Abstract
Muscle repair is regulated by growth factors and cytokines. Low-level laser therapy (LLLT) seems to influence acute inflammation and accelerate skeletal muscle repair. This study verifies the effect of LLLT on the expression of IL-1β in the tibialis anterior (TA) muscle of rats following acute injury. Wistar rats (n = 35) were allocated into three groups: control (without lesion and LLLT, n = 5), injury group (n = 15), and injury + LLLT group (n = 15). The acute injury was induced by the contact with a cooled metal probe (3 mm in diameter) during 10 s, twice, in the same muscle area. LLLT was used three times a week using the InGaAlP laser (660 nm; beam spot of 0.04 cm2, output power of 20 mW, power density of 500 mW/cm2, and energy density of 5 J/cm2 during 10 s). The animals were analyzed at 1, 7, and 14 days following injury. TA muscles samples were used for obtaining total RNA and performing cDNA synthesis. Real-time polymerase chain reactions were realized using IL-1β primer. There was a decrease in IL-1β expression after 7 days in LLLT group in comparison with the no treated group. In conclusion, LLLT was able to decrease IL-1β expression during the skeletal muscle repair following an acute injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent studies on musculoskeletal disorders link local and systemic inflammation to myopathic changes that are accompanied by pain and a decline in function [1]. Following an injury, inflammatory cells reach the muscle tissue and persist there during the repair and regeneration process [2]. This initial cell infiltration includes both neutrophils and macrophages and is associated with an increase in pro-inflammatory cytokines, such as TNFα, IL-1β, and IL-6 [2, 3].
IL-1 is mainly produced by macrophages, monocytes, and dendritic cells. This cytokine has two distinct subtypes, one (IL-1α) that stays primarily in the cytosol of cells and a second one (IL-1β) that becomes active upon cleavage by a protease produced in monocytes [4]. There are many conditions associated with an increase in IL-1 including modulation of inflammation, fever, and cell proliferation. These degenerative effects are more intense when IL-1 acts synergistically with TNF-α to promote the catabolism of lean tissue [5]. IL-1β is also released by injured muscle cells to attract inflammatory cells such as neutrophils and macrophages [3] and plays a critical role in both host defense and muscle tissue remodeling following injury.
Low-level laser therapy (LLLT) has demonstrated to induce improvement on inflammatory and repair process in skeletal muscles [6–9]. However, a number of protocols and different LLLT parameters have been used and the results regarding the modulation of cytokine production are different [6, 8]. Therefore, the aim of the study was verify the influence of LLLT on the mRNA of IL-1β in tibialis anterior (TA) muscle rats following cryoinjury.
Methods
The experiments were carried out in accordance with the guidelines of the National Council for the Control of Animal Experimentation and Universidade Nove de Julho Ethical Committee on Animal Research (approval 13/2007).
Male Wistar rats (body weight 234 ± 37.99 g) were maintained under controlled conditions as previously described [6, 11] and allocated into three groups: control (n = 5, without injury and untreated), injury group (n = 15), and injury + LLLT group (n = 15). Animals from the control group were analyzed at 1 day and injury animals were analyzed at 1, 7, and 14 days following injury. TA muscle was submitted to a cryoinjury as previously described [6, 10, 11]. Animals were anesthetized with 1 ml/kg of 1 % ketamine and 2 % xylazine. The TA muscle was exposed by incision and submitted to cryoinjury by the contact with a cooled metal probe (3 mm in diameter) during 10 s, twice, in the same muscle area. The left TA muscle was injured and the right side was used as control. After cryoinjury, the wounds were sutured.
LLLT procedure
The LLLT equipment used was the InGaAlP diode laser (MMOptics Ltd., São Carlos, SP, Brazil) of 660 nm, a beam spot of 0.04 cm2, an output power of 20 mW, an energy density of 5 J/cm2, and a power density of 500 mW/cm2 for 10 s. The laser beam was placed in contact with the skin surface corresponding to the cryolesioned area and the irradiation was applied at eight points around the cryolesion area as previously described [6, 11].
The total energy used per treatment was 1.6 J, that is, eight application points receiving 0.2 J each. It used a LaserCheck power meter (Coherent, Santa Clara, CA, USA) to verify the output power of the laser device used.
The LLLT was applied three times a week, starting 24 h after injury, with a 24-h interval between sessions. Groups at 1, 7, and 14 days received one, three, and six sessions, respectively. At the end of the treatment, animals were killed and TA muscles were quickly dissected and frozen in liquid nitrogen.
Total RNA was isolated from TA muscles using cold Trizol Reagent (Invitrogen, CA, USA) according to the manufacturer’s instructions. cDNA synthesis was performed using AMV reverse transcriptase (Invitrogen, Brazil) and all samples received treatment with DNase (Invitrogen) to avoid DNA contamination as previously described [6, 11].
Real-time PCR was performed using a 7500 Sequence Detection System (ABI Prism, Applied Biosystems, Foster City, CA) and the SYBRGreen kit (Applied Biosystems, USA). Specific primers for rat IL-1β (forward 5′-CACCTCTCAAGCAGAGCACAG-3′; reverse 5′-GGGTTCCATGGTGAAGTCAAC-3′ (GenBank accession # M98820)) were used for this procedure and a constitutive gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was used to normalize the data using the same amount of cDNA. GAPDH primers were forward 5′-TGCACCACCAACTGCTTAGC-3′ and reverse GCCCCACGGCCATCA-3 (GenBank accession # NM 017008).
To normalize the data for the control, injury, and injury + LLLT, arbitrary units were calculated by subtracting the average Ct (threshold cycle) value of IL-1β mRNA from the average Ct value of the GAPDH as described following: arbitrary unit = 2-∆∆CT and ∆∆TC = IL-1β ∆Ct − GAPDH ∆Ct.
IL-1β mRNA data are presented as mean values and standard deviation. The studied data follow a Gaussian (normal) distribution, as verified by Levene’s test. In this manner, parametric methods were used to detect differences between all possible pairs in the within-group- and within-day analysis. The program GraphPad Prism 4.0 statistical software (GraphPad Software, San Diego, CA, USA) was used for statistical analysis. Statistical significance was assessed by one-way ANOVA and the Tukey test, with the level of significance of P < 0.05.
Results
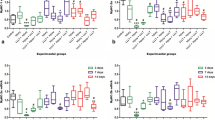
The results of the mRNA IL-1β are presented in Fig. 1. After 1 day following injury, LLLT induced a decrease in IL-1β mRNA expression in comparison to non-LLLT injury groups, although not significantly. There were also no significant differences between experimental groups and the control group.
After 7 days, it noted a significant decrease in IL-1β mRNA expression in the injury + LLLT group in comparison to the injury group without laser treatment. In the same period, there was also a significant increase in IL-1β mRNA in the injury group in comparison to all other groups in all periods, except the injury group at 14 days. At 14 days after injury, there was no significant difference in IL-1β mRNA expression, when comparing LLLT injury and non-LLLT injury groups, or between these groups and the control group.
Discussion
The results of the present study demonstrate that, when using the cryoinjury model, there was a significant increase in IL-1β mRNA in the non-treated injury group 7 days following injury and that LLLT (660 nm, 20 mW, and 5 J/cm2) was able to promote a significant decrease in this expression in the same period. No statistically significant differences between the LLLT and non-LLLT groups were found for the other periods.
There are very few papers on the relationship between LLLT and IL-1β modulation in skeletal muscle following injury. Albertini et al. [8] demonstrated that the mRNA expression of TNF-α, IL-1β, and IL-6 was increased 3 h after carrageenan injection in subplantar muscle tissue of rat paws. LLLT at wavelengths of 660 or 684 nm (30 mW, 7.5 J/cm2) induced a decrease in TNF-α, IL-1β, and IL-6 mRNA expression 3 h after irradiation. In the present study, a nonsignificant increase in IL-1β expression was observed after 1 day in the injury group in comparison to the control group and a nonsignificant decrease was observed in the injury + LLLT group in comparison to the group submitted to injury alone.
These differences in time and significance may be related to differences in the laser parameters and/or the type of injury. Carrageenan subplantar injection induces a biphasic cellular infiltrate. The first peak was seen at 3 h and the delayed peak 48 h after injection. In the early peak, the cellular infiltrate is mainly made up of neutrophils, whereas the infiltrate in the delayed peak is composed of macrophages, eosinophils, and lymphocytes [12–14]. Four hours after injection, carrageenan induced a considerable increase in the expression of IL-1β in the edema fluid of the paw [13].
Neutrophils are the first to respond in acute muscle injury. Typically, a peak in concentration is seen between 6 and 24 h after injury, with a rapid decline [2]. Macrophages reach significantly elevated concentrations at about 24 h post-injury, continuing to increase in numbers until reaching peak concentrations in the muscle at about 4 days, remaining significantly elevated for many days afterward [2]. In the muscle injury model employed in the present study, peak IL-1β RNA expression took place after 7 days, likely produced by macrophages mainly, and LLLT was able to significantly reduce this expression.
In a previous study by our research group using the same irradiation parameters as those employed in the present study, LLLT was found to cause a significant decrease in TNF-α mRNA expression at 1 and 7 days following injury in comparison to the control group, which could explain the decrease in IL-1β presented in this study [6].
Although TNF-α plays a pivotal role in regulating the expression of IL-1β as it is upstream in this cytokine cascade, and has the ability to upregulate IL-1β production [8], it is important to consider that an increase/decrease in gene expression is not always accompanied by a corresponding increase/decrease in protein production as it may be under post-transcriptional or post-translational regulation.
The anti-inflammatory effects of LLLT on muscle tissue have previously been suggested [6, 8, 11, 15]. Accordingly, the present study demonstrates that LLLT was able to modulate IL-1β during muscle repair following an acute injury.
Pro-inflammatory cytokines, such as TNF-α and IL-1β, cause the translocation of NFκβ to the cell nucleus [16, 17]. Once in the nucleus, NF-κB can induce the transcription of iNOS, TNF-α, and IL-1, which may then promote further NF-κB activation, as well as an increase in the expression of other inflammatory mediators, such as IL-6, thereby perpetuating the inflammatory process [16, 17]. Moreover, the activation of NF-κB participates in a key signaling pathway resulting in the inhibition of myogenic differentiation [18–21].
In addition, IL-1β inhibits myoblast differentiation by reducing the IGF-1 ability to promote an increase in the synthesis of myogenin, an important myogenic regulatory factor involved in cell muscle differentiation, and subsequently in increasing myosin expression [22].
It is noteworthy that this study evaluated the effect of LLLT only on gene expression of IL-β. Additional studies involving protein analysis and other experimental periods could complement the data presented.
In conclusion, the present study shows that LLLT was able to decrease IL-1β mRNA expression 7 days after cryoinjury in TA muscle rats, thus favoring the modulation of the inflammatory process, and possibly preventing the inhibition of myogenic differentiation caused by elevated IL-1β concentrations.
References
Barbe MF, Barr AE (2006) Inflammation and the pathophysiology of work-related musculoskeletal disorders. Brain Behav Immun 20:423–429
Tidball JG, Villalta SA (2010) Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol 298:1173–118
Van der Poel C, Gosselin LE, Schertzer JD, Ryall JG, Swiderski K, Wondemaghen M (2011) Ageing prolongs inflammatory marker expression in regenerating rat skeletal muscles after injury. J Inflamm(Lond) 8(41)
Moldoveanu AL, Shephard RJ, Shek PN (2001) The cytokine response to physical activity and training. Sports Med 31:115–144
Liburt NR, Adam AA, Betancourt A, Horohov DW, McKeever KH (2010) Exercise-induced increases in inflammatory cytokines in muscle and blood of horses. Equine Vet J 42:280–288
Mesquita-Ferrari RA, Martins MD Jr, Silva JA, Silva TD, Piovesan RF, Pavesi VC, Bussadori SK, Fernandes KPS (2011) Effects of low-level laser therapy on expression of TNF-α and TGF-β in skeletal muscle during the repair process. Lasers Med Sci 26:335–340
Medrado AR, Pugliese LS, Andrade ZA (2003) Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg Med 32:239–244
Albertini R, Villaverde AB, Aimbire F, Bjordal J, Brugnera A, Mittmann J, Silva JA, Costa M (2008) Cytokine mRNA expression is decreased in the subplantar muscle of rat paw subjected to carrageenan-induced inflammation after low-level laser therapy. Photomed Laser Surg 26:19–24
Souza TO, Mesquita DA, Ferrari RA, Pinto D, JrS CL, Bussadori SK, Fernandes KPS, Martins MD (2011) Phototherapy with low-level laser affects the remodeling of types I and III collagen in skeletal muscle repair. Lasers Med Sci 26:803–814
Miyabara EH, Aoki MS, Soares AG, Moriscot AS (2005) Expression of tropism-related genes in regenerating skeletal muscle of rats treated with cyclosporin-A. Cell Tissue Res 319:479–489
Baptista J, Martins MD, Pavesi VC, Bussadori SK, Fernandes KPS, Mesquita-Ferrari RA (2010) Influence of laser photobiomodulation on collagen IV during skeletal muscle tissue remodeling following injury in rats. Photomed Laser Surg 29:12–17
Huang SS, Chiu CS, Chen HJ, Hou WC, Sheu MJ, Lin YC, Shie PH, Huang GJ (2011) Antinociceptive activities and the mechanisms of anti-inflammation of asiatic acid in mice. Evid Based Complement Alternat Med. doi:10.1155/2011/895857
Luo P, Wong YF, Ge L, Zhang ZF, Liu Y, Liu L, Zhou H (2010) Anti-inflammatory and analgesic effect of plumbagin through inhibition of nuclear factor-κB activation. J Pharmacol Exp Ther 335:735–742
Vinegar R, Truax JF, Selph JL, Johnston PR, Venable AL, McKenzie KK (1987) Pathway to carrageenan-induced inflammation in the hind limb of the rat. Fed Proc 46:118–126
Mafra de Lima F, Costa MS, Albertini R, Silva JA Jr, Aimbere F (2009) Low level laser therapy (LLLT): attenuation of cholinergic hyperreactivity, beta(2)-adrenergic hyporesponsiveness and TNF-alpha mRNA expression in rat bronchi segments in E. coli lipopolysaccharide-induced airway inflammation by a NF-kappaB dependent mechanism. Lasers Surg Med 41:68–74
Ghosh S, May MJ, Kopp EB (1998) NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Ann Rev Immunol 16:225–260
Mourkioti F, Rosenthal N (2008) NF-κB signaling in skeletal muscle: prospects for intervention in muscle diseases. J Mol Med (Berl) 86:747–759
Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS Jr (2000) NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science 289:2363–2366
Langen RC, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM (2001) Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-kappaB. FASEB J 15:1169–1180
Lange RC, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM (2004) Tumor necrosis factor-alpha inhibits myogenic differentiation through MyoD protein destabilization. FASEB J 18:227–237
Jiang Z, Clemens PR (2006) Cellular caspase-8-like inhibitory protein (cFLIP) prevents inhibition of muscle cell differentiation induced by cancer cells. FASEB J 20:2570–2572
Broussard SR, McCusker RH, Novakofski JE, Strle K, Shen WH, Johnson RW, Dantzer R, Kelley KW (2004) IL-1β impairs insulin-like growth factor I-induced differentiation and downstream activation signals of the insulin-like growth factor I receptor in myoblasts. J Immunol 172:7713–7720
Acknowledgments
The authors would like to thank UNINOVE and FAPESP (2011/17638-2; 2011/04452-8) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernandes, K.P.S., Alves, A.N., Nunes, F.D. et al. Effect of photobiomodulation on expression of IL-1β in skeletal muscle following acute injury. Lasers Med Sci 28, 1043–1046 (2013). https://doi.org/10.1007/s10103-012-1233-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1233-x