky SS, Heijl L (1992) Healing following surgical/non-surgical treatment of periodontal disease. J Clin Periodontol 9:115–128. doi:10.1111/j.1600-051X.1982.tb01227.x
Caton JG, Ciancio SG, Blieden TM (2001) Subantimicrobial dose doxycycline as an adjunct to scaling and root planing: post-treatment effects. J Clin Periodontol 28:782–789. doi:10.1034/j.1600-051X.2001.280810.x
Chan Y, Lai CH (2003) Bactericidal effects of different laser wavelengths on periodontopathic germs in photodynamic therapy. Lasers Med Sci 18:51–55. doi:10.1007/s10103-002-0243-5
Soukos NS, Wilson M, Burns T, Speight PM (1996) Photodynamic effects of toluidine blue on human oral keratinocytes and fibroblasts and Streptococcus sanguis evaluated in vitro. Lasers Surg Med 18:253–259. doi:10.1002/(SICI)1096-9101(1996)18:3<253::AID-LSM6>3.0.CO;2-R
Soukos NS, Som S, Abernethy AD, Ruggiero K, Dunham J, Lee C, Doukas AG, Goodson JM (2005) Phototargeting oral black-pigmented bacteria. Antimicrob Agents Chemother 49:1391–1396. doi:10.1128/AAC.49.4.1391-1396.2005
Karu T (1989) Photobiology of low-power laser effects. Health Phys 56:691–704. doi:10.1097/00004032-198905000-00015
Woodruff LD, Bounkeo JM, Brannon WM, Dawes KS, Barham CD, Waddell DL, Enwemeka CS (2004) The efficacy of laser therapy in wound repair: a meta-analysis of the literature. Photomed Laser Surg 22:241–247. doi:10.1089/1549541041438623
Houreld N, Abrahamse H (2007) In vitro exposure of wounded diabetic fibroblast cells to a helium-neon laser at 5 and 16J/cm2. Photomed Laser Surg 25:78–84. doi:10.1089/pho.2006.990
Durmus M, Karaaslan E, Ozturk E, Gulec M, Iraz M, Edali N, Ersoy MO (2003) The effects of single-dose dexamethasone on wound healing in rats. Anesth Analg 97:1377–1380. doi:10.1213/01.ANE.0000080611.29106.9E
Autio P, Oikarinen A, Melkko J, Risteli J, Risteli L (1994) Systemic glucocorticoids decrease the synthesis of type I and type III collagen in human skin in vivo, whereas isotretinoin treatment has little effect. Br J Dermatol 131:660–663. doi:10.1111/j.1365-2133.1994.tb04978.x
Reenstra WR, Veves A, Orlow D, Buras JA (2001) Decrease proliferation and cellular signaling in primary dermal fibroblasts derived from diabetics versus non diabetic sibling controls. Acad Emerg Med 8:519
Bhatti M, MacRobert A, Meghji S, Henderson B, Wilson M (1998) A study of the uptake of toluidine blue O by Porphyromonas gingivalis and the mechanism of lethal photosensitization. Photochem Photobiol 68:370–376. doi:10.1111/j.1751-1097.1998.tb09694.x
Pinheiro AL, Gerbi ME (2006) Photoengineering of bone repair processes. Photomed Laser Surg 24:169–178. doi:10.1089/pho.2006.24.169
No authors listed (2003) Position paper: oral features of mucocutaneous disorders. J Periodontol 74:1545–1556. doi:10.1902/jop.2003.74.10.1545
Acknowledgments
This work is attributed to the Department of Periodontology, Araçatuba Dental School, State University of São Paulo (UNESP) Araçatuba, São Paulo, Brazil. This study was financially supported by CAPES (Coordination for the Improvement of Higher Education Personnel; Brasilia, DF, Brazil) and CNPq (National Council for Scientific and Technological Development; Brasilia, DF, Brazil). The authors would also like to thank Bruno Theodoro Luciano from the Institute of International Relations of the University of Brasilia, for the English review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garcia, V.G., Fernandes, L.A., de Almeida, J.M. et al. Comparison between laser therapy and non-surgical therapy for periodontitis in rats treated with dexamethasone. Lasers Med Sci 25, 197–206 (2010). https://doi.org/10.1007/s10103-009-0678-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-009-0678-z