Abstract
The purpose of this article was to analyze the photobiomodulator role of low-level laser therapy (LLLT) on the skeletal muscle remodeling following cryoinjury in rats, focusing the types I and III collagen proteins. Laser phototherapy has been employed to stimulate repair in different tissues. However, its role in skeletal muscle remodeling is not yet well clarified, especially its effect on the collagen component of the extracellular matrix. Fifty adult Wistar rats were divided into four groups: control, sham, cryoinjury, and laser-treated cryoinjury. Laser irradiation was performed three times a week on the injured region using the InGaAlP (indium-gallium-aluminum-phosphorous) laser (660 nm; beam spot of 0.04 cm2, output power of 20 mW, power density of 0.5 mW/cm2, energy density of 5 J/cm2, 10-s exposure time, with a total energy dose of 0.2 J). Five animals were killed after short-term (days 1 and 7) and long-term (14 and 21) durations following injury. The muscles were processed and submitted to hematoxylin and eosin (H&E) and immunohistochemical staining. The histological slices were analyzed qualitatively, semi-quantitatively, and quantitatively. The data were submitted to statistical analysis using the Kruskal-Wallis test. The qualitative analysis of morphological aspects revealed that the muscle repair were very similar in cryoinjury and laser groups on days 1, 14 and 21. However, at 7 days, differences could be observed because there was a reduction in myonecrosis associated to formation of new vessels (angiogenesis) in the laser-treated group. The analysis of the distribution of types I and III collagen, on day 7, revealed a significant increase in the depositing of these proteins in the laser-treated group when compared to the cryoinjury group. InGaAlP diode laser within the power parameters and conditions tested had a biostimulatory effect at the regenerative and fibrotic phases of the skeletal muscle repairs, by promoting angiogenesis, reducing myonecrosis, and inducing types I and III collagen synthesis, following cryoinjury in rat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Muscle injury is a common phenomenon that may occur as a result of intense physical exercise, trauma, contusion, electrical stimulation, and others. Following injury, the skeletal muscle repair process begins, which consists of several interdependent phases: degeneration, inflammation, regeneration, fibrosis/scar formation, and remodeling. Throughout these phases, there is the activation of different cell types, along with the degradation and synthesis of intracellular proteins and components of the extracellular matrix (ECM) [1–3].
In muscle tissue, the ECM is mainly made up of types I, III, and IV collagen, laminin-2 and 4, fibronectin, tenascin, and proteoglycans, which surround the muscle fibers and offer support, protection, and maintenance of the functional integrity [3, 4]. Studies on changes to the ECM in skeletal muscle in physiological and pathological situations have been carried out to gain a better understanding of the role of each tissue protein. It is also important to develop muscle injury prevention and rehabilitation methods, select the type, load, and duration of exercise in physical training and develop new therapies for congenital and inflammatory muscle diseases. Regarding the collagen component of the ECM, there is a close relationship between an increase in collagen synthesis and the regenerative process that occurs in skeletal muscle as a response to different physical activities [5–8]. An increase in collagen synthesis following exercise may be a physiological adaptation or part of the repair process with or without evident tissue damage [6, 8, 9].
Currently, considerable emphasis has been given to low-level laser therapy (LLLT), which has demonstrated anti-inflammatory, analgesic, and reparative properties. These properties may be related to the action of the laser in the activation of cell metabolism, increase cell proliferation and collagen synthesis, the activation of lymphocytes and angiogenesis [10–13].
Investigations have been carried out to demonstrate the positive effects of LLLT either alone or in combination with other therapies for the treatment of muscle and tendon injuries, rheumatic diseases, osteoarthromyopathies, neuromuscular disorders, sports injuries, among others [14–17]. However, the biological mechanisms that trigger these positive results remain unclear and conflicting results could be observed in the literature [18, 19].
Therefore, the purpose of this article was to analyze the photobiomodulator role of LLLT on the skeletal muscle remodeling following cryoinjury in rats, focusing the types I and III collagen proteins.
Methods
The methodology employed in the present study was designed in compliance with Brazilian National Health Board Resolution 196/96 and received approval from the Ethics Committee of the Universidade Nove de Julho (process number:13/2007). The experimental design respected the principals of the Brazilian College of Animal Experimentation, which is affiliated with the International Council of Laboratory Animal Science (ICLAS) and seeks the improvement of animal experimental conduct based on three basic principles: sensitivity, good sense, and good science.
Animals
Fifty adult male rats (Rattus novegicus albinus, Rodentia mammalia – Wistar lineage) weighing between 250 and 300 g at the beginning of the procedure were maintained under controlled conditions of room temperature (22°C) and relative humidity (40%), with 12-h light/dark cycle. The animals were fed a solid chow and water ad libitum prior to and throughout the experiment.
Experimental groups
The animals were randomly divided into four groups:
- Control group (n = 5):
-
left tibialis anterior (TA) muscle with no surgical procedure or cryoinjury (evaluated on day 1)
- Sham group (n = 5):
-
left TA muscle submitted to surgical procedure without cryoinjury (evaluated on day 7)
- Cryoinjury group (n = 20):
-
left TA muscle submitted to surgical procedure and cryoinjury (evaluated on days 1, 7, 14, and 21)
- Laser group (n = 20):
-
left TA muscle submitted to surgical procedure, cryoinjury and LLLT (evaluated on days 1, 7, 14, and 21).
The 1-day and 7-day groups made up the short-term muscle remodeling evaluations, whereas 14 and 21 days group made up the long-term muscle remodeling evaluations.
The surgical procedures were performed under anesthesia with 1 ml/kg of 1% ketamina-HCL (Dopalen, Vetbrands, São Paulo, Brazil) and 2% xylazine (Anasedan, Vetbrands, São Paulo, Brazil). Surgical exposure of the TA muscle and cryoinjury were performed using the method described by Miyabara et al. (2005) [20]. Two applications of cryoinjury were performed on the muscle belle in situ. Freezing was achieved with the application of a piece of steel (0.4 × 1 cm) previously cooled in liquid nitrogen and placed on the surface of the muscle for 10 s. The procedure was repeated following an interval of 30 s. The surgical wound was closed with a polyamide suture (6–0) and the animals were kept in heated boxes (37°C) for several hours in order to avoid hypothermia.
Laser-treatment protocol
For the laser treatment, the animals were anesthetized with ketamine (Dopalen) and xylazine (Anasedan) and irradiation was performed with an indium-gallium-aluminum-phosphorus (InGaAlP) diode laser (Twin Laser, MM Optics, São Carlos, Brazil) emitted in the visible red range (660 nm), using the point method in continual mode and in contact. Applications were performed on eight points distributed throughout the cryoinjury. The following parameters were used: output power of 20 mW, 0.04 cm2 irradiation area, energy density of 5 J/cm2, and 10-s exposure time, power density of 0.5 mW/cm2 generating a total energy dose of 0.2 J per point. These parameters were selected based on previous demonstrations of the muscle repair stimulation effect of LLLT [21, 22]. Three applications were performed per week. The first application was performed 4 h after the end of the surgical procedure. Prior to each application, a digital power analyzer (Laser Check, Coherent, Inc., Santa Clara, CA, USA) was used to verify the output of the laser and ensure the reliability of the mean energy density emitted.
The animals were killed with an overdose of anesthesia. The muscles were removed and immediately frozen in cooled isopentane and stored in liquid nitrogen. The frozen muscles were cut in cross sections 10 μm in thickness on a cryostat microtome (Leica CM3050, Nussloch, Germany) and placed on glass slides previously treated with 3-aminopropylthrietoxysilane (Sigma Chemical Co., St Louis, MO, USA). The specimens were submitted to hematoxylin and eosin (H&E) staining and immunohistochemical analysis for the detection of types I and III collagen.
Immunohistochemical method
The histological sections were fixed in 20% acetone for 10 min, twice incubated for 15 min in a solution of 6% (v/v) hydrogen peroxide and methanol, rinsed with distilled water and incubated for 10 min in phosphate buffered saline (PBS) 1× and 20 min in PBS1X/2% bovine serum albumin (BSA) to block non-specific antigens. The slides were incubated in the presence of anti-collagen I primary antibody (Santa Cruz, catalog C0807), diluted at 1:100 in PBS1X/BSA solution for 1 h and anti-collagen III primary antibody (ABR, monoclonal mouse, catalog MA1-22147) diluted at 1:50 in PBS1X/ BSA solution for 1 h. The sections were then washed with PBS1X for 10 min, exposed to the secondary antibodies (1:100) (LSAB plus System Horse Radish Peroxidase, Dako) for 30 min, washed again, and incubated with the streptavidin-biotin complex for 30 min. The cuts were incubated with diaminobenzidine tetra-hydrochloride (DAB, Novocastra) and counterstained with Mayer’s hematoxylin. Negative controls were obtained through the substitution of the primary antibodies with non-immune serum.
Qualitative and semi-quantitative analyses
The qualitative and semi-quantitative analyses were performed by a previously calibrated examiner using a conventional light microscope. The examiner had undergone calibration training in advance under supervision by an experienced pathologist. Intra-examiner calibration was performed by means of a second analysis of one in every ten fields observed, applying the intraclass correlation coefficient (p < 0.01) and the Kappa coefficient test (p > 0.7) in order to determine the degree of agreement for quantitative and qualitative analyses, respectively. The examiner was unaware which experimental group of each image belonged.
The qualitative analysis of the histological sections stained with HE consisted of the description of the tissue remodeling phases, involving the presence and type of inflammatory infiltration, edema, necrosis, and new, immature fibers. The location and distribution pattern of the immunolabeling (endomysium, perimysium, epimysium) was recorded. The semi-quantitative analysis consisted of the determination of the aforementioned tissue components as absent (grade 0), slight (grade 1), moderate (grade 2) and intense (grade 3), based on the classification proposed by Walker (2006) [23].
Quantitative histomorphometric analysis of immunohistochemistry
The stained histological cuts were submitted to morphometric analysis for the quantification of types I and III collagen. For such, three histological fields of each specimen were digitalized using a conventional light microscope (Laborval, Zeiss, Germany) containing a CCD camera (Sony, Japan) connected to an image-capturing program (Captivator). The digitalized regions corresponded to the central area of the injury (region A), lateral zone (region B, to the right of the central area of the injury) and deep zone (region C, immediately below the central area of the injury), which were used for the analysis. Magnification was 100×, with standardized light intensity. Manual selection of the immunolabeled areas (positive reaction) was performed, with subsequent automated quantification using the ImageLab 2000 morphometry program (ImageLab, Brazil).
Statistical analysis
The Kruskal-Wallis test was used for the comparison of differences in scores between groups and in each experimental period in both the semi-quantitative and qualitative analyses. Comparisons between groups were made using the median test. All statistical tests were performed using SAS for Windows, v.9.1.3 and GraphPad Prism, v.4.0 programs.
Results
Qualitative analysis (morphological aspects)
H&E staining
The control group exhibited skeletal muscle with normal morphology, characterized by polygonal fibers with multiple nuclei arranged on the periphery of the cell and no signs of injury. The sham group exhibited discreet, predominantly mononuclear inflammatory infiltration, with few degenerated muscle cells (myonecrosis) and focal points of edema in the surface region.
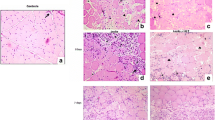
The qualitative analysis of morphological aspects revealed that the muscle repair was very similar in the cryoinjury and laser groups on days 1, 14, and 21. However, at 7 days, differences could be observed because there was a reduction in the inflammatory process and in myonecrosis associated with the emergence of numerous new, immature muscle fibers with a centralized nucleus, indicating tissue regeneration. The myonecrosis still remain most evident in the cryoinjury group (Fig. 1a). The formation of new vessels (angiogenesis) among the new muscle fibers was quite evident in this phase in the laser group (Fig. 1b).
Morphologic findings of cryoinjury (a) and laser (b) groups on 7 days after injury. a The cryoinjury group still exhibiting myonecrosis (*), associated to the emergence of numerous new, immature muscle fibers (arrowhead) with a centralized nucleus, indicating tissue regeneration. b The laser group showing the formation of new vessels (arrows) among the new muscle fibers (arrowheads). (H&E staining, original magnification, ×200)
In addition, in groups that presented no differences between those treated and those non-treated, it could be noted that on day 1 there was intense edema among the muscle fibers and moderate inflammatory infiltration, with neutrophils and macrophages dispersed among the fibers. There were a large number of necrotic muscle cells (myonecrosis), characterized by the rupture and disorganization of muscle fibers. On day 14, the muscle repair was similar between the groups. There was scant edema and inflammatory infiltration and there were a moderate number of muscle cells with a centralized nucleus. On day 21, the muscle of both groups exhibited morphological evidence of complete repair (regeneration), with no signs of inflammation and rare cells with a centralized nucleus.
Immunolabeling of type I and type III collagen
In the immunohistochemical analysis of the control muscles, collagen types I (Fig. 2a) and III (Fig. 2b) were present as a tenuous line enveloping the muscle fibers (endomysium), with more abundant labeling in the perimysium. In the muscles of the cryoinjury and laser groups, there were alterations in the intensity of the immunolabeling of these proteins in both the endomysium and perimysium, depending on the phase of muscle regeneration.
On day 1 following cryoinjury, there was a change in the labeling pattern and distribution of type I (Fig. 3a and b) and III (Fig. 4a and b) collagen in both the perimysium and endomysium. Immunolabeling revealed that both types of collagen were more diffuse among the injured muscle cells, with more intensive labeling. Moreover, some cells with a clear rupture of the sarcolemma exhibited intra-cytoplasmic labeling of these proteins. The immunolabeling for both collagen types was similar in the cryoinjury (Figs. 3a and 4a) and laser groups (Figs. 3b and 4b).
Photomicrographs of immunohistochemical labeling for type I. Cryoinjury group (a) and laser group (b) on day 1, showing diffuse immunohistochemical labeling in the interior of necrotic fibers (*). Cryoinjury group (c) on day 7, with weak labeling in the endomysium (arrows) and moderate labeling in the perimysium (*). Laser group (d) on day 7, with intensive labeling (arrow) on endomysium and perimysium (*). Cryoinjury group (e) and laser group (f) on day 14, with intensive labeling in the endomysium (arrow) and focal points of dispersed labeling in areas with incomplete fusion of myoblasts. g Cryoinjury group on day 21 showing tenuous labeling in endomysium of laser group (arrow) and laser group (h) revealing a more intense immunolabeling (arrow) (magnification: 400×)
Photomicrographs of immunohistochemical labeling for type III. Cryoinjury group (a) and laser group (b) on day 1, showing diffuse immunohistochemical labeling in the interior of necrotic fibers (*). Cryoinjury group (c) on day 7, with weak labeling in the endomysium (arrows) and moderate labeling in the perimysium (*). Laser group (d) on day 7, with intensive labeling (arrow) on endomysium and perimysium (*). Cryoinjury group (e) and laser group (f) on day 14, with intensive labeling in the endomysium (arrow) and focal points of dispersed labeling in areas with incomplete fusion of myoblasts. Cryoinjury group (g) on day 21 showing tenuous labeling in endomysium of laser group (arrow) and laser group (h) revealing a more intense immunolabeling (arrow) (magnification: 400x)
On day 7, the cryoinjury group exhibited weak immunolabeling of type I (Fig. 3c) and III (Fig. 4c) collagen, either delimiting the endomysium of young muscle fibers or in a dispersed manner in areas in which there was the incomplete fusion of myoblasts. The laser group exhibited more intensive immunolabeling to collagen type I (Fig. 3d) and type III (Fig. 4d) in this period.
On day 14, there was moderate labeling for both types of collagen in both the cryoinjury (Figs. 3e and 4e) and laser groups (Figs. 3f and 4f). These proteins enveloped the muscle fibers (endomysium). Focal points of dispersed labeling occurred in sites in areas in which there was the incomplete fusion of myoblasts.
On day 21, the labeling pattern of collagen types I and III in the cryoinjury group (Figs. 3g and 4g) was similar to that of the control muscles. The laser group revealed a more intense immunolabeling for both collagen types (Figs. 3h and 4h) especially delimiting the endomysium.
Semi-quantitative analysis
The semi-quantitative analysis of edema, myonecrosys, inflammatory infiltration , new and immature fibers are illustrated on Fig. 5.
Edema
The analysis of the edema scores revealed an increase in the buildup of interstitial fluid after 1 day at cryoinjury group and laser group. However, a reduction in edema level was observed over the time in both cryoinjury and laser groups. An individual analysis revealed a statistically significant reduction of edema between days 1 and 21 in both cryoinjury and laser groups (p < 0.05).
There was a statistically significant difference between the control and sham groups (p = 0.0009), indicating edema following surgical trauma, as expected. In comparison to the control group, both the cryoinjury and laser groups had higher degrees of edema, with statistically significant differences on days 1, 7, and 14. There were no statistically significant differences in edema between the cryoinjury and laser groups at any of the evaluation times.
Myonecrosis
The analysis of myonecrosis within each group revealed significant differences in the cryoinjury (p = 0.0228) and laser (p = 0.00129) groups. In the cryoinjury group, these differences were between days 1 and 14 and between days 1 and 21, indicating a significant reduction in myonecrosis beginning with day 14. In the laser group, there were significant differences between days 1 and 7, days 1 and 14, and days 1 and 21, indicating a significant reduction in myonecrosis beginning with day 7. There were no differences between the control and sham groups regarding the presence of myonecrosis (p = 0.1380). The cryoinjury and laser groups exhibited higher degrees of myonecrosis in comparison to the control group, with statistically significant differences on days 1 and 7. There was no significant difference in the degree of myonecrosis between the cryoinjury and laser groups at any evaluation time.
Inflammatory infiltration
There was a statistically significant increase in inflammatory infiltration in the cryoinjury group (p = 0.0153), but Dunn’s test did not reveal at which evaluation times this difference occurred. The laser group did not exhibit a statistically significant difference in inflammatory infiltration over time (p = 0.0512). In comparison to the control, the sham group had a significantly higher number of inflammatory cells (p = 0.0009). Comparing the cryoinjury and laser groups with the control, there was a significantly greater degree of inflammatory infiltration in the former groups on days 1 and 7. The cryoinjury group exhibited a significantly greater degree of inflammatory infiltration in comparison to the sham group on day 7 (p = 0.0047). There was no significant difference in the degree of inflammatory infiltration between the cryoinjury and laser groups at any evaluation time.
New, immature muscle fibers
The presence of new, immature muscle fibers, characterized by fibers with a small diameter and a centralized nucleus, was analyzed at each evaluation time. There was a statistically significant difference in both the cryoinjury (p = 0.0017) and laser (p = 0.0147) groups. In both groups, this difference was between days 1 and 7, demonstrating that these cells were absent on day 1 and abundant on day 7 in the area of muscle repair. Comparing the cryoinjury and laser groups with the control, there were significant differences in the number of these fibers between days 7, 14, and 21. There was a significantly greater number of new, immature cells in the cryoinjury (p = 0.0047) and laser (p = 0.0047) groups when compared to the sham group. There was no significant difference in the number of these fibers between the cryoinjury and laser groups at any evaluation time.
Quantitative histomorphometric analysis of immunolabeling for type I and type III collagen
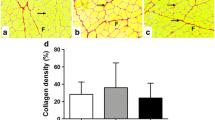
Table 1 shows the average percentage of immunolabeled extracellular matrix (ECM) for type I and III collagen in the histomorphometric analysis according to experimental group and evaluation time.
The control and sham groups exhibit similar results regarding type I collagen immunolabeling. There was a statistically significant difference in collagen type I quantification between the control and cryoinjury group on 14 days (p = 0.022), with an increase in the presence of these proteins in the cryoinjury group. The laser group also exhibited a greater amount of collagen in comparison to the sham group in all the experimental times. In the comparison between the control and laser groups, the latter exhibited a significantly greater amount of collagen depositing at 7 (p = 0.022), 14 (p = 0.022) and 21(p = 0.001) days. The laser group exhibited the most quantity of collagen type I on day 7 (p = 0.0220) when compared to the cryoinjury group at the same period.
The collagen type III analysis showed no difference between the control and sham group. Comparing the control and cryoinjury group, a statistically significant difference was observed on day 7 (p = 0.022) with a decrease of collagen type III component on cryoinjury group followed by increase of this protein on 14 days (p = 0,0013). In the comparison between the control and laser groups, the latter exhibited a significantly greater amount of collagen depositing at 7 (p < 0.0001), 14 (p = 0.0036) and 21 (p < 0.0001) days. The laser group exhibited a higher deposition of collagen type III when compared to cryoinjury group on days 7 (p < 0.0001) and 21(p < 0.0001).
Discussion
Muscle injuries occur through a variety of mechanisms, but the general repair process is similar in most cases [1, 4]. Treatment modalities include surgical repair, rest, cryotherapy, thermotherapy, hydrotherapy, electrotherapy, compression, elevation, immobilization and NSAIDs [24].
Therapeutic advantages of LLLT for inflammatory conditions have been described and the effect of this treatment modality on the regeneration of skeletal muscle has been evaluated in some studies [21, 25, 26]. The present study analyzed the effect of low-level InGaAlP laser (660 nm, power density of 0.5 mW/cm2, energy density of 5 J/cm2 and total energy dose of 0.2 J) on skeletal muscle healing following cryoinjury. The results demonstrated that LLLT has a biostimulatory effect, particularly with regard to the analysis of myonecrosis, angiogenesis, and the remodeling of types I and III collagen.
The complex muscle healing process comprises several phases, including degeneration, inflammation, regeneration, fibrosis/scar formation, and remodeling. The first and second phases involve the formation of hematoma, tissue necrosis, degeneration, and cellular inflammatory response, which occur in the first few days following trauma. The third phase consists of phagocytosis of the degenerated tissue, the regeneration of muscle fibers, tissue production, and vascular growth, which occur around 7–10 days post-injury, with a maximal peak in the second week and a reduction in the third week. The fibrosis and remodeling phases involve the deposition of collagen fibers, maturation of the regenerated fibers, and reorganization of the tissue. Throughout these phases, there is a close relationship between cell–cell and cell–ECM interactions, the aim of which is to morphologically and functionally reestablish the tissue [1–4]. In the present study, all phases of muscle repair were analyzed based on morphology and semi-quantitative methods in the cryoinjury and laser groups. All groups exhibited muscle repair at 21 days. However, in the comparison of histopathological aspects of the cryoinjury and laser groups, the data suggest that laser therapy accelerates muscle repair. The laser group exhibited a reduction in the presence of myonecrosis on day 7 and an increase in new vessels (angiogenesis) among the new muscle fibers, indicating a decrease of the inflammatory phase. A number of studies using different types of laser and parameters report that LLLT decreases myonecrosis [26, 27], and stimulates angiogenesis [28–31] , thereby favoring skeletal muscle healing [32, 33]. These effects could be the result of laser light action on the cellular level causing important biological reactions, such as cell proliferation (fibroblasts and satellite cells) [25, 32, 34], collagen synthesis, the release of growth factors, macrophage/lymphocyte stimulation [22, 35].
Angiogenesis is an important part of wound healing, as it re-establishes circulation at the site of injury, thereby limiting ischemic necrosis and permitting repair. Salate et al. found that 660-nm LLLT was efficient in increasing angiogenesis during tendon repair [29]. However, cell response to LLLT may be dependent on the parameters used. The mean output of 40 mW for 10 s promoted an increase in neovascularization after 3 days of application and a greater number of vessels were observed after 5 days of irradiation with the mean output of 10 mW. On day 7, the group irradiated with 40 mW had a fewer number of vessels than that observed on day 5. Nakano et al. found that LLLT (Ga-Al-As laser; 830 nm; 60 mW; total of 180 s) promoted recovery from disuse muscle atrophy in association with the proliferation of satellite cells and angiogenesis [30]. According to the authors, differences in skeletal muscle reactions depend on the LLLT parameters as well as other conditions, such as the type of muscle fiber and region of muscle irradiated (deep or superficial).
Another important aspect of the inflammatory phase of muscle healing is phagocytosis of the degenerated tissue (myonecrosis) and the synthesis of cytokines and/or growth factors by macrophages. Studies using different types of laser and parameters report that LLLT decreases muscle damage [14, 16, 26, 27, 36, 37]. Iyomasa et al. analyzed the intercellular substance in muscle connective tissue irradiated with 633 nm, 5 or 10 J/cm2 for 7 days and observed that this site was cleaner (devoid of degeneration fragments) in comparison to the control, indicating ultrastructurally that macrophages were activated in the process [38]. A similar result was obtained in the present study with the use of InGaAlP laser (660 nm, 5 J/cm2) after 7 days. It has been shown that 660-nm laser-irradiated macrophages produce conditioned media that contain growth factors capable of modulating the proliferation of fibroblasts. By increasing fibroblast production, the proliferative phase of repair could be accelerated [39]. Using the same injury model and laser parameters used in the present study, Mesquita–Ferrari et al. found a decrease in TGF-β and TNF-α in the LLLT group at 7 days. The decrease in TNF-α mRNA expression observed in the LLLT group at 1 and 7 days likely induces a decrease in the recruitment of polymorphonuclear cells and, consequently, the progression of the inflammatory process [22]. The results of the present study and those reported by Mesquita-Ferrari corroborate the biomodulatory effect of LLLT, which reduces inflammation and accelerates tissue regeneration [11, 14, 17, 18, 33, 40].
The fibrotic phase of muscle healing occurs after around 14 days, with a peak at 21 days, and is characterized by the synthesis of collagen, which is the major component of the ECM of muscles, especially types I and III [4–6]. Type I collagen, which is more commonly found in dense conjunctive tissue, is necessary for the stabilization of the tissue architecture, while type III collagen, which is more commonly found in loose conjunctive tissue, has an important function in tissue elasticity [5, 6]. In skeletal muscle, collagen plays a fundamental role in maintaining the functional integrity of the fibers and the adequate transmission of strength during muscle contraction. In the present study, immunolabeling for both types of collagen was intensive on day 1. However, it was not possible to perform a quantitative analysis, as these proteins did not exhibit a normal arrangement in the injured site and were in a process of degradation together with the injured muscle cells. Therefore, the immunolabeling was indicative of a decomposing ECM. On day 7, there was a significant increase in type I and III collagen in the laser group in comparison to the control and cryoinjury groups. These results indicate that laser accelerates muscle repair through the synthesis of collagen proteins. This finding is in agreement with other studies, in which the best results with LLLT were achieved with 660-nm irradiation in the early stages of wound healing [22, 39–41]. In the present study, the cryoinjury and laser groups exhibited a greater amount of collagen (types I and III) than the control group after 21 days. This indicates that, even when skeletal muscle exhibits morphological aspects of complete repair, the ECM is not totally remodeled and the final phase of muscle healing occurs after 21 days. The laser group exhibited a greater quantity of type III collagen in this period. As type III collagen is associated with tissue elasticity, one may infer that the irradiated group had greater elasticity, which could promote muscle resistance, a lower risk of the recurrence of injury and the prevention of overstretching of the muscle fiber bundles [43].
The effect of LLLT on collagen synthesis is controversial. Some studies have shown an increase in collagen production and wound tensile strength following LLLT therapy [40, 44, 45]. However, other authors report a decrease in collagen production [11, 46, 47]. According to Reddy et al., irradiation with LLLT accelerates collagen synthesis in a number of different biologic models. This effect is believed to be based on the photochemical action of the laser, altering the cellular respiratory chain and oxidoreduction properties of the cell, which results in either cell proliferation, differentiation, or protein synthesis [45]. Yamamoto et al. found that LLLT at a wavelength of 632.8 nm induced an increase in procollagen synthesis in human fibroblast cultures [42]. Pugliese et al. found that Ga-Al-As laser induced biomodulation of collagen and elastic fibers, as evidenced by an increase in the deposition of these fibrillary elements in the animals given laser therapy, and the 4-J/cm2 energy density provided more significant results than 8-J/cm2 [41]. The effects of LLLT on muscles vary considerably among previously published studies and depend on the type of laser used, wavelength and other parameters as well as whether the laser is in direct contact with the muscle or over the skin. Iyomasa et al. found abundant collagen fibers in the intercellular substance of muscles irradiated with 633 nm at energy densities of both 5 and 10 J/cm2 light in comparison with the control [38]. Baptista et al. found that the 660-nm laser promoted an increase in collagen IV in skeletal muscle following cryoinjury within the same timeframe in which an increase in types I and III collagen was observed in the present study [21].
Wavelength, irradiation time, and dosages as well the condition of the treated tissue remain a matter of controversy when considering the efficiency of laser therapy in tissue healing. Further studies should be carried out with different types of laser and different parameters in order to gain a better understanding of the influence of this type of therapy on the distribution and amount of collagen and other components of the ECM during the muscle repair process and establish protocols that can assist in post-trauma muscle rehabilitation
Conclusions
The inGaAlP diode laser (indium-gallium-aluminum-phosphorous) within the power parameters and conditions tested had a biostimulatory effect at the regenerative and fibrotic phases of the skeletal muscle repairs, by promoting angiogenesis, reducing myonecrosis, and inducing types I and III collagen synthesis, following cryoinjury in the rat.
References
Huard J, Li Y, Fu FH (2002) Muscle injuries and repair: current trends in research. J Bone Joint Surg Am 84-A:822–32
Carmeli E, Moas M, Reznick AZ, Coleman R (2004) Matrix metalloproteinases and skeletal muscle: a brief review. Muscle Nerve 29:191–197
Kjaer M (2004) Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84:649–98
Chiquet M, Matthison M, Koch M, Tannheimer M, Chiquet-Ehrismann R (1996) Regulation of extracellular matrix synthesis by mechanical stress. Biochem Cell Biol 74:737–744
Myllyla R, Myllyla VV, Tolonen U, Kivirikko KI (1982) Changes in collagen metabolism in diseased muscle. I. Biochemical studies. Arch Neurol 39:752–755
Han XY, Wang W, Komulainen J, Koskinen SO, Kovanen V (1999) VihkoV, Trackman PC, Takala TE. Increased mRNAs for procollagens and key regulating enzymes in rat muscle following downhill running. Pflugers Arch 437:857–864
Kovanen V, Suominen H, Risteli J, Risteli L (1988) Type IV collagen and laminin in slow and fast skeletal muscle in rats–effects of age and life-time endurance training. Coll Relat Res 8:145–53
Koskinen SOA, Wang W, Ahtikoski AM, Kjaer M, Han XY, Komulainen J, Kovanen V, Takala TES (2001) Acute exercise induced changes in rat skeletal muscle mRNAs and proteins regulating type IV collagen content. Am J Physio Regul Integr Comp Physiol 280:R1292–R1300
Ahtikoski AM, Tuominen H, Korpelainen JT, Takala TE, Oikarinen A (2004) Collagen synthesis and degradation in polyneuropathy and myopathies. Muscle Nerve 30:602–8
Almeida-Lopes L, Rigau J, Zângaro RA, Guiduli-Neto J, Jaeger MMM (2001) Comparison of the low level therapy effects on cultured gingival fibroblasts proliferation using different irradiance and fluence. Lasers Surg Med 29:179–184
Pereira AN, Eduardo CP, Matson E, Marques MM (2002) Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts. Lasers Surg Med 31:263–267
Marques MM, Pereira AN, Fujihara NA, Nogueira FN, Eduardo CP (2004) Effect of low-power laser irradiation on protein synthesis and ultrastructure of human gingival fibroblasts. Lasers Surg Med 34:260–265
Longo L, Evangelista S, Tinacci G, Sesti AG (1987) Effect of diodes-laser silver arsenide–aluminium (Ga–Al–As) 904 nm on healing of experimental wounds. Lasers Surg Med 7:444–447
Liu XG, Zhou YJ, Liu TC, Yuan JQ (2009) Effects of low-level laser irradiation on rat skeletal muscle injury after eccentric exercise. Photomed Laser Surg Aug 21
Lopes-Martins RA, Marcos RL, Leonardo PS, Prianti AC Jr (2006) Muscará MN, Aimbire F, Frigo L, Iversen VV, Bjordal JM Effect of low-level laser (Ga-Al-As 655 nm) on skeletal muscle fatigue induced by electrical stimulation in rats. J Appl Physiol 101:283–8
Sussai DA, Carvalho Pde T, Dourado DM, Belchior AC, dos Reis FA (2010) Low-level laser therapy attenuates creatine kinase levels and apoptosis during forced swimming in rats. Lasers Med Sci 25:115–20
Reinoso C, Cremonezzi D, Moya M, Soriano F, Palma J, Campana V (2010) Helium–neon laser reduces the inflammatory process of arthritis. Photomed Laser Surg 28:125–129
Giuliani A, Fernandez M, Farinelli M, Baratto L, Capra R, Rovetta G, Monteforte P, Giardino L, Calzà L (2004) Very low level laser therapy attenuates edema and pain in experimental models. Int J Tissue React 26:29-37.
Brosseau L, Wells G, Marchand S, Gaboury I, Stokes B, Morin M, Casimiro L, Yonge K, Tugwell P (2005) Randomized controlled trial on low level laser therapy (LLLT) in the treatment of osteoarthritis (OA) on the hand. Laser Surg Med 36:210–219
Miyabara EH, Aoki MS, Soares AG, Moriscot AS (2005) Expression of tropism-related genes in regenerating skeletal muscle of rats treated with cyclosporin-A. Cell Tissue Res 319:479–489
Baptista J, Martins MD, Pavesi VC, Bussadori SK, Fernandes KP, Pinto Júnior Ddos S, Ferrari RA (2011) Influence of laser photobiomodulation on collagen IV during skeletal muscle tissue remodeling after injury in rats. Photomed Laser Surg 29:11–7
Mesquita-Ferrari RA, Martins MD, Silva JA Jr, da Silva TD, Piovesan RF, Pavesi VC, Bussadori SK, Fernandes KP (2011) Effects of low-level laser therapy on expression of TNF-α and TGF-β in skeletal muscle during the repair process. Lasers Med Sci 26:335–40
Walker RA (2006) Quantification of immunohistochemistry-issues concerning methods, utility and semiquantitative assessment I. Histochemistry 49:406–410
Reilly T, Ekblom B (2005) The use of recovery methods post-exercise. J Sports Sci 23:619–627
Bibikova A, Oron U (1995) Regeneration in denervated toad (bufoviridis) gastrocnemius muscle and the promotion of the process by low energy laser irradiation. Anat Rec 241:123–128
Barbosa AM, Villaverde AB, Sousa LG, Munin E, Fernandez CM, Cogo JC, Zamuner SR (2009) Effect of low-level laser therapy in the myonecrosis induced by Bothrops jararacussu snake venom. Photomed Laser Surg 27:591–7
Dourado DM, Favero S, Baranauskas V, Cruz-Hofling MA (2003) Effects of the GaAs laser irradiation on myonecrosis caused by Bothrops moojeni snake venom. Lasers Surg Med 33:352–357
Deveci D, Marshall JM, Egginton S (2002) Chronic hypoxia induces prolonged angiogenesis in skeletal muscles of rat. Exp Physiol 87:287–291
Salate AC, Barbosa G, Gaspar P, Koeke PU, Parizotto NA, Benze BG, Foschiani D (2005) Effect of In-Ga-Al-P diode laser irradiation on angiogenesis in partial ruptures of Achilles tendon in rats. Photomed Laser Surg 23:470–5
Nakano J, Kataoka H, Sakamoto J, Origuchi T, Okita M, Yoshimura T (2009) Low-level laser irradiation promotes the recovery of atrophied gastrocnemius skeletal muscle in rats. Exp Physiol 94:1005–15
Smith LE, Shen W, Perruzzi C, Soker S, Kinose F, Xu X, Robinson G, Driver S, Bischoff J, Zhang B, Schaeffer JM, Senger DR (1999) Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med 5:1390–1395
Weiss N, Oron U (1992) Enhancement of muscle regeneration in the rat gastrocnemius by low energy laser irradiation. Anat Embryol 186:497–503
Amaral AC, Parizotto NA, Salvini TF (2001) Dose-dependency of low-energy HeNe laser effect in regeneration of skeletal muscle in mice. Lasers Med Sci 16:44–51
Gallegly JC, Turesky NA, Strotman BA, Gurley CM, Peterson CA, Dupont-Versteegden EE (2004) Satellite cell regulation of muscle mass is altered at old age. J Appl Physiol 97:1082–1090
Yu W, Naim JO, McGowan M, Ippolito K, Lanzafame RJ (1997) Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria. Photochem Photobiol 66:866–871
Leal Junior EC, Lopes-Martins RA, de Almeida P, Ramos L, Iversen VV, Bjordal JM (2010) Effect of low-level laser therapy (GaAs 904 nm) in skeletal muscle fatigue and biochemical markers of muscle damage in rats. Eur J Appl Physiol 108:1083–1088
Cressoni MD, Dib Giusti HH, Casarotto RA, Anaruma CA (2008) The effects of a 785-nm AlGaInP laser on the regeneration of ratanterior tibialis muscle after surgically-induced injury. Photomed Laser Surg (Ahead of print). doi:10.1089/pho.2007.2150
Iyomasa DM, Garavelo I, Iyomasa MM, Watanabe IS, Issa JP (2009) Ultrastructural analysis of the low level laser therapy effects on the lesioned anterior tibial muscle in the gerbil. Micron 40:413–8
Youn SR, Dyson M (1990) The effect of therapeutic ultrasound on angiogenesis. Ultrasound Med Biol 16:261–269
Silveira PC, Silva LA, Fraga DB, Freitas TP, Streck EL, Pinho R (2009) Evaluation of mitochondrial respiratory chain activity in muscle healing by low-level laser therapy. J Photochem Photobiol B 95:89–92
Pugliese LS, Medrado AP, Reis SR, Andrade Zde A (2003) The influence of low-level laser therapy on biomodulation of collagen and elastic fibers. Pesqui Odontol Bras 17:307–13
Yamamoto Y, Kono T, Kotani H, Kasai S, Mito M (1996) Effect of low-power laser irradiation on procollagen synthesis in human fibroblasts. J Clin Laser Med Surg 14:129–32
Purslow PP (1989) Strain-induced reorientation of an intramuscular connective tissue network: implications for passive muscle elasticity. J Biomech 22:21–31
Yew DT, Li WW, Pang KM, Mok YC, Au C (1989) Stimulation of collagen formation in the intestinal anastomosis by low dose He-Ne laser. Scanning Microsc 3:379–85
Reddy GK, Stehno-Bittel L, Enwemeka CS (1998) Laser photostimulation of collagen production in healing rabbit Achilles tendons. Lasers Surg Med 22:281–7
Fillipin LI, Mauriz JL, Vedovelli K, Moreira AJ, Zettler CG, Lech O, Marroni NP, Gonzalez-Gallego J (2005) Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized Achilles tendon. Lasers Surg Med 37:293–300
Morrone G, Guzzardella Ga, Orienti L, Giavaresi G, Fini M, Rocca M, Torricelli P, Martini L, Giardino R (1998) Muscular trauma treated with a Ga-Al-As diode laser: In vivo experimental study. Lasers Med Sci 13:293–298
Acknowledgments
The authors are grateful to the Brazilian fostering agency Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (process no 07/55439-6). We thank Richard Boike for his helpful comments and expertise with the English grammar.
Author disclosure statement
The authors declare that there are no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Souza, T.O.F., Mesquita, D.A., Ferrari, R.A.M. et al. Phototherapy with low-level laser affects the remodeling of types I and III collagen in skeletal muscle repair. Lasers Med Sci 26, 803–814 (2011). https://doi.org/10.1007/s10103-011-0951-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-011-0951-9