Abstract
Nd:YAG laser and its association with fluoride have been proposed as an option for the prevention of dental erosion. This study evaluated the progression of existing dentin erosive lesions after treatment with different Nd:YAG laser (1064 nm) protocols, associated or not with fluoride. Erosive lesions were created with 1 % citric acid for 10 min in human dentin specimens. They were randomly assigned into eight groups (n = 15): no treatment (control), 1-min application of 2 % sodium fluoride gel (NaF), Nd:YAG1 (Nd:YAG laser irradiation 0.5 W; 50 mJ; ~41.66 J/cm2; 10 Hz; 40 s; in contact), Nd:YAG2 (0.7 W; 70 mJ; ~62.50 J/cm2; 10 Hz; 40 s; in contact), Nd:YAG3 (1 W; 100 mJ; ~54,16 J/cm2; 10 Hz; 40 s; 1 mm unfocused), NaF + Nd:YAG1, NaF + Nd:YAG2, and NaF + Nd:YAG3. After treatment, the specimens were submitted to a 5-day erosion-remineralization cycling model, 6×/day. Dentin surface loss (SL) was evaluated with optical profilometry after the formation of the initial lesion; after treatment; and after days 1, 3, and 5. Data were statistically analyzed (alpha = 0.05). Significant differences were observed among the groups in all testing times (p < 0.001), except after initial lesion formation. Loss of dentin surface was observed after irradiation with all Nd:YAG laser protocols (p < 0.05). The association fluoride and laser did not differ significantly from laser alone. NaF showed the lowest values of SL and Nd:YAG2 and NaF + Nd:YAG2, the highest. Within the limitations of an in vitro study, it was concluded that laser irradiation, according to the parameters used, was not an appropriated approach to prevent dentin erosion progression, even when it was associated with fluoride.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental erosion prevalence has increased in recent years [1] and has been associated, among other factors, to the increased consumption of acidic drinks [2–4]. The erosive process occurs by the frequent contact of acids with tooth surfaces [5]. At initial stages, it causes only superficial demineralization. As it progresses, bulk enamel loss takes place, leaving a softened demineralized layer. In advanced stages, dentin is often exposed, which can lead to undesirable consequences, such as dentin hypersensitivity [6].
The first strategy to manage this condition is patient education, which includes a reduction in the frequency of acid exposure [7]. Another recommended approach is the application of topical fluoride products, which can increase the acid resistance of the tooth surfaces [8]. Fluoride compounds, such as sodium fluoride and amine fluoride, can protect the dental surfaces against erosive demineralization most likely through the formation of CaF2-like precipitates [9]. However, some studies demonstrated that such precipitates might not be as resistant to the low pH of the erosive challenge, which could limit its protective effects [9, 10]. Attempts to further increase the acid resistance of the dental surface were made with polyvalent fluorides, with best evidence for stannous fluoride [10].
Other potential approach is the irradiation of the dental surface with high-power lasers, such as the Nd:YAG, combined or not with fluoride [11–13]. In dentin, Nd:YAG laser irradiation can melt the hydroxyapatite structure, which, upon cooling, can re-solidify forming larger crystals than the ones in the initial structure [14]. As a result, a glazed and non-porous surface with occluded dentinal tubules will occur, and this is also relevant for dentin hypersensitivity treatment, as shown by many studies [15–17]. Some investigations suggest improved demineralization protection when laser was combined with fluoride [13, 18]. It was postulated that laser irradiation would increase fluoride deposition and fluoride uptake by the dental structures [19, 20].
Laser irradiation on dentin surfaces may be affected by several factors, such as the laser wavelength, power, energy output, time of irradiation, as well as the dentin surface conditions [14]. According to Zapletalová et al. [21], the absorption of all wavelengths of laser irradiation can also be influenced by free molecules of water, proteins, and pigments of the substrate. For Nd:YAG laser irradiation, most of the investigations about dental erosion and dentin hypersensitivity used protocols in the range of 0.5–1 W power, 10 Hz of repetition rate, and 50–100 mJ of energy [11–13, 15–17]. However, to the authors’ knowledge, no study has evaluated the effectiveness of Nd:YAG laser protocols on the progression of erosion in previously formed dentin lesions. This is important because, in dentin, unlike in enamel, the erosion process is affected by the organic collagen-rich matrix, which can significantly reduce the development of the erosive lesion [22], and this modified substrate can potentially influence the effects of laser irradiation.
Studies investigating irreversible loss of dental hard tissues have been made using optical profilometric analysis [23–25]. This methodology involves the scanning of the specimen’s topography, which usually includes a protected reference area and the treated area. These areas are then compared using a specific software that calculates the depth of the lesion and the volume of tooth loss. One limitation of this method would be the measurement of eroded dentin surfaces, because the exposed collagen fibrils can undergo shrinkage [26]; therefore, it is recommended to scan the specimens in moist conditions [27].
In this study, it was hypothesized whether the effects of Nd:YAG laser irradiation, with parameters previously proposed for enamel and sound dentin, may be still relevant for the inhibition of the dentin erosive lesion progression. A second hypothesis was that Nd:YAG laser irradiation would improve sodium fluoride’s protection against the progression of dentin erosion.
The purpose of this study was to evaluate the effectiveness of the most commonly used Nd:YAG laser protocols for dental erosion in sound dentin and dentin hypersensitivity, as well as their combination with fluoride, in the control of dentin erosion progression.
Materials and methods
Experimental design
This study followed the complete randomized design, for the study of a single experimental factor (dentin treatment), at eight levels (n = 15): (1) control (no treatment); (2) 2 % sodium fluoride (NaF) gel (Flugel, DFL®, Rio de Janeiro, Brazil); (3) Nd:YAG laser irradiation (Power Laser™ ST6, Lares Research, Chico, CA, USA), protocol 1 (0.5 W; 50 mJ; ~41.66 J/cm2; 10 Hz; 40 s; in contact); (4) Nd:YAG laser irradiation, protocol 2 (0.7 W; 70 mJ; ~62.50 J/cm2; 10 Hz; 40 s; in contact); (5) Nd:YAG laser irradiation, protocol 3 (1 W; 100 mJ; ~54.16 J/cm2; 10 Hz; 40 s; 1 mm unfocused); (6) NaF gel followed by Nd:YAG1; (7) NaF gel followed by Nd:YAG2; and (8) NaF gel followed by Nd:YAG3. Eroded dentin specimens were the experimental units. Dentin surface loss, evaluated by optical profilometry, was the response variable, and it was evaluated in five different experimental times: after the initial lesion formation (T0); immediately after the treatments (T1); and after the first (T2), third (T3), and fifth (T4) days of erosive cycling.
Specimen preparation
Human third molars were used in this study, after the approval of the Local Ethics Committee (CAAE317.635). Teeth were stored in 0.1 % thymol solution, under refrigeration at 4 °C, until the beginning of the experiment. They were initially cleaned with 11–12/13–14 Gracey curettes and submitted to prophylaxis with Robinson’s brush coupled in a low-speed handpiece, pumice stone, and water. After cleaning, specimens were stored in distilled water at 4 °C.
The buccal surface of each tooth was grinded in a polishing machine (Ecomet 3 Machine, Buehler LTD, Lake Buff, Illinois, USA), using #180-grit silicon carbide abrasive papers, under continuous cooling, until the exposure of the dentin surface. The teeth were sectioned into 4 mm × 4 mm × 2 mm dentin slabs, using a microtome (Isomet, Buehler, Lake Bluff, Illinois, USA). The slabs were embedded in acrylic resin (VariDur, Buehler, Lake Bluff, Illinois, USA) and grinded using the following sequence of abrasive papers: 500-, 1200-, 2400-, and 4000-grit (MD-Fuga, Struers Inc., Cleveland, OH, USA), under water cooling, and polished using felt discs soaked with diamond suspension (1 μm; Struers Inc., Cleveland, OH, USA). Subsequently, the specimens were immersed in an ultrasonic bath with detergent solution (2 % micro-90 liquid soap, International Product Corporation, Burlington, NJ, USA), for 3 min, and thoroughly rinsed afterwards. Specimens without fractures or any other visual imperfections were then selected. The selected specimens were kept under moist environment, at 4 °C.
Profilometric analysis
For standardization, before the profilometric analysis, the specimens were left to dry for 10 min [28]. Profilometric analysis was performed with an optical profilometer (Proscan 2100, Scantron, Venture Way, Taunton, UK). The instrument sensor scanned an area of 2 mm long (X-axis) by 1 mm wide (Y-axis), located at the center of specimen. The equipment was set to go through 200 steps in the X-axis, with each step measuring 0.01 mm. In the Y-axis, there were 20 steps measuring 0.05 mm each. Using a specific software (Proscan Application software version 2.0.17), the lesion depth was calculated based on subtraction of the average height of the test area, from the average height of the reference surfaces. The result was expressed in micrometers. Specimens with curvature values superior to 0.3 μm were discarded, and the selected specimens received adhesive unplasticized polyvinyl chloride (UPVC, Graphic Tape, Chartpak, Leeds, USA) tapes on their polished surfaces, leaving a central window of 4 mm × 1 mm exposed to subsequent testing.
Initial lesion creation
To create the initial lesion, specimens were fixed to the lid of cell culture plates (CLS3512—Corning, Corning, NY, USA) with sticky wax, in order to perform the test procedures for each specimen independently. They were immersed in 1 % citric acid (Sigma-Aldrich, St. Louis, MO, USA; pH ~2.3) [29] for 10 min, at room temperature. The specimens were rinsed with distilled water and submitted to the second profilometric analysis (T0). For each profilometric analysis, the specimens were removed from the plates and cleaned to avoid interference. The tapes were also removed and replaced afterwards. Once the initial erosion had developed, the specimens were randomly divided into the eight experimental groups.
Sodium fluoride (2 %) application
The specimens from the fluoride group received an application of neutral 2 % sodium fluoride (NaF) gel without pigments (Flugel, DFL®, Rio de Janeiro, Brazil), according to manufacturer’s recommendation. The excess of gel was removed with cotton roll. For the groups treated with Nd:YAG laser associated with fluoride, the gel was applied before irradiation and the excess also removed.
Nd:YAG laser irradiation
Irradiation with the Nd:YAG laser (Power Laser™ ST6, Lares Research®, Chico, CA, USA) was performed perpendicular to the specimen surface, using a 400-μm quartz fiber in x- and y-axis directions in the lesion’s region. This procedure was performed in four 10-s irradiations (two in each direction). An interval of 10 s between the irradiations was necessary for thermal relaxation of the dentinal tissue. Laser irradiation was performed manually with scanning movements and pulse duration of 120 μs. The protocols Nd:YAG1 (0.5 W; 50 mJ; ~41.66 J/cm2; 10 Hz) and Nd:YAG2 (0.7 W; 70 mJ; ~62.50 J/cm2; 10 Hz) were performed in contact and focused mode. The protocol Nd:YAG3 (1 W; 100 mJ; ~54.16 J/cm2; 10 Hz) was performed at a distance of 1 mm, unfocused [29]. This distance was set with the aid of an endodontic file fixed to the handpiece of the laser. Before all irradiation procedures, power output measurements were taken with a power meter (Coherent, Newport, USA), without power loss during any of the irradiations performed.
After the different treatments, a third profilometric analysis was performed, denominated T1.
Erosive cycling
Samples were re-mounted on the lid of cell culture plates for the erosive cycling procedure, which consisted of 3-min immersion in 1 % citric acid (pH ~2.3) followed by a 60-min immersion in artificial saliva (pH 7). This procedure was repeated six times a day, for 5 days. The specimens were stored in artificial saliva overnight. During the remineralization period, the plates were placed on an orbital shaker, set at 35 rpm (Orbital Agitador, A-9000-B, BrLabs®, Campinas, Brazil). The citric acid solution was changed after each demineralization episode (six times per day). Artificial saliva was changed at the beginning of each cycle (once a day). At the end of the first, third, and fifth days, new profilometric analyses were made (T2, T3, and T4, respectively).
Statistical analysis
Dentin surface loss data were analyzed for normal distribution and homocesdasticity with Shapiro-Wilks and Levene tests, respectively. As the premise of homocesdasticity was not satisfied, the comparisons among groups within each experimental time were performed with Kruskal-Wallis and Tukey tests (Table 1), and the comparison among the different experimental times within each group were performed with Friedman and Tukey tests (Table 2). The software Minitab, version 16.1, was used for all calculations. The significance level was set at 5 %.
Results
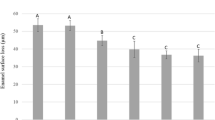
The means (SD) of dentin loss (in μm) for the groups in each experimental time are shown in Table 1. For T0, all groups showed initial erosion lesions in the range of 2–5 μm of depth, mean (SD) value of 3.38 (0.32), with no significant differences among them. For all other experimental times, the groups differed significantly from each other (p < 0.001).
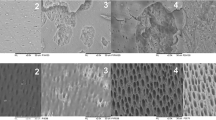
In T1 (after treatments), all laser groups presented significantly higher dentin loss than the control group, which did not differ significantly from NaF. Groups NaF + Nd:YAG2 and Nd:YAG2 showed the highest values of dentin loss, with no significant difference between them. Nd:YAG2 was not significantly different from NaF + Nd:YAG3, which in turn did not significantly differ from NaF + Nd:YAG1, Nd:YAG1, and Nd:YAG3. Carbonization and tissue loss were observed after irradiation with all of the laser protocols, but in different levels.
In T2 (after the first day of the erosive cycle), the control only showed significant difference from Nd:YAG2 and NaF + Nd:YAG2, which had the highest surface loss, with no significant difference between them. Nd:YAG3 was not statistically different from the control and NaF. Considering the association of fluoride and laser, there was no significant difference between NaF + Nd:YAG1 and NaF + Nd:YAG3. Group NaF + Nd:YAG2 continued to be the most aggressive.
In T3 (after the third day of the erosive cycle), the control showed significantly higher dentin loss than NaF, which presented the lower dentin loss, but it was not significantly different from NaF + Nd:YAG3. Groups Nd:YAG2 and NaF + Nd:YAG2 presented the highest dentin loss; however, Nd:YAG1 and all the other groups were not statistically different from the control.
In T4 (after 5 days of erosive cycling), NaF had significantly lower dentin loss than the control, which in turn presented significantly lower dentin loss than Nd:YAG2 and NaF + Nd:YAG2. The other groups were not significantly different from the control.
Regarding the comparison among experimental times, within groups, for the control and NaF, T0 did not significantly differ from T1. From T1, there was a progressive and statistically significant increase in the dentin loss values. For the laser groups, T1 showed significant higher dentin loss than T0. From T1, there was also a progressive and statistically significant increase in the dentin loss, except for group NaF + Nd:YAG2, in which T0 was lower than T1, which was not significantly different from T2. Experimental times T3 and T4 exhibit the highest dentin loss with no significant difference between them.
Discussion
Among the available high-power lasers, Nd:YAG laser is considered as the “gold standard” for the treatment of dentin hypersensitivity and its effectiveness was already demonstrated in several investigations [16, 30–33]. Since dental erosion and dentin hypersensitivity are often related, it would be interesting if the laser could not only treat dentin hypersensitivity by sealing the dentinal tubules but also control the progression of dentin erosion. There are many safe protocols with positive results reported in the literature for the treatment of dentin hypersensitivity. It was reported that when the thickness of the dentin exposed to Nd:YAG laser irradiation for 10 s, not exceeding parameters of 1 W and 10 Hz, is of at least 1 mm, no pulp damage can be expected [34]. Besides tubule occlusion, a previous investigation showed that the surface of dentin treated with Nd:YAG laser was more resistant to acid demineralization [35]. The authors related this fact to several possibilities, such as the removal of impurities from the crystal lattice during heating, which may reduce dentin’s solubility; the reduction in the surface area to be attacked by the acid, due to a change in crystals morphology; and the reduction on dentin’s permeability and thus the acid diffusion pathways within its structure. However, there are only few studies testing the anti-erosive effect of Nd:YAG laser in dentin [11, 36], and to the authors’ knowledge, no investigations were yet conducted to determine the laser effect on the progression of dental erosion lesions. In view of this and considering the high prevalence of dental erosion and the necessity of finding a treatment protocol that would decrease the progression of erosive lesions, this study selected three different protocols of Nd:YAG laser to be tested on a previously eroded dentin surface [29]. The difference between the protocols was the density of energy, which can influence laser penetration in the irradiated surface. The proper laser wavelength and energy output may change the dentin’s surface, and this is thought to reduce the progression of demineralization.
In the present study, Nd:YAG laser irradiation was not able to reduce further demineralization of eroded dentin, rejecting the first hypothesis of this study. In fact, carbonization and tissue loss were observed after treatment with all laser protocols tested. Nd:YAG laser irradiation is highly absorbed by pigments [37]. It can be hypothesized that the laser led to tissue carbonization at the first 10 s of irradiation, since the initial acid attack created a demineralized dentin surface, with exposure of its protein content. Thus, the resulting black spots at the dentin surface may have contributed to additional tissue removal in the next 30 s of irradiation. It should be noted that these protocols were previously suggested by the literature for the prevention of dental erosion and for the treatment of cervical dentin hypersensitivity.
Considering that the protocols used in the present investigation were already tested in other studies, in which no tissue removal was reported [15, 38], we may infer that the same Nd:YAG laser parameters suitable for sound dentin cannot be used for eroded dentin. This has an especial importance in the cases of dentin hypersensitivity, where erosion is recognized as one of its main etiological factor [39] and where Nd:YAG laser irradiation is often suggested as treatment [16]. For these cases, the use of low-energy density parameters could be an alternative that warrants further investigations.
Since in the present study, laser irradiation resulted in surface removal, the specimens from the laser groups already started the erosion-remineralization cycle with higher surface loss; therefore, the effect of Nd:YAG laser irradiation on the progression of dentin erosion, if any, might have been dissembled. Evidence of this phenomenon can be observed in the group Nd:YAG3, which despite starting the cycle with higher surface loss than the control, after the first day of cycling, there was no significant difference between them and this trend was kept until the end of the experiment.
Likewise, Nd:YAG laser irradiation was not able to improve fluoride’s protection against dentin erosion, rejecting the second hypothesis of this study. This outcome is in agreement with the findings of Magalhães et al. [11]; however, it should be mentioned that the previous study used sound instead of eroded dentin. Similar to other studies, in the groups where fluoride and laser were associated, the NaF gel was applied before Nd:YAG laser irradiation [11, 24, 40], due to the laser’s potential to increase fluoride uptake [41], as well as the formation of CaF2-like material [42]. Nevertheless, although the synergistic effect between fluoride and Nd:YAG laser irradiation on the prevention of enamel erosion was already shown in a few studies [12, 13], for dentin, the results so far do not support this effect. In the case of the present study, it can be supposed that the laser irradiation could have removed the fluoride deposits formed after gel application, thus hampering the protective action of fluoride.
In contrast to the Nd:YAG laser, the NaF gel used alone was the group that most reduced dentin erosion progression (21 % of reduction in comparison to the control group) by the end of the experiment. Fluoride treatment is an established solution for the prevention of demineralization. However, as suspected, this effect was only limited and supports the need for searching more effective treatment options.
Although Nd:YAG laser irradiation was not able to prevent further demineralization on eroded dentin, it may be speculated that the use of more conservative protocols (with lower outputs and energy densities), and consequently, less possibility of structural loss and thermal damage, may show improved effects and should be considered in future investigations. New in vitro studies should be performed in order to achieve laser protocols that can be safely used for the progression of dentin erosion in the clinic.
Conclusions
The Nd:YAG laser protocols tested caused ablation with unexpected dentin removal; therefore, they are not suitable to be used in eroded dentin. Sodium fluoride gel (2 %) was the only treatment able to reduce further demineralization on eroded dentin.
References
Jaeggi T, Lussi A (2014) Prevalence, incidence and distribution of erosion. Monogr Oral Sci 25:55–73
O'Sullivan EA, Curzon ME (2000) A comparison of acidic dietary factors in children with and without dental erosion. ASDC J Dent Child 67(3):186–192
Bartlett DW, Fares J, Shirodaria S, Chiu K, Ahmad N, Sherriff M (2011) The association of tooth wear, diet and dietary habits in adults aged 18–30 years old. J Dent 39(12):811–816
Murakami C, Oliveira LB, Sheiham A, Nahas Pires Correa MS, Haddad AE, Bonecker M (2011) Risk indicators for erosive tooth wear in Brazilian preschool children. Caries Res 45(2):121–129
Imfeld T (1996) Dental erosion. Definition, classification and links. Eur J Oral Sci 104(2(Pt 2)):151–155
Lussi A, Schlueter N, Rakhmatullina E, Ganss C (2011) Dental erosion—an overview with emphasis on chemical and histopathological aspects. Caries Res 45(Suppl 1):2–12
Magalhaes AC, Wiegand A, Rios D, Honorio HM, Buzalaf MA (2009) Insights into preventive measures for dental erosion. J Appl Oral Sci 17(2):75–86
Magalhaes AC, Wiegand A, Rios D, Buzalaf MA, Lussi A (2011) Fluoride in dental erosion. Monogr Oral Sci 22:158–170
Ganss C, Schlueter N, Klimek J (2007) Retention of KOH-soluble fluoride on enamel and dentine under erosive conditions—a comparison of in vitro and in situ results. Arch Oral Biol 52(1):9–14
Huysmans MC, Young A, Ganss C (2014) The role of fluoride in erosion therapy. Monogr Oral Sci 25:230–243
Magalhaes AC, Rios D, Machado MA, Da Silva SM, de F Lizarelli R, Bagnato VS, Buzalaf MAR (2008) Effect of Nd:YAG irradiation and fluoride application on dentine resistance to erosion in vitro. Photomed Laser Surg 26(6):559–563
Sobral MA, Lachowski KM, de Rossi W, Braga SR, Ramalho KM (2009) Effect of Nd:YAG laser and acidulated phosphate fluoride on bovine and human enamel submitted to erosion/abrasion or erosion only: an in vitro preliminary study. Photomed Laser Surg 27(5):709–713
Rios D, Magalhaes AC, Machado MA, da Silva SM, Lizarelli Rde F, Bagnato VS, Buzalaf MAR (2009) In vitro evaluation of enamel erosion after Nd:YAG laser irradiation and fluoride application. Photomed Laser Surg 27(5):743–747
Lan WH, Chen KW, Jeng JH, Lin CP, Lin SK (2000) A comparison of the morphological changes after Nd-YAG and CO2 laser irradiation of dentin surfaces. J Endod 26(8):450–453
Naylor F, Aranha AC, de P Eduardo C, Arana-Chavez VE, Sobral MA (2006) Micromorphological analysis of dentinal structure after irradiation with Nd:YAG laser and immersion in acidic beverages. Photomed Laser Surg 24(6):745–752
Lopes AO, Aranha AC (2013) Comparative evaluation of the effects of Nd:YAG laser and a desensitizer agent on the treatment of dentin hypersensitivity: a clinical study. Photomed Laser Surg 31(3):132–138
Palazon MT, Scaramucci T, Aranha AC, Prates RA, Lachowski KM, Hanashiro FS, Youssef MN (2013) Immediate and short-term effects of in-office desensitizing treatments for dentinal tubule occlusion. Photomed Laser Surg 31(6):274–282
Zezell DM, Boari HG, Ana PA, Eduardo Cde P, Powell GL (2009) Nd:YAG laser in caries prevention: a clinical trial. Lasers Surg Med 41(1):31–35
Goodman BD, Kaufman HW (1977) Effects of an argon laser on the crystalline properties and rate of dissolution in acid of tooth enamel in the presence of sodium fluoride. J Dent Res 56(10):1201–1207
Gao XL, Pan JS, Hsu CY (2006) Laser-fluoride effect on root demineralization. J Dent Res 85(10):919–923
Zapletalova Z, Perina J Jr, Novotny R, Chmelickova H (2007) Suitable conditions for sealing of open dentinal tubules using a pulsed Nd:YAG laser. Photomed Laser Surg 25(6):495–499
Hara AT, Ando M, Cury JA, Serra MC, Gonzalez-Cabezas C, Zero DT (2005) Influence of the organic matrix on root dentine erosion by citric acid. Caries Res 39(2):134–138
Scaramucci T, Hara AT, Zero DT, Ferreira SS, Aoki IV, Sobral MA (2011) In vitro evaluation of the erosive potential of orange juice modified by food additives in enamel and dentine. J Dent 39(12):841–848
Steiner-Oliveira C, Nobre-dos-Santos M, Zero DT, Eckert G, Hara AT (2010) Effect of a pulsed CO2 laser and fluoride on the prevention of enamel and dentine erosion. Arch Oral Biol 55(2):127–133
Scaramucci T, Borges AB, Lippert F, Zero DT, Aoki IV, Hara AT (2015) Anti-erosive properties of solutions containing fluoride and different film-forming agents. J Dent 43(4):458–465
Attin T, Wegehaupt FJ (2014) Methods for assessment of dental erosion. Monogr Oral Sci 25:123–142
Attin T, Becker K, Roos M, Attin R, Paque F (2009) Impact of storage conditions on profilometry of eroded dental hard tissue. Clin Oral Investig 13(4):473–478
Borges AB, Scaramucci T, Lippert F, Zero DT, Hara AT (2014) Erosion protection by calcium lactate/sodium fluoride rinses under different salivary flows in vitro. Caries Res 48(3):193–199
Ganss C, Klimek J, Schaffer U, Spall T (2001) Effectiveness of two fluoridation measures on erosion progression in human enamel and dentine in vitro. Caries Res 35(5):325–330
Gelskey SC, White JM, Pruthi VK (1993) The effectiveness of the Nd:YAG laser in the treatment of dental hypersensitivity. J Can Dent Assoc 59(4):377–378, 383–376
Lan WH, Liu HC (1996) Treatment of dentin hypersensitivity by Nd:YAG laser. J Clin Laser Med Surg 14(2):89–92
Lier BB, Rosing CK, Aass AM, Gjermo P (2002) Treatment of dentin hypersensitivity by Nd:YAG laser. J Clin Periodontol 29(6):501–506
Dilsiz A, Aydin T, Canakci V, Gungormus M (2010) Clinical evaluation of Er:YAG, Nd:YAG, and diode laser therapy for desensitization of teeth with gingival recession. Photomed Laser Surg 28(Suppl 2):S11–S17
White JM, Fagan MC, Goodis HE (1994) Intrapulpal temperatures during pulsed Nd:YAG laser treatment of dentin, in vitro. J Periodontol 65(3):255–259
Kinney JH, Haupt DL, Balooch M, White JM, Bell WL, Marshall SJ, Marchall GW Jr (1996) The threshold effects of Nd and Ho: YAG laser-induced surface modification on demineralization of dentin surfaces. J Dent Res 75(6):1388–1395
Watanabe T, Fukuda M, Mitani A, Ting CC, Osawa K, Nagahara A, Satoh S, Fujimura T, Takahashi S, Iwamura Y, Murakami T, Nogichi T (2013) Nd:YAG laser irradiation of the tooth root surface inhibits demineralization and root surface softening caused by minocycline application. Photomed Laser Surg 31(12):571–577
Matos AB, Azevedo CS, da Ana PA, Botta SC, Zezell DM (2012) Laser technology for caries removal. In: Ming-Yu L (ed) Contemporary approach to dental caries, Intech, pp 291–312
Farmakis ET, Beer F, Kozyrakis K, Pantazis N, Moritz A (2013) The influence of different power settings of Nd:YAG laser irradiation, bioglass and combination to the occlusion of dentinal tubules. Photomed Laser Surg 31(2):54–58
Aw TC, Lepe X, Johnson GH, Mancl L (2002) Characteristics of noncarious cervical lesions: a clinical investigation. J Am Dent Assoc 133(6):725–733
Vlacic J, Meyers IA, Walsh LJ (2007) Laser-activated fluoride treatment of enamel as prevention against erosion. Aust Dent J 52(3):175–180
Tagomori S, Morioka T (1989) Combined effects of laser and fluoride on acid resistance of human dental enamel. Caries Res 23(4):225–231
Chin-Ying SH, Xiaoli G, Jisheng P, Wefel JS (2004) Effects of CO2 laser on fluoride uptake in enamel. J Dent 32(2):161–167
Acknowledgments
The authors would like to thank FAPESP (State of São Paulo Research Foundation, Grants #2012/20632-9 and #2011/17699-1), LELO – FOUSP (Special Laboratory of Lasers in Dentistry at the School of Dentistry of the University of São Paulo, Brazil), and the Oral Health Research Institute (Indiana University School of Dentistry) for the support and Adam Kelly and Joseph Joseph for the technical support.
Compliance with ethical standards
ᅟ
Conflict of interest
The authors declare that they have no competing interests.
Funding
This study was funded by FAPESP (State of São Paulo Research Foundation, Grants #2012/20632-9 and #2011/17699-1), through scholarships and grants for research.
Ethics approval
Human third molars were used in this study after the approval of the Local Ethics Committee (CAAE317.635).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
João-Souza, S.H., Scaramucci, T., Hara, A.T. et al. Effect of Nd:YAG laser irradiation and fluoride application in the progression of dentin erosion in vitro. Lasers Med Sci 30, 2273–2279 (2015). https://doi.org/10.1007/s10103-015-1802-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-015-1802-x