Abstract
Increasing rates of non-carious cervical lesions due to dental erosion, exposure of dentinal tubules, and hypersensitivity to environmental stimuli have led to the development of new prevention strategies. This study evaluated the effects of a low-intensity diode laser (λ = 808 nm) on the dentinal chemical composition and prevention of demineralization. In addition, the study monitored temperature changes during the course of irradiation. Forty dentin specimens were randomly allocated into four groups (n = 10): G1 – No treatment (control), G2 – irradiated with 15 J/cm2, G3 – irradiated with 30 J/cm2, and G4 – irradiated with 60 J/cm2. Each specimen was partially covered with nail varnish, treated according to the group irradiation levels, and exposed to an erosive challenge (1.0 M hydrochloric acid) for 5 min. Afterwards, dentin loss was profilometrically analyzed and examined by scanning electron microscopy (SEM) combined with energy dispersive X-ray (EDX). Intrapulpal temperatures were measured during the dentin irradiation. One-way ANOVA and Tukey tests (p < 0.05) were performed to assess differences. For all irradiated groups, intrapulpal temperature changes were less than 3°C. The G2 group showed statistically significant differences when compared to the other groups, representing the lowest temperature increase. A quantitative element analysis via EDX did not significantly differ (p < 0.05) for Ca, P, F, O, or C between the four groups when measured after irradiation/erosion. The mean wear rates (± SD, μm) were 35.66 ± 7.28; 40.70 ± 5.03; 38.17 ± 10.81 and 25.25 ± 6.87 for G1–G4, respectively. The G4 group statistically differed from all other groups representing the lowest wear rate. These results suggest that dentin irradiation, using a diode laser with levels set at 60 J/cm2, may induce inhibitory effects on root dentin demineralization without causing any harmful thermal effects. However, the exact mechanism of the action of the laser remains unclear.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Various lifestyle changes and the advent of new dietary habits may be related to the increased loss of hard dental substrates by corrosive processes for many individuals today [1, 2]. The higher consumption of foods, drinks, and medicines with dental demineralizing properties, as well as the prevalence of chronic conditions such as gastroesophageal reflux disease [3] and feeding disorders as bulimia and anorexia [4] have all contributed to a higher incidence of non-carious lesions. Dental erosion involves a multifactorial pathogenesis where chemical, biological, and behavioral factors are related to the outcome, which is progressive and irreversible demineralization of the outer layer of the dental tissues [5, 6].
Severe corrosive injuries may affect not only the enamel surface but can also lead to the exhibition of the coronary or root dentin [7]. Demineralization in dentin occurs much more quickly than in enamel because the former has a lower mineral content [8]. An aging population [9, 10], coupled with cumulative damages from years of exposure to both mechanical and environmental factors (e.g., gingival recession, abrasion, abfraction, and erosion) that lead to the loss of enamel and/or cementum, has led to an increased incidence of dentinal sensitivity due to the exposure of dentinal tubules [11–13].
Because the prevalence of dental erosion has been increasing, appropriate diagnostic methods and approaches to control this pathogeneses are important [1, 14–16]. Preventive strategies for dental erosion include dietetic advising, stimulation of salivary flow, optimization of fluoride regimes, modification of erosive drinks, and appropriate approaches to oral hygiene. All of these strategies aim to minimize the demineralization resulting from the action of non-bacterial acids [17–19]. However, controlling all etiological factors related to dental erosion is difficult because most of them are dependent on patient compliance. Thus, other strategies, such as laser treatment, have been developed to prevent or arrest dentin erosion and, consequently, hypersensitivity [20].
Several studies have demonstrated that high-power laser irradiation (Nd:YAG, argon, CO2, and Er:YAG) may reduce the progression of the demineralization process. However, despite this beneficial effect, such lasers may also cause undesirable thermal alterations [21, 22] and are often also more expensive and less accessible.
In the search for alternatives to protect dental tissues from constant exposure to high acidity, researchers have shown that the use of the diode lasers at wavelengths in the visible and near-infrared regions may lead to an increase in the resistance of teeth against demineralization [22, 23]. The effects of diode laser irradiation in mineralized dental tissues have not been explored in depth, whereas more emphasis has been placed on investigating the caries-preventive effects of high-intensity lasers used in clinical dentistry settings.
Therefore, the aim of the present in vitro study is to investigate the effects of a low-intensity diode laser on dentin demineralization and chemical composition, as well as the possible temperature changes during the laser treatment.
Materials and methods
Experimental design and specimen preparation
The Research and Ethics Committee of the Federal University of Ceará Medical School (Process # 143/2009) approved the collection and use of extracted human teeth for all of the experiments conducted in this in vitro study. Initially, 60 extracted human third molars that had more than two-thirds of the formed roots stored in 0.01% (w/v) thymol solution at 4°C were used.
Forty dentin slabs (4 × 4 × 2 mm) were cut from 60 roots using water-cooled diamond saw disks (Extec Corp., Enfield, CT, USA) and a cutting machine (IsoMet Low Speed Saw, Buehler, Lake Bluff, IL, USA). Next, the slabs were embedded in Pre-30 self-polymerized acrylic resin cylinders to facilitate handling (Arotec S.A. Indústria e Comércio, Cotia, SP, Brazil), serially flattened with water-cooled abrasive discs (320, 600, and 1,200-grit Al2O3 papers; Buehler), and polished with felt paper and diamond spray (1 μm; Buehler). This procedure resulted in the removal of a layer of dentin about 200 μm in depth.
The surface microhardness was determined by performing five indentations (Knoop diamond, 25 g, 5 s, FM100, Future Tech, Tokyo, Japan) in the center of the dentin surface, for selection and randomized distribution purposes. Dentin slabs with microhardness values ranging from 53–65 Knoop hardness number (KHN) (59.15 ± 5.91 KHN) were randomly assigned according to a computer-generated randomization list [24] into the following four groups (n = 10): (1) control, (2) laser treatment with 15 J/cm2, (3) laser treatment with 30 J/cm2, and (4) laser treatment with 60 J/cm2.
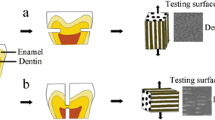
To provide matched pairs, each slab was then divided into two halves. One half of each specimen served as the test surface for laser irradiation. The other half of each specimen was covered with an acid-resistant varnish in a dark color (Colorama, CEIL Coml. Exp. Ind. Ltd., São Paulo, SP, Brazil) and was not treated any further. The application of the acid-resistant varnish was performed not only over one-half of the exposed area of each slab but also over the surrounding acrylic resin. This procedure enabled the removal of the whole varnish layer without affecting the dentin surface control area, as the beginning of the removal procedure was carried out in the acrylic resin with the application of light pressure using a scalpel blade.
The specimens were stored in humid conditions throughout the study to prevent the dentin surface from drying.
Pellicle formation
Whole saliva was collected 1 h after breakfast from 20 healthy volunteers. The subjects chewed paraffin wax for 5 min to stimulate saliva secretion. Next, the saliva from the first minute of chewing was swallowed while the rest was collected and subsequently introduced into centrifuge tubes [25]. The saliva samples were then centrifuged for 10 min at 2,000 rpm in a pre-cooled centrifuge (4°C) (MPW Med-Instruments, MPW-350R, Warsaw, Poland) and filtered by a vacuumed filtration device (0.45 Stericup Express, Millipore, Billerica, MA, USA). The clear fluid located above the sediments was pooled and used for pellicle formation [26]. Each slab group was immersed independently in clarified saliva (30 ml per group) and incubated under agitation at 100 rpm for 24 h at room temperature (24 ± 1°C) prior to the laser treatment.
Laser treatment
The samples from groups 2, 3, and 4 were irradiated with a low-level gallium-aluminum-arsenide (GaAlAs) diode laser 'Whiteness lase II' (DMC, São Carlos, SP, Brazil) at a wavelength of 808 nm in the infrared spectrum. This laser consisted of a class 3B system with a continuous wave and an adjustable output energy ranging from 30 to 100 mW and a spot size of 0.6 mm. A power meter 'Lasermate' (Coherent Inc, Santa Clara, CA, USA) was used to measure the maximum output power.
To promote an area of radiation that included the whole surface of the selected area, the laser probe was held perpendicular to the tooth surface (Fig. 1). Energy densities of 15 J/cm2 and higher were used for each treatment, since this energy density was shown to be optimal in a previous study [22]. Therefore, the average energy densities were calculated to be 15, 30, and 60 J/cm2. Using the output power of 100 mW, the necessary exposure times in order to reach the chosen densities were calculated according the following formula: \( {\hbox{density of energy }} = {\hbox{ power }}\left( {\hbox{W}} \right){\hbox{ x time }}\left( {\hbox{s}} \right){ }/{\hbox{ Area }}\left( {{\hbox{c}}{{\hbox{m}}^{{2}}}} \right) \).
Erosive challenge
Over the surface of each sample, 5 μl of 1.0 M hydrochloric acid (Synth, Diadema, SP, Brazil) was statically applied for 5 min [22]. This procedure simulates an acid challenge, representing the normal values similar to the conditions of gastric reflux (gastric secretions pH < 4) [27].
Subsequently, all of the specimens were rinsed with deionized water and dried with absorbent paper. The varnish was carefully removed using acetone and a scalpel blade without touching the dentin surface [20]. The specimens were then analyzed by mechanical profilometry.
Profilometry
The levels of dentin wear were determined in relation to the reference surfaces (covered by the nail varnish), using a profilometer Hommel Tester T1000 (Hommelwerke GmbH, Germany). At intervals of 100 μm, five profile traces (1.5 mm in length) were recorded for each specimen. These profilometric traces were taken by moving the stylus from the reference surface to the exposed surface. For each sample, the mean values obtained from the five traces were calculated.
Energy dispersive X-ray (EDX) spectrometry
Three dentin samples from each group were selected for this analysis. This technique was used to detect alterations in the distribution of principal elements present in the sample. The specimens from each group were mounted on aluminum stubs that were coated with carbon. Five points in each surface were randomly selected around the surface area. Normalized high-resolution spectra of the main elements’ concentration in weight % were performed and later calculated by an energy-dispersive X-ray spectrometer (EDX), using the backscattered electron detector attached to a scanning electron microscope TESCAN SEM (Model VEGA II\XMU, Brno, Czech Republic) operating at 30 kV. Data acquisition and analysis were performed using Quantax 800 software (Bruker AXS, Karlsruhe, Germany).
Temperature measurements
For this analysis, one 1-mm cylindrical preparation was made using a #4 steel-spherical drill (Maillefer, Ballaigues, Switzerland) in a low-speed hand piece on the inferior surface of the acrylic resin cylinder, where each dentin slab was inserted. The preparation was extended until the dentin slab was reached in order to allow contact between the thermocouple and the dentin slab [28]. One K-type thermocouple (wire diameter of 0.25 mm) was coupled to a digital portable thermometer (model MT 600, Minipa, São Paulo, Brazil) with a resolution of 0.1°C, basic precision of 0.1 ± 0.7%, and the capacity to measure temperatures ranging from 0.1 to 200°C. Additionally, a thermally conductive paste (Implastec, Votoratim, São Paulo, Brazil) was used to attach the thermocouple to the slab maintaining thermal contact with the internal surface of the slab [29].
During irradiation, the samples were positioned over a device to assure that they were submerged in a water bath (37 ± 0.1°C) during the testing procedures (Fig. 1). This method effectively stabilized the internal baseline temperature at 37°C. This also minimized the effects of ambient temperature changes and provided a consistent initial temperature for each data set [29, 30]. The thermocouple wire was connected to a data logger (Temp Monitor Software Minipa, SP, Brazil) and the mean of the differences between the baseline and the highest temperature was calculated for each specimen (ΔT parameter) and for each group.
Statistical analysis
Mean values of wear per group, ΔT, and elemental concentration were calculated. To assess the effectiveness of the treatments, the dependent variables were analyzed; the assumptions of equality of variances (Levene's test) and normal distribution of errors (Shapiro Wilk test) were verified. Profilometry and ΔT data were analyzed by one-way ANOVA followed by a Tukey-Kramer test. Data measuring element concentration in normalized wt% were subjected to a paired t test because each group’s reference surfaces were compared to the irradiated surfaces. The significance level was set at 5%. The software program BioStat 2007 Professional (Analyst Soft Robust business solutions company, Vancouver, British Columbia, Canada) was used.
Results
The diode laser treatment at 60 J/cm2 (G4) significantly reduced dentin loss (p <0.05) when compared to the control (G1) and the other irradiated groups (G2 and G3). In contrast, significant differences for groups G2 and G3 were not identified when compared to the control group, and they did not significantly differ from each other (Fig. 2).
In the EDX analysis, the primary elements observed in the analysis of dentin were calcium (Ca), phosphorus (P), oxygen (O), and carbon (C). Minor quantities of other elements were also detected but were not considered in the present study. The Ca, P, O, and C wt% detected in the samples are summarized in Table 1. A quantitative element analysis revealed no statistically significant differences in Ca, P, C, and O content between the reference and irradiated halves of the specimens in any groups (p > 0.05). Thus, a representative change in structural composition is unlikely.
Figure 3 presents ΔT results according to the groups. Group G4 (60 J/cm2) had the highest ΔT value, which was statistically different from G2 (15 J/cm2), but similar to G3 (30 J/cm2). The lowest ΔT value was found for group G2, which did not show a statistically significant difference from group G3.
Discussion
In this study, the effectiveness of the low-power infrared laser (LPIRL) in preventing dentin erosion was investigated. We compared the use of LPIRL alone, at three different energy densities. Some degree of protection against erosive challenges was found for the group lased with 60 J/cm2.
Although high-power lasers may significantly increase the acid resistance of enamel [21, 31, 32], similar effects have not been observed on dentin, potentially minimizing its use as a strategy to control dental erosion [33]. In addition, due to their inherent cost of instrumentation, high-power lasers are not yet widely employed in routine dentistry, especially in developing countries. Consequently, low-power red and near-infrared lasers (such as the 808-nm diode laser) appear to be alternative approaches, because reports in literature suggest that their application can possibly induce modification of the organic matrix content of enamel, which may then lead to an increased resistance against demineralization. This is particularly important when considering that maintenance of the organic matrix in the dentin lesion might be important in reducing the rate of erosive progression [34]. Another important point to consider is the weight composition of dentin, which is 70% mineral hydroxyl apatite, 20% organic material, and 10% water. This is different from enamel, which consists of 96% mineral with water and organic material composing the remainder [35].
Only a few studies have investigated the effects of diode lasers on acid resistance, either with or without a fluoride varnish [22]. Indeed, to the best of our knowledge, there have been no studies involving the use of low-level lasers to irradiate dentin as a protective mechanism against erosive challenge to dental tissues. Accordingly, our results are not directly comparable to other available studies. However, one may speculate that the interaction between laser energy and dentin may have caused protein denaturation, which can result in the blockage of the diffusion pathway in dentin, a process similar to that in dental enamel irradiated by high-power lasers [36]. Indeed, the consolidation of the protein component of the organic matrix after denaturation might have produced a significant reduction in the crystal surface area that is made available to acid decalcification [37]. It should be emphasized that no dose-response was observed with the laser application because higher wear values were found for groups G2 and G3 when compared to the control group. It is possible that, in these groups, the power density threshold needed to induce changes on dentin was not reached.
With regard to the EDX results, the lower Ca and P contents after treatment confirm the profilometric results, which showed loss of dentin tissue after acid challenge caused by particularly aggressive demineralization, when compared to those contents found in the control halves of the specimens. However, even with lower decreases in Ca and P percentages for irradiated groups when compared to controls, these losses were detected only by profilometry analysis when dentin was irradiated with 60 J/cm 2. This may be partially explained by the great variability found in EDX results even in the control halves of the specimens. Thus, it can be suggested that EDX may be a useful tool for selecting specimens with standard concentrations of chemical constituents. Nevertheless, the information obtained by EDX involves only the presence of entrapped molecular elements and is unable to show molecular characteristics. Specifically, it is unable to recognize different mineral phases or discriminate between the same minerals of various origins. Consequently, since the same chemical elements are present in both the irradiated and unirradiated enamel samples, this technique might not have been the most appropriate strategy for detecting changes caused by laser irradiation. As such, these assumptions should be confirmed by additional research [38] using another analysis such as Raman spectroscopy.
When using lasers in vivo, the thermal effects on pulpal tissues are a cause of concern. Low-power lasers are advantageous for having a lower possibility of promoting thermal damages to the dental tissue when compared to higher-powered lasers. According to the present study, even when the laser was used at 60 J/cm2 (the highest energy density applied), temperature increases of less than 3°C were found, which is considered a temperature threshold capable of causing reversible inflammatory responses within the pulp, based on previous studies on root dentin [39].
Conclusions
These results suggest that dentin irradiation with a diode laser operating at 60 J/cm2 may induce inhibitory effects on root dentin demineralization without causing any harmful thermal effect, which supports the application of this type of laser to conservative dentistry. However, the exact mechanism of the action of the laser remains unclear.
References
Lussi A, Jaeggi T (2008) Erosion—diagnosis and risk factors. Clin Oral Invest 12(Suppl 1):5–13. doi:10.1007/s00784-007-0179-z
Abrahamsen TC (2005) The worn dentition – pathognomonic patterns of abrasion and erosion. Int Dent J 55:268–276
Gregory-Head BL, Curtis DA, Kim L, Cello J (2000) Evaluation of dental erosion in patients with gastroesophageal reflux disease. J Prost Dent 83(6):675–680. doi:10.1067/mpr.2000.107193
Dynesen AW, Bardow A, Petersson B, Nielsen LR, Nauntofte B (2008) Salivary changes and dental erosion in bulimia nervosa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106(5):696–707. doi:10.1016/j.tripleo.2008.07.003
Bartlett DW (2005) The role of erosion in tooth wear: a etiology, prevention and management. Int Dent J 55:277–284. doi:10.1177/154405910608500405
Lussi A, Hellwig E, Zero D, Jaeggi T (2006) Erosive tooth wear: diagnosis, risk factors and prevention. Am J Dent 19(6):319–325
Magalhães AC, Wiegand A, Rios D, Honório HM, Buzalaf MAR (2009) Insights into preventive measures for dental erosion. J Appl Oral Sci 17(2):75–86
Mellberg JR (1986) Demineralization and remineralization of root surface caries. Gerodontology 5(1):25–31. doi:10.1111/j.1741-2358.1986.tb00380.x
Wathen WF (1997) The dentulous, aging patient: what should we do? Quintessence Int 28(4):225
Shay K (2004) The evolving impact of aging America on dental practice. J Contemp Dent Pract 5(4):101–110
Al-Sabbagh M, Andreana S, Ciancio SG (2004) Dentinal hypersensitivity: review of aetiology, differential diagnosis, prevalence, and mechanism. J Int Acad Periodontol 6(1):8–12
Vieira AH, Santiago SL (2009) Management of dentinal hypersensitivity. Gen Dent 57(2):120–126
Bamise CT, Olusile AO, Oginni AO (2008) An analysis of the etiological and predisposing factors related to dentin hypersensitivity. J Contemp Dent Pract 9(5):52–59
Lussi A, Jaeggi T, Zero D (2004) The role of diet in the aetiology of dental erosion. Caries Res 38(1):34–44. doi:10.1159/000074360
Borcic J, Anic I, Urek MM, Ferreri S (2004) The prevalence of non-carious cervical lesions in permanent dentition. J Oral Rehabil 31(2):117–123. doi:10.1046/j.0305-182X.2003.01223.x
Mahoney EK, Kilpatrick NM (2003) Dental erosion: Part 1. Aetiology and prevalence of dental erosion. N Z Dental J 99(2):33–41
Van Rijkom H, Ruben J, Vieira A, Huysmans MC, Truin GJ, Mulder J (2003) Erosion inhibiting effect of sodium fluoride and titanium tetrafluoride treatment in vitro. Eur J Oral Sci 111:253–257. doi:10.1034/j.1600-0722.2003.00031.x
Amaechi BT, Higham SM (2005) Dental erosion: possible approaches to prevention and control. J Dent 33(3):243–252. doi:10.1016/j.jdent.2004.10.014
Lussi A (2009) Dental erosion—novel remineralizing agents in prevention or repair. Adv Dent Res 21:13–16. doi:10.1177/0895937409335592
Magalhães AC, Rios D, Machado MA, Da Silva SM, Lizarelli Rde F, Bagnato VS, Buzalaf MA (2008) Effect of Nd:YAG irradiation and fluoride application on dentin resistance to erosion in vitro. Photomed Laser Surg 26(6):559–563. doi:10.1089/pho.2007.2231
Rios D, Magalhães AC, Machado MA, da Silva SM, Lizarelli Rde F, Bagnato VS, Buzalaf MA (2009) In vitro evaluation of enamel erosion after Nd:YAG laser irradiation and fluoride application. Photomed Laser Surg 27(5):743–747. doi:10.1089/pho.2007.2231
Vlacic J, Meyers IA, Walsh LJ (2007) Laser-activated fluoride treatment of enamel as prevention against erosion. Aust Dent J 52(3):175–180. doi:10.1111/j.1834-7819.2007.tb00485.x
Vlacic J, Meyers I, Kim JA, Walsh LJ (2007) Laser-activated fluoride treatment of enamel against an artificial caries challenge: comparison of five wavelengths. Aust Dent J 52(2):101–105. doi:10.1111/j.1834-7819.2007.tb00472.x
Barone A, Aldini NN, Fini M, Giardino R, Calvo-Guirado JL, Covani U (2008) Xenograft versus extraction alone for ridge preservation after tooth removal: a clinical and histomorphometric study. J Periodont 79(8):1370–1377. doi:10.1902/jop.2008.070628
Holbrook WP, Furuholm J, Gudmundsson K, Theodórs A, Meurman JH (2009) Gastric reflux is a significant causative factor of tooth erosion. J Dent Res 88(5):422–426. doi:10.1177/0022034509336530
Nekrashevych Y, Stösser L (2003) Protective influence of experimentally formed salivary pellicle on enamel erosion. Caries Res 37:225–231. doi:10.1159/000070449
Fackler WK, Vaezi MF, Richter JE (2001) Ambulatory gastric pH monitoring: proper probe placement and normal values. Aliment Pharmacol Ther 15(8):1155–1162. doi:10.1046/j.1365-2036.2001.01011.x
Lee BS, Lin YW, Chia JS, Hsieh TT, Chen MH, Lin PC, Lan WH (2006) Bactericidal effects of diode laser on Streptococcus mutans after irradiation through different thickness of dentin. Lasers Surg Med 38(1):62–69. doi:10.1002/lsm.20279
de Paula DM, Melo MAS, Lima JPM, Nobre-dos-Santos M, Zanin ICJ, Rodrigues LKA (2010) In vitro assessment of thermal changes in human teeth during photodynamic antimicrobial chemotherapy performed with red light sources. Laser Phys 20(6):1475–1480. doi:10.1134/S1054660X10110046
Yazici AR, Khanbodaghi A, Kugel GJ ((2007) Effects of an in-office bleaching system (ZOOM) on pulp chamber temperature in vitro. J Contemp Dent Pract 8:19–26
Walsh LJ (2003) The current status of laser applications in dentistry. Aust Dent J 48(3):146–155. doi:10.1111/j.1834-7819.2003.tb00025.x
Rodrigues LKA, Featherstone JDB, Nobre-dos-santos M (2006) In situ mineral loss inhibition by CO2 laser and fluoride. J Dent Res 85(7):617–621. doi:10.1177/154405910608500707
Steiner-Oliveira C, Nobre-Dos-Santos M, Zero DT, Eckert G, Hara AT (2010) Effect of a pulsed CO2 laser and fluoride on the prevention of enamel and dentine erosion. Arch Oral Biol 55:123–127. doi:10.1016/j.archoralbio.2009.11.010
Hara AT, Ando M, Cury JA, Serra MC, González-Cabezas C, Zero DT (2005) Influence of the organic matrix on root dentine erosion by citric acid. Caries Res 39(2):134–138. doi:10.1159/000083159
Cate AR Ten (1998) Oral Histology: development, structure, and function, 5th edn. Mosby, London
Liu Y, Hsu CY (2007) Laser-induced compositional changes on enamel: A FT-Raman study. J Dent 35(3):226–230. doi:10.1016/j.jdent.2006.08.006
Fleming S, Tawashi R (1977) Dissolution retardation of dental enamel with special reference to the protein matrix. Can J Pharmaceut Sci 12:55–59
Paula SS, Soares LES, Santo AME, Martin AA, Cavalli V, Liporoni PCS (2009) FT-Raman and energy dispersive X-ray fluorescence spectrometric analyses of enamel submitted to 38% hydrogen peroxide bleaching, an acidic beverage, and simulated brushing. Photomed Laser Surg 28(3):391–396. doi:10.1089/pho.2008.2426
Norbert Gutknecht N, Franzen R, Meister J, Vanweersch L, Mir M (2005) Temperature evolution on human teeth root surface after diode laser assisted endodontic treatment. Lasers Med Sci 20(2):99–103. doi:10.1007/s10103-005-0347-9
Acknowledgments
The authors thank IPDI (Institute of Research, Development and Innovation) for the EDX measurements. This research was financially supported by Grant # 152.01.00/09 from the State of Ceará Research Foundation (FUNCAP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de-Melo, M.A.S., Passos, V.F., Alves, J.J. et al. The effect of diode laser irradiation on dentin as a preventive measure against dental erosion: an in vitro study. Lasers Med Sci 26, 615–621 (2011). https://doi.org/10.1007/s10103-010-0865-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-010-0865-y