Abstract
To evaluate the effect of Er,Cr:YSGG laser, associated with fluoride application, on the prevention/control of dentin erosion. Dentin slabs were embedded in acrylic resin, flattened, and polished. Half of the specimens were previously eroded (10 min immersion in 1% citric acid solution) and half were kept sound. The specimens (n = 10 each substrate) were randomly allocated into the experimental groups, according to the following treatments: control (no treatment); APF gel (1.23% F, 1 min); Er,Cr:YSGG laser irradiation (P1: 0.25 W, 20 Hz, 2.8 J/cm2, tip S75, beam diameter of 750 μm, 1 mm away from the surface); Er,Cr:YSGG laser irradiation (P2: 0.50 W, 20 Hz, 5.7 J/cm2, tip S75, beam diameter of 750 μm, 1 mm away from the surface); APF gel + Er,Cr:YSGG laser P1 and; APF gel + Er,Cr:YSGG laser P2. Afterwards, the specimens underwent an erosion-remineralization cycling, consisting of a 5-min immersion into 0.3% citric acid, followed by 60-min exposure to artificial saliva. This procedure was repeated 4×/day, for 5 days. Surface loss (SL, in μm) was determined by optical profilometry. Specimens from each group were analyzed by environmental scanning electron microscopy (n = 3). Data were statistically analyzed (α = 0.05). For the eroded specimens, APF gel presented the lowest SL, being different from the control. For the sound specimens, none of the groups differed from the control, except for Er,Cr:YSGG laser P2, which presented the highest SL. When substrates were compared, only the eroded specimens of the control and APF + Er,Cr:YSGG laser P1 Groups showed higher SL. Selective structure removal was observed for the laser-treated groups. None of the Er,Cr:YSGG laser parameters were effective in the prevention/control dentin erosion. The laser was also unable to enhance the protection of fluoride against dentin erosion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental erosion is a condition that has received increasing attention from both, professionals and researchers, in the last few years. Many studies have been conducted in an attempt to reach better understanding of this process, along with its preventive/treatment therapies [1]. Induced by recurrent episodes of acid exposure, dental erosion can be defined as a loss of the dental hard tissues, without bacterial involvement [2, 3]. In this condition, the superficial layer of the tooth surface is dissolved. In enamel, first there is a loss of surface hardness, resulting in a softened layer, a few micrometers in depth. With continuous exposure to acid or due to the incidence of mechanical impacts, this layer can be lost [4, 5]. In dentin, at least in vitro, as the demineralization advances, it leaves a portion of insoluble collagen matrix intact, which has been shown to be relatively resistant to mechanical impacts [6, 7].
To prevent or control the progression of dental erosion, oral care products containing fluoride have been recommended [8,9,10,11]. Professional products have higher concentrations of fluoride [8], and many studies have indicated that they may present some effectiveness against erosion [8, 12, 13]. However, associating in-office fluoridated products with other therapies, such as high power lasers, has been suggested as a possible alternative to control erosion progression [9].
When combined with fluoride, high-power laser irradiation may potentially increase the deposition and incorporation of fluoride by the dental substrates [14,15,16,17], thus helping to control demineralization. There are several studies showing the ability of Nd:YAG, CO2, Argon, and Er:YAG lasers, by themselves, to increase the acid resistance of dental surfaces, leading to a decrease in the caries process [17,18,19]. However, when dental erosion is concerned, especially in the dentin substrate, the studies have shown controversial results, demanding further investigations. While de-Melo et al. [20] observed that diode laser irradiation might reduce the effect of erosion on root dentin specimens, some other studies have failed to find a significant protective effect of high power lasers against dentin erosion [12, 13, 21, 22].
Another high power-laser that has been used in the prevention and control of dental caries [23], but which was not fully explored in the context of dental erosion is the Er,Cr:YSGG laser (2.78 μm). Due to its high absorption by water and hydroxyl ions of hydroxyapatite, this laser is suitable for use in dentistry for cavity preparation, removal of caries lesions, soft tissue surgeries, among other applications [23]. With sub-ablative parameters, Er,Cr:YSGG laser irradiation can cause chemical, physical, and crystallographic changes in the dental hard tissues, increasing their acid resistance [23, 24]. In a previous study, in enamel, the irradiation with Er,Cr:YSGG laser with 8.5 J/cm2 resulted in significantly lower hardness loss compared with the control, after a cariogenic challenge [23]. This study also observed that when laser irradiation was used before acidulated phosphate fluoride (APF) application, there was an increase in the amount of fluoride on the enamel surface, when compared with the group that had been treated with fluoride only. Moslemi et al. [25] observed that the combination of APF with the Er,Cr:YSGG laser resulted in greater reduction of enamel demineralization, regardless of the order of application of the treatments. In relation to dental erosion, De Oliveira et al. [26] found that the irradiation of enamel surfaces with Er,Cr:YSGG laser, at a pulse frequency of 30 Hz and power of 0.50 W, was able prevent enamel erosion. In this study, no association with fluoride was tested. An investigation by our study group (non-published data) observed that irradiating enamel specimens with Er,Cr:YSGG laser (0.50 W, 20 Hz, 5.7 J/cm2) after fluoride application significantly reduced enamel erosion. However, there is still little information on the anti-erosive effect of this laser on dentin, especially on previously eroded dentin.
In view of the aforementioned, the aim of this in vitro study was to evaluate the effect of different protocols of Er,Cr:YSGG laser, either associated with fluoride application, or not, for the prevention and control of dentin erosion. The null hypotheses were (1) the different laser protocols would not be able to prevent or control the progression of dentin erosion and (2) the different laser protocols would not be able to increase the protective effect of fluoride against dentin erosion.
Materials and methods
Study design
This study followed a factorial 6 × 2 design. The study design and experimental groups are presented on Table 1.
Specimen preparation
For this study, bovine roots were used. Root dentin slabs (4 mm × 4 mm × 2 mm) were sectioned using a microtome (Isomet, Buehler, Lake Bluff, IL, USA) and embedded in acrylic resin (Varidur, Buehler). The resulting blocks were ground flat and polished, using the following sequence of abrasive papers: 800, 1200, 2400, and 4000 grit (Buehler), under constant water-cooling. At the end of each polishing procedure, the specimens underwent an ultrasonic bath with deionized water for 3 min. Specimens with no fractures or any other visual imperfections were selected. In half of the specimens (60 specimens), to make an initial erosion lesion, unplasticized polyvinyl chloride (UPVC) tapes were placed on their polished surfaces, leaving a central window of 4 mm × 1 mm exposed. Then the specimens were immersed in 1% citric acid solution (Sigma Aldrich, St. Louis, MO, USA, pH~2.4), at room temperature for 10 min. After immersion, the specimens were rinsed with deionized water. Afterwards, all the specimens were analyzed with an optical profilometer (as previously described [27]), to select those with curvature below 0.3 μm for the sound specimens, and surface loss values from 3 to 5 μm for the eroded specimens. The selected specimens were randomly assigned into the 12 experimental groups (n = 10).
Treatments
For the treatments, the tapes were placed on the polished surfaces of the sound specimens as well. APF gel application was performed with the aid of swabs, for 1 min, and removed with cotton rolls [23]. The Er,Cr:YSGG laser equipment used presented a wavelength of 2.78 μm, pulse width of 60 μs (H mode), fixed repetition rate of 20 Hz, and a power rate that can range from 0 to 6 W. The energy was delivered through an optical fiber with a beam diameter of 430 μm, with a sapphire tip of 750 μm in diameter and 6 mm in width (S75), 1 mm away from the surface, focused mode. For parameter 1, the following protocol were used: power of 0.25 W, repetition rate of 20 Hz, energy density of 2.8 J/cm2; for parameter 2: power of 0.50 W, repetition rate of 20 Hz, and an energy density of 5.7 J/cm2. Ten-second irradiations were performed, making three horizontal sweeping movements, under 30% air cooled without water, covering the entire surface of the lesion formed or the surface that was going to be submitted to cycling. APF gel was applied immediately before laser irradiation.
Erosive challenge
After treatments, all specimens were attached to the lids of 12-well cell culture plates, using sticky wax. Specimens were immersed in 0.3% citric acid (Sigma Aldrich, Darmstadt, Germany - natural pH~2.6), for 5 min, followed by a 60-min immersion in artificial saliva (0.213 g/l CaCl2 2H2O; 0.738 g/l KH2PO4; 1.114 g/l KCl; 0.381 g/l NaCl; 12 g/l Tris buffer, pH adjusted to 7.0 with 1 M HCl solution) [27]. This cycle was repeated four times a day for 5 days. All experimental procedures were conducted at room temperature. The acid was renewed after each episode of exposure, and the artificial saliva was renewed each day, before the cycle began.
SL assessment
For post-treatment and post-cycling, the tapes were removed from the specimens and their surfaces were analyzed. An area of 2 mm long (x axis) × 1 mm wide (y axis) was scanned with an optical profilometer (Proscan 2100, Scantron, Venture Way, Tauton, UK). The scan covered the treated area and protected reference surfaces on both sides. The step size was set at 0.01 mm and the number of steps at 200 in the x-axis and at 0.1 mm and 10, respectively, in the y-axis. The depth of the treated area was calculated based on subtracting the average height of the test area from the average heights of the two reference surfaces by using the dedicated software (Proscan Application software v. 2.0.17, Venture Way, Tauton, UK). The specimens were scanned in a moistened condition to prevent collagen shrinkage [28].
Environmental scanning electron microscopy evaluation
During post-treatments and post-cycling, three randomly selected specimens from each group were subjected to ESEM to qualitatively verify the superficial morphology of the specimens. Representative micrographs were taken at × 2000 magnification, by using Analy observation conditions, at the centre of each specimen with 15 Kv. No specimen preparation was required. In the qualitative assessment, the surface characteristics of micrographs were evaluated.
Statistical analysis
The SL data were tested for normality and homoscedasticity by Shapiro-Willks and Brown-Forsythe, respectively. Since data did not follow a normal distribution, they were analyzed by Kruskal-Wallis, Tukey, and Mann-Whitney tests, at a level of significance of 5%. The software SigmaPlot 13.0 (Systat Software Inc., Chicago, IL, USA) was used for the calculations.
Results
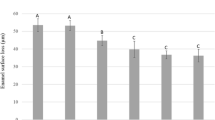
At baseline, the mean (SD) curvature for all the specimens was 0.12 μm (0.08). After initial lesion, the mean (SD) surface loss of the specimens was 3.17 (0.93). The medians of all groups, after treatments, are presented on Table 2. For the eroded and sound substrates, a small increase in surface loss occurred in the groups in which the laser was used, for both parameters. For the sound specimens, this effect was observed only when the laser was used with parameter 2. Surface loss values in the eroded specimens were higher than those in the sound type, for all the groups (p < 0.05).
The medians of surface loss, after the erosive cycling, for all experimental groups are shown in Table 3. For the eroded specimens, none of the groups differed significantly from the control (C), except for the fluoride group (F). F presented the lowest surface loss, with no difference from laser P1 and F + laser P1 (p < 0.05). For the sound specimens, none of the groups differed significantly from the control as well, except for laser P2, which presented significantly higher surface loss (p < 0.05). When sound and eroded specimens were compared, only the control and F + laser P1 groups showed significant difference between substrates.
In the ESEM images, post-treatment (Fig. 1), of the control group, a regular surface, with a few occluded dentin tubules could be observed. The images of the eroded control group showed a more irregular dentin surface, with opened tubules. In the APF gel group, a similar pattern was observed for both substrates, except for the presence of some particles over dentin, which were suggestive of calcium fluoride deposits, because of the size of the particles in relation to the dentinal tubules. In the laser-treated groups, with both parameters 1 and 2, it was possible to observe that a possible dentin conditioning occurred, enlarging the dentin tubule entrances. In some parts, it seemed that the laser removed portions of the dentin, forming a crater. For the eroded substrate, it seemed that the citric acid either removed the crater, or it was not formed.
After cycling (Fig. 2), this surface pattern remained for all groups, however, more discreetly.
Discussion
According to the results of the present study, both of our null hypotheses were accepted because, irrespective of the substrate, no laser protocol showed an ability to protect dentin from erosion, not even when associated with fluoride.
In a previous investigation performed by our study group (unpublished data), the use of an Er,Cr:YSGG laser, either combined with fluoride, or not, was tested against enamel erosion progression. Promising results were observed with the association of fluoride and this laser with parameter 2 (Er,Cr:YSGG laser P2 0.5 W, 5.7 J/cm2). A significant reduction in enamel surface loss was found, which encouraged us to test the anti-erosive effects of this laser in dentin as well. As dentin has distinct morphological aspects, it was reasonable to suppose that the interaction between the laser and this tissue would be also different. Dentin contains more water than enamel, which in turn, has a higher mineral content. In addition, erosion progresses differently in dentin than it does in enamel. In view of these factors, the laser parameters used should be individualized for each tissue.
The Er,Cr:YSGG laser energy is highly absorbed by water and hydroxyapatite, acting on the dental hard tissues through explosive thermo-mechanical ablation. In this mechanism, the water molecules within the hydroxyapatite crystals absorb the incident radiation, and the water vaporization results in increased internal pressure and micro explosions occur, leading to substrate ejection in the form of inorganic particles and to precise irradiated tissue removal [29, 30]. When used under the ablative threshold, it was suggested that the heating caused by the Er,Cr:YSGG laser irradiation would cause chemical and crystallographic changes on dental mineralized tissues, resulting in an increase in their acid resistance [31, 32]. Some other studies have suggested that laser irradiation could promote the formation of micro spaces in enamel, enhancing the incorporation or diffusion of fluoride through its structure, thus allowing the formation of a fluoride reservoir that would be relevant for dentin erosion protection [33, 34]. However, the results of the present study, testing this laser on dentin (with the same parameters previously tested on enamel) were less promising. The authors believe that the parameters used were extremely aggressive for dentin, especially when it was eroded, because this substrate contains more water than enamel. As Er,Cr:YSGG laser is absorbed by water to a larger extent, consequently, it caused more ablation of the surface. This fact could be observed in the profilometer measurements performed after treatment, in which different degrees of dentin surface loss occurred after laser irradiation. In the ESEM images was also possible to observe indications that the tissue was ablated.
As far as fluoride application was concerned, it only showed a protective effect for the eroded specimens; however, this effect was limited. This could be due to the low frequency of gel application, as it was applied only once before cycling. Monovalent fluoride compounds act on erosion mainly by surface protection, through the deposition of precipitates such as CaF2-like, which would act as a first barrier against erosive acids. These deposits can also serve as a reservoir, releasing fluoride and calcium into the medium at the time of the erosive challenge [12, 13]. However, due to the high aggressiveness of the erosive challenges, the protection offered by fluorides is usually of short duration, requiring frequent application, which may not compatible with the mode of use of professional products. Although the mechanism of action of fluoride on enamel and dentin are quite similar, the concentration of CaF2-like deposits in dentin was found to be sevenfold higher than it was in enamel, which could be explained by the smaller size of the hydroxyapatite crystals in dentin, resulting in a larger surface area, therefore a more reactive mineral phase [35]. Additionally, dentin is a more acid-soluble substrate than enamel [36], resulting in more calcium being released by the APF treatment, which would react with fluoride and precipitates as CaF2-like material [37]. However, despite all these characteristics, the presence of organic matrix was found to be a key factor for the effectiveness of fluoride against dentin erosion. Organic matrix is not only capable of slowing down demineralization, but in the presence of high amounts of fluoride, it is capable of stopping the process of erosion [38]. This may explain why fluoride only showed a protective effect for the eroded specimens, which probably had the organic matrix exposed because of the initial exposure to acid to create an initial lesion.
For the association between high power lasers and fluoride, some studies showed a significant synergism in reducing enamel demineralization and increasing fluoride retention [23]. One study showed that the firmly bound fluoride integrated into the crystalline structure may increase crystal stability and acid resistance [39]. In addition, the tightly bound fluoride can server as a fluoride reservoir [15, 39]. In a previous investigation performed by our group (non-published data), a significant reduction in enamel surface loss was observed when compared with the control (without treatment) with the combined use of Er,Cr:YSGG laser and fluoride, but not when these treatments were tested separately. Nevertheless, in the present study, this synergism was not observed. Although some reduction in surface loss was found for the sound specimens irradiated with the Er,Cr:YSGG laser protocol P1, when compared with APF + Er,Cr:YSGG laser P1, both treatments did not significantly differ from the control. Perhaps for dentin, this synergic effect warrants further investigations with the use of lower energy laser protocols.
In the present study, eroded and sound substrates were used. The option taken was to test both substrates, because most of the studies testing high power lasers against dentin erosion were performed on sound dentin specimens [21, 22, 40]. However, it is unlikely that this would be the substrate irradiated in the clinical scenario. It is likely that high power laser irradiation would be recommended to prevent erosion progression on already eroded surfaces. Additionally, one previous study by our group showed that the laser protocols usually applied on sound dentin might not be feasible for use on eroded dentin [12]; thus, it is necessary to establish different protocols for each substrate. Indeed, the parameter P1 used herein (parameter 1: 0.25 W, 20 Hz, 2.8 J/cm2) was unable to promote much change in the sound dentin, but some ablation occurred in the eroded substrate.
Conclusion
Considering the limitations of this in vitro study, it could be concluded that the Er,Cr:YSGG laser parameters tested were not effective in the control of dentin erosion progression. Laser irradiation was also not able to increase the protective effect of fluoride against dentin erosion.
Reference
Lussi A, Carvalho TS (2014) Erosive tooth wear: a multifactorial condition of growing concern and increasing knowledge. In: Monographs in oral science. pp 1–15
Eccles JD (1978) The treatment of dental erosion. J Dent 6:217–221. https://doi.org/10.1016/0300-5712(78)90245-2
Addy M, Shellis RP (2014) Interaction between attrition, abrasion and erosion in tooth wear. Monogr Oral Sci 20:17–31. https://doi.org/10.1159/000093348
Lussi A, Schlueter N, Rakhmatullina E, Ganss C (2011) Dental erosion - an overview with emphasis on chemical and histopathological aspects. Caries Res 45:2–12. https://doi.org/10.1159/000325915
Amaechi BT, Higham SM (2001) Eroded enamel lesion remineralization by saliva as a possible factor in the site-specificity of human dental erosion. Arch Oral Biol 46:697–703. https://doi.org/10.1016/S0003-9969(01)00034-6
Ganss C, Hardt M, Blazek D et al (2009) Effects of toothbrushing force on the mineral content and demineralized organic matrix of eroded dentine. Eur J Oral Sci 117:255–260. https://doi.org/10.1111/j.1600-0722.2009.00617.x
Ganss C, Schlueter N, Hardt M et al (2007) Effects of toothbrushing on eroded dentine. Eur J Oral Sci 115:390–396. https://doi.org/10.1111/j.1600-0722.2007.00466.x
Murakami C, Bönecker M, Corrêa MSNP et al (2009) Effect of fluoride varnish and gel on dental erosion in primary and permanent teeth. Arch Oral Biol 54:997–1001. https://doi.org/10.1016/j.archoralbio.2009.08.003
João-Souza SH, Bezerra SJC, Borges AB et al (2015) Effect of sodium fluoride and stannous chloride associated with Nd:YAG laser irradiation on the progression of enamel erosion. Lasers Med Sci 30:2227–2232. https://doi.org/10.1007/s10103-015-1791-9
Scaramucci T, Borges AB, Lippert F et al (2013) Sodium fluoride effect on erosion-abrasion under hyposalivatory simulating conditions. Arch Oral Biol 58:1457–1463. https://doi.org/10.1016/j.archoralbio.2013.06.004
Scaramucci T, Borges AB, Lippert F et al (2015) Anti-erosive properties of solutions containing fluoride and different film-forming agents. J Dent 43:458–465. https://doi.org/10.1016/j.jdent.2015.01.007
João-Souza SH, Scaramucci T, Hara AT, Aranha ACC (2015) Effect of Nd:YAG laser irradiation and fluoride application in the progression of dentin erosion in vitro. Lasers Med Sci:30. https://doi.org/10.1007/s10103-015-1802-x
Steiner-Oliveira C, Nobre-dos-Santos M, Zero DT et al (2010) Effect of a pulsed CO2 laser and fluoride on the prevention of enamel and dentine erosion. Arch Oral Biol 55:127–133. https://doi.org/10.1016/j.archoralbio.2009.11.010
Goodman BD, Kaufman HW (1977) Effects of an argon laser on the crystalline properties and rate of dissolution in acid of tooth enamel in the presence of sodium fluoride. J Dent Res 56:1201–1207
Gao X-L, Pan J-S, Hsu C-Y (2006) Laser-fluoride effect on root demineralization. J Dent Res 85:919–923. https://doi.org/10.1177/154405910608501009
Tepper SA, Zehnder M, Pajarola GF, Schmidlin PR (2004) Increased fluoride uptake and acid resistance by CO2 laser-irradiation through topically applied fluoride on human enamel in vitro. J Dent 32:635–641. https://doi.org/10.1016/j.jdent.2004.06.010
Liu Y, Hsu C-YS, Teo CMJ, Teoh SH (2013) Potential mechanism for the laser-fluoride effect on enamel demineralization. J Dent Res 92:71–75. https://doi.org/10.1177/0022034512466412
Tavares JG, Eduardo C de P, Burnett LH et al (2012) Argon and Nd:YAG lasers for caries prevention in enamel. Photomed Laser Surg 30:433–437. https://doi.org/10.1089/pho.2011.3104
Esteves-Oliveira M, El-Sayed KF, Dörfer C, Schwendicke F (2016) Impact of combined CO2 laser irradiation and fluoride on enamel and dentin biofilm-induced mineral loss. Clin Oral Investig. https://doi.org/10.1007/s00784-016-1893-1
De-Melo MAS, Passos VF, Alves JJ et al (2011) The effect of diode laser irradiation on dentin as a preventive measure against dental erosion: an in vitro study. Lasers Med Sci 26:615–621. https://doi.org/10.1007/s10103-010-0865-y
Magalhães AC, Rios D, Machado MADA et al (2008) Effect of Nd:YAG irradiation and fluoride application on dentine resistance to erosion in vitro. Photomed Laser Surg 26:559–563. https://doi.org/10.1089/pho.2007.2231
Wiegand A, Magalhães AC, Navarro RS et al (2010) Effect of titanium tetrafluoride and amine fluoride treatment combined with carbon dioxide laser irradiation on enamel and dentin erosion. Photomed Laser Surg 28:219–226. https://doi.org/10.1089/pho.2009.2551
Ana PA, Tabchoury CPM, Cury JA, Zezell DM (2012) Effect of Er,Cr:YSGG laser and professional fluoride application on enamel demineralization and on fluoride retention. Caries Res 46:441–451. https://doi.org/10.1159/000333603
Fried D, Visuri SR, Featherstone JD et al (1996) Infrared radiometry of dental enamel during Er:YAG and Er:YSGG laser irradiation. J Biomed Opt 1:455–465. https://doi.org/10.1117/12.250668
Moslemi M, Fekrazad R, Tadayon N et al (2009) Effects of ER,Cr:YSGG laser irradiation and fluoride treatment on acid resistance of the enamel. Pediatr Dent 31:409–413
de Oliveira RM, de Souza VM, Esteves CM et al (2017) Er,Cr:YSGG laser energy delivery: pulse and power effects on enamel surface and erosive resistance. Photomed Laser Surg 35:639–646. https://doi.org/10.1089/pho.2017.4347
Pereira LGS, Joao-Souza SH, Bezerra SJC et al (2017) Erratum to: Nd:YAG laser irradiation associated with fluoridated gels containing photo absorbers in the prevention of enamel erosion (Lasers in Medical Science, (2017), 32, 7, (1453-1459), 10.1007/s10103-017-2226-6). Lasers Med. Sci 32:1461
Attin T, Becker K, Roos M et al (2009) Impact of storage conditions on profilometry of eroded dental hard tissue. Clin Oral Investig 13:473–478. https://doi.org/10.1007/s00784-009-0253-9
Meister J, Franzen R, Forner K et al (2006) Influence of the water content in dental enamel and dentin on ablation with erbium YAG and erbium YSGG lasers. J Biomed Opt 11:34030. https://doi.org/10.1117/1.2204028
Ramos TM, Ramos-Oliveira TM, de Freitas PM et al (2015) Effects of Er:YAG and Er,Cr:YSGG laser irradiation on the adhesion to eroded dentin. Lasers Med Sci 30:17–26. https://doi.org/10.1007/s10103-013-1321-6
Ramalho KM, Hsu C-YS, de Freitas PM et al (2015) Erbium lasers for the prevention of enamel and dentin demineralization: a literature review. Photomed Laser Surg 33:301–319. https://doi.org/10.1089/pho.2014.3874
de Freitas PM, Rapozo-Hilo M, Eduardo C de P, JDB F (2010) In vitro evaluation of erbium, chromium:yttrium-scandium-gallium-garnet laser-treated enamel demineralization. Lasers Med Sci 25:165–170. https://doi.org/10.1007/s10103-008-0597-4
Zamataro CB, Ana PA, Benetti C, Zezell DM (2013) Influence of Er,Cr:YSGG Laser on CaF 2-like products formation because of professional acidulated fluoride or to domestic dentifrice application. Microsc Res Tech 76:704–713. https://doi.org/10.1002/jemt.22221
Nammour S, Demortier G, Florio P et al (2003) Increase of enamel fluoride retention by low fluence argon laser in vivo. Lasers Surg Med 33:260–263. https://doi.org/10.1002/lsm.10219
ten Cate JM, Damen JJ, Buijs MJ (1998) Inhibition of dentin demineralization by fluoride in vitro. Caries Res 32:141–147
Hoppenbrouwers PM, Driessens FC, Borggreven JM (1986) The vulnerability of unexposed human dental roots to demineralization. J Dent Res 65:955–958. https://doi.org/10.1177/00220345860650071101
Falcão A, Masson N, Leitão TJ et al (2016) Fluoride rinse effect on retention of CaF2 formed on enamel/dentine by fluoride application. Braz Oral Res 30. https://doi.org/10.1590/1807-3107BOR-2016.vol30.0031
Ganss C, Klimek J, Starck C (2004) Quantitative analysis of the impact of the organic matrix on the fluoride effect on erosion progression in human dentine using longitudinal microradiography. Arch Oral Biol 49:931–935. https://doi.org/10.1016/j.archoralbio.2004.05.010
Geraldo-Martins VR, Lepri CP, Faraoni-Romano JJ, Palma-Dibb RG (2014) The combined use of Er,Cr:YSGG laser and fluoride to prevent root dentin demineralization. J Appl Oral Sci 22:459–464. https://doi.org/10.1590/1678-775720130570
Jordão MC, Forti GM, Navarro RS et al (2016) CO2 laser and/or fluoride enamel treatment against in situ/ex vivo erosive challenge. J Appl Oral Sci 24:223–228. https://doi.org/10.1590/1678-775720150399
Acknowledgments
The authors would like to thank FAPESP (São Paulo Research Foundation, for the scholarship 2016/25883-0), and LELO (Special Laboratory of Lasers in Dentistry from the Department of Restorative Dentistry at the School of Dentistry of the University of São Paulo, Brazil).
Funding
The work was supported by the São Paulo Research Foundation (FAPESP, scholarship no. 2016/25883-0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Bezerra, S.J.C., Trevisan, L.R., Viana, I.E.L. et al. Er,Cr:YSGG laser associated with acidulated phosphate fluoride gel (1.23% F) for prevention and control of dentin erosion progression. Lasers Med Sci 34, 449–455 (2019). https://doi.org/10.1007/s10103-018-2609-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-018-2609-3