Abstract
The purpose of this study was to evaluate the effects of low-level laser therapy (LLLT) on morphological aspects, IL-6 and IL-1β expressions, as well as the distribution and organization of collagen in the tibialis anterior (TA) muscle of elderly rats submitted to cryoinjury. Histological photomicrographs were taken of TA muscles stained with HE and picrosirius red. Immunohistochemistry was used for the evaluation of IL-6 and IL-1β. Male Wistar rats, aged 20 months, were distributed into three groups: (1) control animals not injured or treated with LLLT (n = 5), (2) cryoinjury without LLLT treatment (n = 15), and (3) cryoinjury treated with infrared LLLT (n = 15). LLLT was applied to the TA 2 h after of the injury induction and consisted of daily applications until the sacrifice (1, 3, and 7 days). The following parameters were used: λ = 780 nm, power density 1 W/cm2, output power 40 mW, 10 s per point, 8 points, and 3.2 J of total energy. In the histomorphological analysis, the treated group exhibited a significant decrease in inflammatory infiltrate (p < 0.001) as well as an increase immature fibers and new blood vessels at 7 days compared to the untreated group (p < 0.05). Furthermore, treatment induced a better collagen distribution and organization at 7 days in comparison to the untreated group (p < 0.05). In conclusion, LLLT demonstrated a modulatory effect on the muscle repair process in elderly animals with regard to the collagen remodeling and morphological aspects of muscle tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is a biological process of changes over time that may result in functional decline and eventually in the death [1]. Skeletal muscle comprises 40 to 45% of body weight and constitutes dynamic tissue with a high capacity in terms of remodeling, repair, and regeneration [2]. In humans, 40% of muscle mass is lost between 20 and 80 years of age [3,4,5]. During the aging process, there is less replacement of damaged muscle fibers by new fibers and the muscle inflammatory response becomes more prolonged. This sequence of events results in the loss of muscle mass, a reduced number of muscle fibers, reductions in both strength and the quality of movements, concomitant fibrosis, and extracellular matrix deposition [6,7,8,9]. Such factors favor the occurrence of injuries in older adults and reduce the regenerative capacity of skeletal muscle [10].
The pro-inflammatory state that occurs during aging is believed to be related to the development of sarcopenia, as elevated levels of cytokines diminish the synthesis of muscle protein. Indeed, high plasma levels of IL-6, TNF, and IL-1 are found in older adults [3], which are associated with muscle loss and lower strength. IL-6, in particular, plays a role in accelerating sarcopenia and frailty [11, 12]. With the increase in life expectancy, it is essential to establish new therapeutic resources in clinical practice to improve the quality of the muscle repair process, motor quality, and injury recovery time in older adults [10].
The comprehension of aging process may assist the development of new treatment strategies for age-associated diseases, and animal models represent an important tool for preclinical biomedical research [1]. Low-level laser therapy (LLLT) has been widely administered as a therapeutic resource in studies involving humans and/or experimental models [13, 14]. When applied to injured muscle tissue, LLLT is able to reduce pain, edema, leukocyte influx, and myonecrosis as well as alter the expression of cytokines, inflammatory enzymes, and collagen remodeling, thereby accelerating the repair process [15,16,17,18,19,20,21,22].
The use of LLLT is well grounded in the literature due to its effect on inflammatory disorders, tissue regeneration, and skeletal muscle repair. LLLT has positive modulatory effects on muscle precursor cells and collagen remodeling [22,23,24]. However, the few studies that associate LLLT with skeletal muscle regeneration in older animals differ with regard to the dosimetric parameters employed [25, 26]. Although LLLT may represent an important therapeutic tool in elderly population, studies in this area are still limited.
Therefore, purpose of the present study was to evaluate the effects of LLLT (780 nm, 40 mW, and 10 J/cm2) on morphological aspects and the remodeling of connective tissue in the TA muscle of elderly rats submitted to cryoinjury.
Methods
Animals
Male Wistar rats (aged 20 months, body mass 400 ± 15 g) were randomly divided into three groups: (1) control group not submitted to injury or treatment (n = 5), (2) cryoinjury without treatment (n = 15), and (3) cryoinjury treated with infrared LLLT 780 nm (n = 15). The control group was euthanized on the first day after the onset of the experiment. Animals from the untreated and treated cryoinjury groups were euthanized on days 1, 3, and 7 (n = 5 per day) following the induction of injury.
Cryoinjury procedure
The surgical procedures were performed as described by Miyabara et al. [27] and Mesquita-Ferrari et al. [18]. The animals were weighed and anesthetized with an intraperitoneal injection of a mixture proportional to the body mass of 80 mg/kg ketamine (Dopalen, Vetbrands, São Paulo, Brazil) and 10 mg/kg of xylazine (Anasedan, Vetbrands, São Paulo, Brazil). The TA muscle was surgically exposed, and cryoinjury was performed with two applications (10 s each) of a flat-ended metal rod (3 mm) previously cooled in liquid nitrogen directly to the ventral surface of the muscle. After the procedure, the incision was sutured with polyamide thread and the animals were kept in heated cages to avoid hypothermia. At the end of the experimental protocol, the animals were euthanized with an overdose of ketamine (240 mg/kg) and xylazine (30 mg/kg) and the TA muscles were removed.
Laser irradiation
The laser device was an aluminum-gallium-arsenide (AlGaAs) Twin Laser® (MMOptics, São Carlos, SP, Brazil) with a beam spot of 0.04 cm2, an output power of 40 mW, a wavelength of 780 nm, an energy density of 10 J/cm2, a power density of 1 W/cm2, and an exposure time of 10 s. The laser beam was placed in contact with the skin surface corresponding to the cryoinjured area, and radiation was applied to eight points within the area. The energy per point was 0.4 J, giving a total of 3.2 J per treatment. A LaserCheck power meter (MM Optics, São Carlos, SP, Brazil) was used to determine the output of the equipment. The experiments were performed with standardized procedures. Laser irradiation was initiated 2 h following injury and performed daily until sacrifice (1, 3, or 7 days).
Qualitative and quantitative morphological analysis of TA muscle
Muscle samples were collected, hemisected in the middle of injured area, and fixed in 10% buffered formalin. The samples were then embedded in paraffin and cut transversely to a thickness of 10 μm using a microtome (Leica RM2125, Nussloch, Germany). Hematoxylin-eosin (HE) staining was used for routine histological examination under conventional light microscopy (Zeiss Axioplan 2, Germany). The qualitative analysis of the histological sections stained with HE involved a description of the presence and type of inflammatory infiltrate, edema, myonecrosis, immature muscle fibers, and blood vessels, which were quantified per area [22, 28]. Three histological sections of the muscle were examined and counted for each animal. In each cut, five areas corresponding to 50% of the injury total area were photographed (magnification × 400) using a conventional light microscope (Zeiss Axioplan 2, Germany). Total inflammatory cells, myonecrosis, blood vessels, and immature (new) muscle fibers were determined using the “cell counter” plug-in of the ImageJ software (National Institutes of Health, USA).
Analysis of collagen distribution and organization
Additional cuts were stained with picrosirius red (Sigma, St. Louis, MO, USA) using the method described by Junqueira et al. [29] and examined with the aid of a polarized light microscope (Pol-Interferential Photomicroscope, Model 61282, Carl Zeiss, Germany). The images were analyzed using the ImageJ program (National Institutes of Health, USA). The relative area occupied by collagen was calculated in relation to the area of the total cut, as described by Hadi et al. [28] and Alves et al. [22].
Immunohistochemical analysis for IL-6 and IL-1β
After being embedded in paraffin, TA muscle specimens were cut into sections measuring 5 μm in thickness. Dewaxing was performed with xylene, and the samples were immersed in alcohol, followed by incubation in a 3% hydrogen peroxide solution diluted in Tris-buffered saline (TBS) (pH 7.4). Incubation was then performed with 3% goat serum (20 min), and the samples were blocked by immersion in citrate buffer solution (pH 6.0) for 20 min at 95 °C for antigen recovery. The slides were then incubated with anti-IL-6 and anti-IL-1β antibodies.
The samples were stored in a humidified chamber at 4 °C overnight, washed in TBS, and incubated for 30 min with N-Histoin Simple Stain (Ni-chirei Biosciences Inc., Tokyo, Japan). The samples were incubated with 3,3-diaminobenzidine and chromogenic solution (Dako) for 5 min at room temperature, stained with hematoxylin, and covered. Primary antibodies were suspended in PBS/1% bovine serum albumin. In the microscopic analysis, five fields representing 50% of the injured area were photographed (Leica Micro-Systems, Wetzlar, Germany) at a magnification of × 400. Image analysis was performed using the cell counter plug-in of the ImageJ program (free software, National Institutes of Health, Bethesda, MD, USA). Values were obtained from an average of integrated optical density (IOD).
Statistical analysis
The data were analyzed with the aid of GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA). The Kolmogorov-Smirnov test was used to determine the data distribution (normal or non-normal). Data with parametric distribution were submitted to the one-way analysis of variance (ANOVA) followed by Tukey’s test for comparisons among groups. Confidence levels were adjusted to 95% (p < 0.05).
Results
Qualitative morphological analysis of TA muscle
The histological slices of the TA muscle in the control group exhibited normal morphology (fibers with peripheral nuclei; absence of lesions and inflammatory process) (Fig. 1a). The injury group without laser treatment exhibited moderate infiltration (neutrophils and mononuclear cells) between muscle fibers, areas of myonecrosis (necrotic fibers), and edema between fibers at days 1 and 3 (Fig. 1b, d). The injured group submitted to LLLT exhibited less inflammatory infiltrate and myonecrosis compared to the untreated injury group in the same period (Fig. 1c, e). At 7 days, the untreated injury group exhibited intense inflammatory infiltrate and myonecrosis (Fig. 1f), whereas the treated injury group had more preserved histological muscle fibers, with less myonecrosis and inflammatory infiltrate (Fig. 1g).
Sections of histological photomicrography of anterior tibialis muscles, stained with HE (original magnification × 100). Arrow indicates inflammatory cell infiltrate; asterisk indicates edema; the caret symbol indicates mature blood vessels; the triangle indicates myonecrosis, and the square indicates immature muscle fibers. a Control muscle: normal muscle morphology. b One-day lesion: inflammatory infiltrate, edema, and myonecrosis. c Injury + LLLT after 1 day: presence of edema and mild inflammatory infiltrate. d Injury after 3 days: edema, myonecrosis, and adjacent inflammatory infiltrate. e Lesion + LLLT after 3 days: edema, myonecrosis, and leukocyte infiltration (arrow). fInjury after 7 days: intense inflammatory infiltrate, myonecrosis, and presence of blood vessels. g Injury + LLLT: myonecrosis, presence of immature muscle fibers and blood vessels, and decreased amount of inflammatory infiltrate
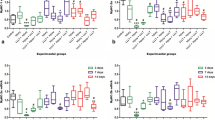
Quantitative morphological analysis of TA muscle
The control group (uninjured and untreated) exhibited normal histomorphological aspects of the TA muscle, with no inflammatory infiltrate or myonecrosis, differing significantly from the other groups (p < 0.05). The quantitative analysis (Fig. 2) revealed that laser therapy had an effect on the muscle fibers and was able to reduce the amount of myonecrosis after 1 day of treatment compared to the untreated injury group (p < 0.001).
At 3 days, the lower amount of myonecrosis and inflammatory cells in the group treated with LLLT was non-significant and there was no difference in the number of blood vessels between the untreated and treated groups. At day 7, the group submitted to laser therapy exhibited a significant reduction in the amount of inflammatory infiltrate and myonecrosis compared to the untreated group (p < 0.001) and a significant increase occurred in the number of blood vessels and immature fibers.
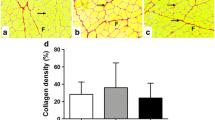
Qualitative and quantitative analysis of collagen fibers
The control group exhibited normal tissue with organized, properly distributed collagen in the endomysium, perimysium, and epimysium (Fig. 3). At days 1 and 3, the untreated and treated (LLLT) injury groups were similar with regard to collagen distribution, exhibiting fibers dispersed in the region of the inflammatory infiltrate and necrotic fibers in cell spaces. At day 7, the treated injury group exhibited better collagen organization compared to the untreated injury group, which exhibited disorganized collagen. No differences were found in the amount of collagen in the untreated and treated groups at days 1, 3, and 7, but the group submitted to LLLT exhibited better collagen organization at day 7 compared to the other treated and untreated injury groups. Moreover, an increase in the concentration of collagen was found in the untreated and treated injury groups compared to the control group at day 7 (p < 0.05).
Photomicrograph of histological section of the muscle stained with picrosirius red with or without polarized light (original magnification × 400). The figures illustrate images used to quantify the percentage of collagen fibers per area, made under a polarized light microscope. a Muscle control, showing normal morphology. b Injury group after 1 day. c Injury + LLLT group after 1 day. d Injury group after 3 days. e Injury + LLLT group after 3 days. f Injury group after 7 days. g Lesion + LLLT group after 7 days
Immunohistochemical analysis of IL-6 and IL-1β expressions in cells of TA muscle
The expression of IL-6 was significantly lower in the control group compared to the injury groups at days 1, 3, and 7 (p < 0.05), whereas no significant differences were found between the untreated and treated injury groups (Fig. 4). The expression of IL-1β also differed significantly between the control and injury groups at days 1, 3, and 7 (p < 0.05). Moreover, no differences were found among the non-treated injury group in comparison to the treated injury group in all periods (Fig. 5).
a Representative images of histological sections of muscles stained with immunohistochemistry of IL-6 at 1, 3, and 7 days after cryoinjury (scale bar 50 μm). b Relative immunostaining of IL-6 in injured area at 1, 3, and 7 days. Values expressed as mean ± SD (ANOVA/Tukey); n = 5 animals per group; *p < 0.05 compared to control group
a Representative images of histological sections of muscles stained with immunohistochemistry of IL-1β at 1, 3, and 7 days after cryoinjury (scale bar: 50 μm). b Relative immunostaining of IL-1β in injured area at 1, 3, and 7 days. Values expressed as mean ± SD (ANOVA/Tukey); n = 5 animals per group; *p < 0.05 compared to control group
Discussion
The present study was conducted to evaluate the modulation of acute inflammation by LLLT in a muscle injury model involving elderly rats. LLLT is known to be a photobiomodulation therapy, and the choice of irradiation and treatment parameters, such as wavelength, power output, beam area, total energy, irradiation time, frequency of treatment, mode of application, and the onset of treatment, can be crucial to achieving positive effects in the treatment of muscle injuries. Pertille et al. [26] used irradiation and treatment parameters different from the current study: infrared laser, wavelength 830 nm, output power 30 mW, and total energy 0.96 J; the treatment was initiated 24 h after injury induction, and only two points on the muscle were irradiated. Our study used an infrared laser (wavelength 780 nm, output power 40 mW, total energy 3.2 J) with total energy and treatment protocol different from other studies. The treatment was initiated 2 h after injury induction, was performed daily, and multiple points (eight) were irradiated on the muscle. These points are extremely important to the study of the muscle repair process because the administration of light at different times and energies can exert an influence on each stage of the repair process by modulating different cell types and events that have a direct effect on the results.
The cryoinjury procedure has previously been performed by research groups, and many studies published in the literature have demonstrated that LLLT has an effect on the skeletal muscle repair process, despite the different dosimetric parameters employed [30, 31]. It is a well-known experimental model that allows the induction of injury on the ventral surface of the muscle with similar characteristics, cleanly, causing less variation in the severity of the injury. This makes cryoinjury a highly reproducible model that mimics the natural muscle response to injury as well as the regeneration capacity [22]. However, the pressure of the iron bar against muscle tissue to induce cryoinjury was not measured, and this should be considered as a limitation.
Furthermore, most studies are either restricted to young and adult animals or do not cite the age of the animal used in the research. In contrast, very few studies have employed older animals [25, 26], probably due to the difficulty in acquiring or keeping older specimens for a prolonged time. Moreover, many of these animals do not withstand the consequences of old age, which leads to premature losses. However, there is a need to deepen knowledge on this age group, since the dynamics of aging affect the tissue repair process. Therefore, to study the effects of LLLT on the elderly in comparison to effects described for young and adult animals, we maintained the same protocols and dosimetric parameters used in previous studies [23].
Effect of laser on skeletal muscle regeneration
The process of skeletal muscle regeneration involves three interrelated phases (degeneration, inflammation/repair, and fibrosis/remodeling), which lead to the structural and functional recovery of injured muscle. In young and adult animals, the repair process occurs in a fast, orderly manner, with an initial influx of cytokines, followed by the removal of necrotic tissue, increased local vascularization, the activation of satellite cells, the formation of new functional muscle fibers, the reorganization of scar tissue, and the functional recovery of the injured muscle [6, 22, 32]. In the elderly, the repair process of injured muscle decreases markedly, since aging causes cellular alterations that affect the capacity for regeneration [33] and the progressive decline in muscle mass leads to a decrease in the capacity of skeletal muscle to synthesize new proteins [3, 6, 34,35,36,37,38]. Thus, muscle regeneration is slower in older animals [6, 39, 40]. This difference in comparison to young and adult animals may also be related to the reduction in the vascular supply to skeletal muscle, less potent chemotactic stimuli, and persistent inflammation [39, 41,42,43,44].
Mesquita-Ferrari et al. [18] demonstrated that LLLT has a positive effect on the skeletal muscle repair process, as it influences the production of pro-inflammatory and antiinflammatory cytokines, reduces the amount of inflammatory infiltrate, enhances the synthesis and organization of collagen, and maintains the functional integrity of muscle fibers [30, 31, 45]. Pertille et al. [26] evaluated the effect of laser therapy using a muscle contusion model in 18-month-old rats. LLLT (GaAlAs; wavelength 830 nm; continuous emission; output power 30 mW; beam area 0.07 cm2; energy density 4 J/cm2) was administered using the punctual method on two points—one immediately on top of the injury and another on the distal third of the muscle at a distance of 1 cm. Exposure time was 16 s on each point. Treatment was initiated 24 h after the contusion. After 21 days of treatment, a significant reduction in inflammation/regeneration area occurred in the group treated with laser compared to the only untreated injury group, whereas no significant difference between groups was found regarding the cross-sectional area of the fibers undergoing regeneration.
Morphological analysis of skeletal muscle
Vatansever et al. [25] evaluated the effects of LLLT (λ = 830 nm; energy: 0.87 J; exposure time: 29 s) after cryoinjury to the TA muscle of male Wistar rats aged 3 and 10 months (juveniles and adults, respectively). The morphological analysis revealed no difference in muscle regeneration between irradiated and non-irradiated adult rats. However, the expression of myogenic regulatory factor was upregulated, with the increased expression of MyoD mRNA in adult mice. LLLT induced an increase in the maturation of satellite cells into myoblasts and myotubes, reducing muscle atrophy in irradiated adult rats. These results are important to the development of new strategies and therapeutic modalities.
Melo Rambo et al. [46] analyzed the effect of LLLT (wavelength 660 nm; power 30 mW; total energy 2 J) on the healing of skin wounds in young and elderly rats. The histological analysis showed that the repair process is slower in elderly animals compared to young animals, especially in the inflammatory phase (nuclear polymorphism, macrophages and lymphocytes) and the proliferation phase (re-epithelialization, angiogenesis and fibrogenesis). However, the repair process was enhanced in both treated groups compared to control groups. The authors found that LLLT was effective at decreasing the expression of pro-inflammatory mediators (IL-1-β and TNF-α) throughout the experimental period, although an increase was found in the expression of the antiinflammatory cytokine IL-10, with higher levels in young animals compared to older animals.
The present findings are in agreement with data described in the literature. A decrease in inflammatory infiltrate was found in the injured elderly animals submitted to LLLT for 7 days. In previous studies, the same occurred in young adult animals submitted to the same injury model, but the reduction occurred as early as the third day of treatment [22, 23]. No significant difference in myonecrosis occurred, but a reduction was found in the elderly animals after 7 days of treatment with LLLT. The effect on the increase in blood vessels and immature fibers was apparently slower in elderly animals (7 days) than that demonstrated by our research group using young adult animals (3 days).
Analysis of collagen fibers and IL-6 and IL-1β expressions
In the present study, LLLT did not cause a significant difference in collagen area, as previously observed in young animals [23], with respect to the remodeling of the extracellular matrix during the muscle repair process in elderly rats. However, treatment promoted better organization of the collagen bundles after 7 days, which was previously observed at 3 days in young animals [23]. These findings demonstrate that LLLT exerted a positive effect on skeletal muscle repair in elderly rats.
IL-6 is produced by skeletal muscle and is associated with stimulation of muscle growth and hypertrophic myogenesis by regulating the proliferative capacity of muscle satellite cells. In the present study, IL-6 expression was increased in the groups submitted to injury compared to the control, but no difference was found between the untreated and treated groups. IL-1β is a chemotactic cytokine secreted by injured muscle cells that plays a critical role in host defense and the remodeling of muscle tissue after injury. In the present study, the expression of this cytokine in the group submitted to LLLT was increased at day 3 in comparison to days 1 and 7.
Conclusion
LLLT positively modulated the muscle repair process in elderly animals, reducing the amount of inflammatory infiltrate and myonecrosis as well as stimulating the formation of both new immature fibers and blood vessels. Furthermore, LLLT promoted improvements in the organization and remodeling of collagen fibers. It is important to emphasize that older animals responded more slowly to LLLT compared to data on young adult animals published in the literature.
References
Mitchell SJ, Scheibye-Knudsen M, Longo DL, de Cabo R (2015) Animal models of aging research: implications for human aging and age-related diseases. Annu Rev Anim Biosci 3:283–303
Ceafalan LC, Popescu BO, Hinescu ME (2014) Cellular players in skeletal muscle regeneration. Biomed Res Int 957014
Evans WJ, Paolisso G, Abbatecola AM, Corsonello A, Bustacchini S, Strollo F, Lattanzio F (2010) Frailty and muscle metabolism dysregulation in the elderly. Biogerontology 11(5):527–536
Lexell J, Taylor CC, Sjostrom M (1988) What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year old men. J Neurol Sci 84:275–294
Reid KF, Fielding RA (2012) Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev 40(1):4–12
Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Muñoz-Cánoves P (2011) Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle 1(1):21
Jang YC, Sinha M, Cerletti M, Dall'Osso C, Wagers AJ (2011) Skeletal muscle stem cells: effects of aging and metabolism on muscle regenerative function. Cold Spring Harb Symp Quant Biol 76:101–111
Brooks NE, Myburgh KH (2014) Skeletal muscle wasting with disuse atrophy is multi-dimensional: the response and interaction of myonuclei, satellite cells and signaling pathways. Front Physiol 5:99
Peake J, Della Gatta P, Cameron-Smith D (2010) Aging and its effects on inflammation in skeletal muscle at rest and following exercise-induced muscle injury. Am J Physiol Regul Integr Comp Physiol 298(6):R1485–R1495
Sobrian SK, Walters E (2014) Enhanced satellite cell activity in aging skeletal muscle after manual acupuncture-induced injury. Chin Med 5:22–33
Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB (2002) Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci 57(5):M326–M332
Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, Colbert LH, Pahor M, Rubin SM, Tylavsky FA, Visser M, Health ABC Study (2009) Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A BiolSci Med Sci 64(11):1183–1189
Fukuda TY, Tanji MM, Silva SR, Sato MN, Plapler H (2013) Infrared low-level diode laser on inflammatory process modulation in mice: pro- and anti-inflammatory cytokines. Lasers Med Sci 28(5):1305–1313
Silveira PC, Silva LA, Fraga DB, Freitas TP, Streck EL, Pinho R (2009) Evaluation of mitochondrial respiratory chain activity in muscle healing by low-level laser therapy. J Photochem Photobiol B 95(2):89–92
Dourado DM, Favero S, Baranauskas V, da Cruz-Hofling MA (2003) Effects of the Ga-As laser irradiation on myonecrosis caused by Bothrops moojeni snake venom. Lasers Surg Med 33(5):352–357
Barbosa AM, Villaverde AB, Guimaraes-Souza L, Ribeiro W, Cogo JC, Zamuner SR (2008) Effect of low-level laser therapy in the inflammatory response induced by Bothrops jararacussu snake venom. Toxicon 51(7):1236–1244
Barbosa AM, Villaverde AB, Sousa LG, Munin E, Fernandez CM, Cogo JC, Zamuner SR (2009) Effect of low-level laser therapy in the myonecrosis induced by Bothrops jararacussu snake venom. Photomed Laser Surg 27(4):591–597
Mesquita-Ferrari RA, Martins MD, Silva JA Jr, da Silva TD, Piovesan RF, Pavesi VC, Bussadori SK, Fernandes KP (2011) Effects of low-level laser therapy on expression of TNF-α and TGF-β in skeletal muscle during the repair process. Lasers Med Sci 26(3):335–340
De Souza TO, Mesquita DA, Ferrari RA, Dos Santos Pinto D Jr, Correa L, Bussadori SK, Fernandes KP, Martins MD (2011) Phototherapy with low-level laser affects the remodeling of types I and III collagen in skeletal muscle repair. Lasers Med Sci 26(6):803–814
Baptista J, Martins MD, Pavesi VC, Bussadori SK, Fernandes KP, Pinto Junior D dos S, Ferrari RA (2011) Influence of laser photobiomodulation on collagen IV during skeletal muscle tissue remodeling after injury in rats. Photomed Laser Surg 29(1):11–17
Fernandes KP, Alves AN, Nunes FD, Souza NH, Silva JA Jr, Bussadori SK, Ferrari RA (2013) Effect of photobiomodulation on expression of IL-1β in skeletal muscle following acute injury. Lasers Med Sci 28(3):1043–1046
Alves AN, Fernandes KP, Deana AM, Bussadori SK, Mesquita-Ferrari RA (2014) Effects of low-level laser therapy on skeletal muscle repair: a systematic review. Am J Phys Med Rehabil 93(12):1073–1085
Alves AN, Fernandes KP, Melo CA, Yamaguchi RY, França CM, Teixeira DF, Bussadori SK, Nunes FD, Mesquita-Ferrari RA (2013) Modulating effect of low level-laser therapy on fibrosis in the repair process of the tibialis anterior muscle in rats. Lasers Med Sci 29(2):813–821
França CM, de Loura Santana C, Takahashi CB, Alves AN, De Souza Mernick AP, Fernandes KP, de Fátima Teixeira da Silva D, Bussadori SK, Mesquita-Ferrari RA (2013) Effect of laser therapy on skeletal muscle repair process in diabetic rats. Lasers Med Sci 28(5):1331–1338
Vatansever F, Rodrigues NC, Assis LL, Peviani SS, Durigan JL, Moreira FM, Hamblin MR, Parizotto NA (2012) Low intensity laser therapy accelerates muscle regeneration in aged rats. Photonics Lasers Med 1(4):287–297
Pertille A, Macedo AB, Oliveira CP (2012) Evaluation of muscle regeneration in aged animals after treatment with low-level laser therapy. Rev Bras Fisioter 16(6):495–501
Miyabara EH, Aoki MS, Soares AG, Moriscot AS (2005) Expression of tropism-related genes in regenerating skeletal muscle of rats treated with cyclosporin-A. Cell Tissue Res 319(3):479–489
Hadi AM, Mouchaers KT, Schalij I, Grunberg K, Meijer GA, Vonk-Noordegraaf A et al (2011) Rapid quantification of myocardial fibrosis: a new macro-based automated analysis. Cell Oncol (Dordr) 34:343–354
Junqueira LC, Montes GS, Sanchez EM (1982) The influence of tissue section thickness on the study of collagen by the Picrosirius-polarization method. Histochemistry 74(1):153–156
Rodrigues NC, Brunelli R, Abreu DC, Fernandes K, Parizotto NA, Renno AC (2014) Morphological aspects and Cox-2 expression after exposure to 780-nm laser therapy in injured skeletal muscle: an in vivo study. Braz J PhysTher 18(5):395–401
Pires D, Xavier M, Araújo T, Silva JA Jr, Aimbire F, Albertini R (2011) Low-level laser therapy (LLLT; 780 nm) acts differently on mRNA expression of anti- and pro-inflammatory mediators in an experimental model of collagenase-induced tendinitis in rat. Lasers Med Sci 26:85–94
Mauro A (1961) Satellite cells of skeletal fibers. J Biophys Biochem Cytol 9:493–495
Blau HM, Cosgrove BD, Ho AT (2015) The central role of muscle stem cells in regenerative failure with aging. Nat Med 21(8):854–862
Smythe GM, Shavlakadze T, Roberts P, Davies MJ, McGeachie JK, Grounds MD (2008) Age influences the early events of skeletal muscle regeneration: studies of whole muscle grafts transplanted between young (8 weeks) and old (13-21 months) mice. Exp Gerontol 43(6):550–562
Albright JW, Albright JF (2000) Soluble receptors and other substances that regulate proinflammatory cytokines in young and aging humans. J Gerontol A BiolSci Med Sci 55:20–25
Herbst A, Johnson CJ, Hynes K, McKenzie D, Aiken JM (2013) Mitochondrial biogenesis drives a vicious cycle of metabolic insufficiency and mitochondrial DNA deletion mutation accumulation in aged rat skeletal muscle fibers. PLoS One 8(3):e59006
van der Poel C, Gosselin LE, Schertzer JD, Ryall JG, Swiderski K, Wondemaghen M, Lynch GS (2011) Ageing prolongs inflammatory marker expression in regenerating rat skeletal muscles after injury. J Inflamm (Lond) 8(1):41
Prasad S, Sung B, Aggarwal BB (2012) Age-associated chronic diseases require age-old medicine: role of chronic inflammation. Prev Med 54(Suppl):S29–S37
Tidball JG (2005) Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 288(2):R345–R353
Arsic N, Zacchigna S, Zentilin L, Ramirez-Correa G, Pattarini L, Salvi A, Sinagra G, Giacca M (2004) Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol Ther 10:844–854
Grounds MD, Sorokin L, White J (2005) Strength at the extracellular matrix- muscle interface. Scand J Med Sci Sports 15:381–391
Kääriäinen M, Järvinen T, Järvinen M, Rantanen J, Kalimo H (2000) Relation between myofibers and connective tissue during muscle injury repair. Scand J Med Sci Sports 10(6):332–337
Wynn TA (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214:199–210
Carlson BM, Faulkner JA (1996) The regeneration of noninnervated muscle grafts and marcaine-treated muscles in young and old rats. J Gerontol A BiolSci Med Sci 51(1):B43–B49
Pinheiro AL, Soares LG, Aciole GT, Correia NA, Barbosa AF, Ramalho LM et al (2011) Light microscopic description of the effects of laser phototherapy on bone defects grafted with mineral trioxide aggregate, bone morphogenetic proteins, and guided bone regeneration in a rodent model. J Biomed Mater Res A 98(2):212–221
Melo Rambo CS, Silva JA Jr, Serra AJ, Ligeiro AP, de Paula Vieira R, Albertini R, Leal-Junior EC, de Tarso Camillo de Carvalho P (2014) Comparative analysis of low-level laser therapy (660 nm) on inflammatory biomarker expression during the skin wound-repair process in young and aged rats. Lasers Med Sci 29(5):1723–1733
Funding
This work was supported by UNINOVE and the following Brazilian fostering agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (www.cnpq.br; process number: 305739/2014 RAMF, 311078/2015-0 KPSF, 305905/2014-7 SKB), Coordenação de Aperfeiçoamento de Pessoal do Nível Superior (www.capes.gov.br; process numbers: 1510536 BGR), and Fundação de Amparo à Pesquisa do Estado de São Paulo (www.fapesp.br; process number: 2014/12381-1 RAMF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was developed at the research laboratory of the Postgraduate Program in Biophotonics Applied to Health Sciences using a methodology in accordance with international ethical standards for animal experimentation (National Research Council, 1996).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and informed consent
The protocol of this study plan was approved by the Ethics Committee for Animal Research of University Nove de Julho (no. An0002/2014). This study does not include human participants.
Rights and permissions
About this article
Cite this article
de Brito, A., Alves, A.N., Ribeiro, B.G. et al. Effect of photobiomodulation on connective tissue remodeling and regeneration of skeletal muscle in elderly rats. Lasers Med Sci 33, 513–521 (2018). https://doi.org/10.1007/s10103-017-2392-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-017-2392-6