Abstract
This study evaluated the functional and quantitative differences between the early and delayed use of phototherapy in crushed median nerves. After a crush injury, low-level laser therapy (GaAs) was applied transcutaneously at the injury site, 3 min daily, with a frequency of five treatments per week for 2 weeks. In the early group, the first laser treatment started immediately after surgery, and in the delayed group, after 7 days. The grasping test was used for functional evaluation of the median nerve, before, 10, and 21 days after surgery, when the rats were killed. Three segments of the median nerve were analyzed histomorphometrically by light microscopy and computer analysis. The following features were observed: myelinated fiber and axon diameters, myelin sheath area, g-ratio, density and number of myelinated fibers, and area and number of capillaries. In the proximal segment (site of crush), the nerves of animals submitted to early and delayed treatment showed myelinated fiber diameter and myelin sheath area significantly larger compared to the untreated group. In the distal segment, the myelin sheath area was significantly smaller in the untreated animals compared to the delayed group. The untreated, early, and delayed groups presented a 50, 57, and 81% degree of functional recovery, respectively, at 21 days after injury, with a significant difference between the untreated and delayed groups. The results suggest that the nerves irradiated with low-power laser exhibit myelinated fibers of greater diameter and a better recovery of function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recovery from injuries to peripheral nerves eventually occurs in most cases and can be a very slow and frequently incomplete process. Numerous attempts have been made to enhance and accelerate the recovery of injured peripheral nerves and various tools have been experimentally tested, one of the methods studied is the use of phototherapy [1]. The phototherapy has been applied clinically in cases of pain, wound, inflammation, and soft-tissue injury, occurs at irradiation intensities so low that any resulting biological effects are due to physical and/or chemical changes associated with the interaction of cells and tissues with the laser irradiation, and not simply as a result of heating. In the case of peripheral nerve injury, the phototherapy has been used as an affective adjuvant to both conservative and surgical treatment. In nerve-cell cultures, laser phototherapy has been used to stimulate activation of cells [2, 3].
Several studies have shown the positive effects of phototherapy on peripheral nerve regeneration in crush injuries [4–8], in end-to-side [9] and end-to-end neurorrhaphy [10–12], tubulization repair [13, 14], and acellular nerve allograft [15]. There are some similarities among the protocols used to start treatment. Most studies have adopted a treatment protocol based on the early use of phototherapy, on the first postoperative day, with positive results [1]. The only study [16] in which the beginning of treatment was delayed to the second postoperative week led to negative results.
The purpose of the present study was to investigate if there were functional (evaluated by grasping test [17]) and morphometric (achieved by light microscopy and computer analysis) differences between the early (starting on the first postoperative day) and delayed (starting on second postoperative week) use of phototherapy of crushed median nerves and to observe the effects of these methods on three segments of median nerve.
Materials and methods
Animals and surgical procedure
The animal protocol was approved by the Ethics Committee for Animal Experimentation of the Faculty of Medicine of Ribeirão Preto, University of São Paulo (protocol number: 050/2006). Twenty-four female Wistar rats weighing on average 250 g were used. Before and after the operation the animals were kept in plastic cages in rooms with a 12-h light-dark cycle, with free access to food and water. The animals were anesthetized with an intraperitoneal injection of xylazine (20 mg/kg) and ketamine (100 mg/kg). Under aseptic conditions and after hair trimming, the right median nerve was exposed 10 mm above the elbow and a crush injury was induced using a standard hemostat in which the second notch was utilized for maintaining the nerve crush. The same was maintained closed, during 2 min, in order to crush the nerve. The skin was closed with 4–0 silk sutures. The animals were randomly divided into three groups of eight animals each: group 1 did not receive the laser treatment (untreated group), group 2 received the laser treatment starting on the first postoperative day (early group), and group 3 received the laser treatment starting 1 week after the injury (delayed group).
Phototherapy
The equipment used herein was a pulsed gallium-arsenide (GaAs) laser (BIOSET®) with wavelength of 904 nm, power of 40 mW, energy density of 9 J/cm2 and total delivered energy for irradiated groups was 7.2 J. Laser treatment was applied transcutaneously to the site of surgery, in a single point, the incidence angle of beam was kept perpendicular (90°) to the irradiation surface, and the irradiated area was same size as the laser spot (0.2 cm2). The therapeutic procedure was begun immediately after surgery in the early group and in the delayed group after 7 days, with a frequency of 5 days per week, for 2 weeks. All animals were treated the same way.
Assessment of nerve function recovery
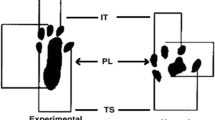
The recovery of median nerve function was assessed by means of the grasping test [17]. Briefly, the rats were gently lifted by the tail and allowed to grasp a grid connected to an ordinary electronic balance. While grasping the grid, the animal continued to be lifted by the tail with increasing firmness until it lost its grip. At this precise moment, the value shown by the balance was recorded. Before surgery, the function of the right median nerve was assessed in all animals in order to obtain baseline control values (presurgery). All animals were then retested on postoperative day 10 and at the end of the experiment (day 21). The contralateral forepaw was temporarily prevented by wrapping it around with an adhesive tape. The tests were made by a single, skilled investigator who was blinded to the experimental group to which each animal belonged.
Histology and quantitative morphology
Twenty-one days after surgery, under general anesthesia, the site of crush was located through a stitch made in the neighboring muscle, and the median nerve segment (proximal segment) was removed after being fixed in situ with 2.5% glutaraldehyde solution in 0.025 M cacodylate buffer. The distal segment (forearm, distal 1/3) of the median nerve and the lateral proper digital nerve of the third finger (the more distal branch of the median nerve) were also removed. After removal, the nerves were kept in the same fixative solution for an additional 48 h, postfixed with 1% osmium tetroxide for 12 h, and processed for epoxy resin embedding (Poly/Bed 812®, Polysciences Inc., Warrington, PA, USA). After careful positioning of the nerve fragments in the embedding molds, transverse sections were cut at 0.25 μm, stained with 1% Toluidine blue, and observed under an Axiophot photomicroscope (Carl Zeiss, Jena, Germany).
The images were acquired via a digital camera (TK-1270, JVC, Victor Company of Japan, Ltd, Tokyo, Japan) and were analyzed using an IBM/PC. Analysis of the sections was blind. The cross-sectional areas of the fascicles were obtained manually, and the number of fascicles was counted. The luminal area and number of capillaries were also obtained. The endoneurial area occupied by the capillaries was measured. In the study of the myelinated fibers, the endoneurial space was observed using an optical set with an oil immersion lens (100×), optovar (1.6×), and a camera with lens of 0.5× and an 8× magnification, which provided images with excellent resolution. The images were scanned using an automatic motorized microscope stage (Carl Zeiss, Jena, Germany), without overlap of the microscopic fields. Thirty percent of such microscopic fields were randomly studied in proximal and distal segments of the median nerves. All microscopic fields of the lateral proper digital nerve of the third finger were analyzed. The morphometric parameters for the myelinated fibers of the nerve segments were obtained as described previously [18, 19]. Briefly, the total number of myelinated fibers present in each microscopic field was identified by visual inspection and counted. The numerical density of the myelinated fibers was calculated. The minimal diameter of the myelinated fibers was measured using image-analysis software (KS 400, Kontron 2.0, Eching Bei München, Germany). Only fibers of circular shape were measured. Both the axonal minimal diameter and the total fiber minimal diameter were measured, and the ratio between the two diameters, the g-ratio (a measure of the degree of myelination), was obtained [20, 21]. The myelin sheath area was calculated for each myelinated fiber area measured. Histograms of the distribution of the myelinated fiber and axon populations were constructed. A regression analysis was generated to determine the relationship between the diameter of the axon and that of the myelinated fiber (shown for the proximal and distal segments of the median nerves).
Statistical analysis
Functional and morphometric data are presented as mean ± SD. Morphometric data were compared between groups by the non-parametric Kruskal–Wallis test. Two-tailed and functional data was compared by the non-parametric Kruskal–Wallis test followed by the post hoc Dunn test. Statistical analysis was performed using the Statistical Package for the Social Science (SPSS), version 15.0. Differences were considered significant at p ≤ 0.05.
Results
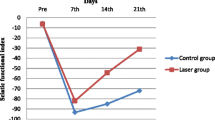
The results of the grasping test assessment are reported in Table 1. On the 10th day after injury, the three groups presented similar values of muscle strength, corresponding to only 7% (untreated and delayed groups) and 6% (early group) of normal values. The untreated, early, and delayed groups presented 50, 57, and 81% of the normal strength, respectively, on the 21st day after injury. There was a significant difference (p = 0.03) between the untreated and delayed groups on the 21st day.
Figure 1 shows photomicrographs of the proximal (i.e., injury site) and distal segments of the median nerve and the lateral proper digital nerve of the third finger of the three groups. In the early and delayed groups, the proximal segments showed myelinated fibers of larger diameter. In the distal segments that difference was less evident, with the delayed group appearing to have some myelinated fibers of larger diameter. The lateral proper digital nerves of the third finger showed an expressive reduction of myelinated fibers without visual differences among groups.
Photomicrographs of transverse semi-thin sections of the proximal (a, b, and c) and distal (d, e, and f) segments and of the lateral proper digital nerve of the third finger (g, h, and i) in the untreated (a, d, and g), early (b, e, and h) and delayed (c, f, and i) groups. Note presence of a higher number of myelinated fibers with a large diameter in the early and delayed groups in proximal segments, myelinated fibers with a large diameter in the delayed groups in distal segments, and absence of myelinated fibers in the lateral proper digital nerve of the third finger in the three groups (Toluidine blue). Scale bars = 10 μm (a, b, c, d, e, and f), 50 μm (g), 30 μm (h and i)
The morphometric data obtained for the proximal and distal segments of the median nerve of the three groups are presented in Table 2. In the proximal segments, the axon diameter (p = 0.04), the myelinated fiber diameter (p = 0.03), and the myelin sheath area (p = 0.02) were significantly larger in the delayed group compared to the untreated group. The myelinated fiber diameter (p = 0.03) and the myelin sheath area (p = 0.02) were significantly larger in the early group compared to the untreated one. The delayed group tended to present a larger number of capillaries in the proximal segments. However, there was no difference among groups in the total endoneurial area occupied by the capillaries, which did not exceed 1.14% (proximal segments) and 0.26% (distal segments). In the distal segment, the myelin sheath area (p = 0.05) was larger in the delayed group compared to the untreated group. The absence of visual differences in the lateral proper digital nerves of the third finger among groups was confirmed by statistical analysis. The distribution of the myelinated fibers and axons diameters in the proximal segments of the early and delayed groups showed fibers of larger diameter compared to the untreated group (Fig. 2). Regression analysis obtained by the least-squares method (Fig. 3) showed the relationship between the axon and myelinated fiber diameters. Regression coefficients larger than 0.90 were obtained for all nerves studied. The g-ratio obtained in most of the myelinated fibers was 0.7 to 0.8.
Discussion
Phototherapy has had positive effects on regenerative medicine mainly related to skin and nerves [22], however, the beneficial effects of laser therapy on neuromuscular recovery have been observed [23] and the potential application of photocurrent—therapy with participation of light and electrical stimulation—has been studied in regenerative medicine [22]. The effect of low-level laser therapy irradiation on the peripheral nerve system has been studied in experimental axonotmesis models as well as in neurotmesis, which requires surgical repair to allow nerve regeneration. Generally, phototherapy is applied transcutaneously at the site of injury with continuous light emission, wavelengths of less than 904 nm, and starting on the first postoperative day [1], and having positive results in most studies. Previous studies have obtained negative [16] and null [24] results using a GaAs laser at a wavelength of 904 nm and with pulsed emission. Chen et al. [16] created a gap of 10 mm in the sciatic nerve of Sprague-Dawley rats and treated this injury with silicone rubber tubes and a low-level pulsed laser. The stimulation began 1 week after the nerve repair and continued for 7 weeks. After 8 weeks of recovery, the middle regions of the regenerated nerve in the chamber were studied. The qualitative and quantitative data revealed that the control group had a more mature ultrastructural nerve organization with a higher number of myelinated axons. Bagis et al. [24] used low-level laser irradiation in crushed sciatic nerves of Wistar rats 24 h after injury for seven consecutive days. As done in the present study, the animals were killed 21 days after the injury. No qualitative difference in the pattern of regenerated nerve fibers was observed between the control and laser-irradiated nerves. The choice nerve in the study was the median nerve, which is more superficial than the sciatic nerve. One reason for the positive results obtained here is the greater effectiveness of the method in more superficial nerves such as the median nerve. The treatment was applied with an interval of 2 days (Saturday and Sunday), as done in clinical application, such as the parameters of low-level laser therapy utilized. It is possible that the differences between this study and the study of Chen et al. [16] were due to the distinct experimental models. Câmara et al. [25] used a laser at 904 nm (AsGa) for recovery of crushed sciatic nerve in Wistar rats. The laser therapy was initiated on the post-surgical first day, with applications once a day for 14 and 21 days. Histological analysis showed an increase in the number of Schwann cells, myelinic axons with a large diameter and neurons, making the nerve recovery more rapid and efficient.
Low-level laser therapy's effects on the components of the nerve have been described by Anders et al. [26]. Regarding the neuronal components, they refer to the induction of massive axonal sprouting and outgrowth in cultured neuronal cells in in vitro studies [27] and to the post-traumatic chromatolysis and neuronal atrophy of motoneurons, which are less extensive in the rat crushed sciatic nerve [28]. The biological basis of this effect can be represented by an increased synthesis of various molecules with a neuroprotective effect such as calcitonin gene-related peptide [7] and transforming growth factor beta-1 [29] and by the demonstration of suppressed nitric oxide activity (neurotoxic agent). Besides, in the non-neuronal component, low-level laser irradiation can stimulate the in vitro proliferation of rat Schwann cells [30], macrophages [31], and fibroblasts [32], which play a role in the regeneration process.
This experimental model is useful since the median nerve and finger flexor function can be easily assessed by the grasping test [17]. Rats usually have no contractures or autotomy, the distance to the target organs is short in the rat forelimb, and the time required for functional recovery is less than in the hindlimbs [17, 33]. The median nerve was studied in three regions: proximal (site of injury) and distal (forearm, distal 1/3) segments, and the lateral proper digital nerve of the third finger. This model allows observing not only the direct effects of the laser but also the speed of regeneration evaluated morphologically and clinically. On the 21st day, the number of myelinated fibers in the proximal segment of the three experimental groups was close to normal [9, 34, 35]. In the distal segments (30 mm away from the site injury) that number was only 30% of the normative data previously published [35]. The minimal diameters of axons and myelinated fibers and the myelin sheath area were smaller in the studied groups compared to normal nerves [35] and this difference was much more marked in the distal segments. The values of the early and delayed groups were closer to normal. The lateral proper digital nerves of the third finger, approximately 53 mm away from the injury site, showed a significant reduction of myelinated fiber number because the duration of the experiment did not allow these fibers to reach that nerve branch. The dimensional parameters utilized in this study, myelin sheath, fiber size, and fiber diameter/axon diameter ratio, are among the major factors when the regeneration of nerve fibers is investigated [36, 37], and a generally observed convention is that the minor axis is most representative of the true diameter [19, 38–40]. A disadvantage of this study is the small number of rats. However, this is in compliance with the norms of the Ethics Committee for Animal Experimentation of the Faculty of Medicine of Ribeirão Preto. There is a dead-weight device for crushing the rats' nerves [8], but the way in which the crushing lesions were produced in this study (with hemostatic tweezers) has also been utilized in a variety of other studies [4–7, 28, 41].
Conclusions
According to the results obtained for the proximal and distal segments of the median nerve and the return of function within 21 days, the treatment is effective in terms of the speed of nerve regeneration. The results obtained in this study suggest that the nerves irradiated transcutaneously at the site of injury with low-level laser therapy, mainly in the delayed treatment, exhibit myelinated fibers of greater diameter and a better return to function.
References
Gigo-Benato D, Geuna S, Rochkind S (2005) Phototherapy for enhancing peripheral nerve repair: a review of the literature. Muscle Nerve 31:694–701
Bae C-S, Lim S-C, Kim K-Y, Song C-H, Pak S, Kim S-G, Jang C-H (2004) Effect of Ga-As laser on the regeneration of injured sciatic nerves in the rat. In Vivo 18:489–496
Rochkind S (2009) Phototherapy in peripheral nerve regeneration: from basic science to clinical study. Neurosurg Focus 26:1–6
Anders JJ, Borke RC, Woolery SK, Van de Merwe WP (1993) Low-power laser irradiation alters the rate of regeneration of the rat facial nerve. Lasers Surg Med 13:72–82
Khullar SM, Brodin P, Messelt EB, Haanaes HR (1995) The effects of low-level laser treatment on recovery of nerve conduction and motor function after compression injury in the rat sciatic nerve. Eur J Oral Sci 103:299–305
Rochkind S, Nissan M, Alon M, Shamir M, Salame K (2001) Effects of laser irradiation on the spinal cord for the regeneration of crushed peripheral nerve in rats. Lasers Surg Med 28:216–219
Shin DH, Lee E, Hyun JK, Lee SJ, Chang YP, Kim JW, Choi YS, Kwon BS (2003) Growth-associated protein-43 is elevated in the injured rat sciatic nerve after low-power laser irradiation. Neurosci Lett 344:71–74
Barbosa RI, Marcolino AM, Guirro RRJ, Mazzer N, Barbieri CH, Fonseca MCR (2010) Comparative effects of wavelengths of low-power laser in regeneration of sciatic nerve in rats following crushing lesion. Lasers Med Sci 25:423–430
Gigo-Benato D, Geuna S, de Castro RA, Tos P, Fornaro M, Boux E, Battiston B, Giacobini-Robecchi MG (2004) Low-power laser biostimulation enhances nerve repair after end-to-side neurorrhaphy: a double-blind randomized study in the rat median nerve model. Lasers Med Sci 9:57–65
Shamir MH, Rochkind S, Sandbank J, Alon M (2001) Double-blind randomized study evaluating regeneration of the rat transected sciatic nerve after suturing and postoperative low-power laser treatment. J Reconstr Microsurg 17:133–137
Mohammed IF, Kaka LN (2007) Promotion of regenerative processes in injured peripheral nerve induced by low-level laser therapy. Photomed Laser Surg 25:107–111
Reis FA, Belchior ACG, Carvalho PTC, Silva BAK, Pereira DM, Silva IS, Nicolau RA (2009) Effect of laser therapy (660 nm) on recovery of the sciatic nerve in rats after injury through neurotmesis followed by epineural anastomosis. Lasers Med Sci 24:741–747
Miloro M, Halkias LE, Mallery S, Travers S, Rashid RG (2002) Low-level laser effect on neural regeneration in Gore-Tex tubes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 93:27–34
Rochkind S, Leider-Trejo L, Nissan M, Shamir MH, Kharenko O, Alon M (2007) Efficacy of 780-nm laser phototherapy on peripheral nerve regeneration after neurotube reconstruction procedure (double-blind randomized study). Photomed Laser Surg 25:137–143
Zhang L-X, Tong X-J, Yuan X-H, Sun X-H, Jia H (2010) Effects of 660-nm gallium-aluminum-arsenide low-energy laser on nerve regeneration after acellular nerve allograft in rats. Synapse 64:152–160
Chen YS, Hsu SF, Chiu CW, Lin JG, Chen CT, Yao CH (2005) Effect of low-power pulsed laser on peripheral nerve regeneration in rats. Microsurgery 25:83–89
Bertelli JA, Mira JC (1995) The grasping test: a simple behavioral method for objective quantitative assessment of peripheral nerve regeneration in the rat. J Neurosci Methods 58:151–155
Fazan VPS, Fazan R, Salgado HC, Barreira AA (1999) Morphology of the aortic depressor nerve myelinated fibers in normotensive Wistar-Kyoto and spontaneously hypertensive rats. J Auton Nerv Syst 77:399–404
Jeronimo A, Jeronimo CAD, Rodrigues Filho OA, Sanada LS, Fazan VPS (2005) Microscopic anatomy of the sural nerve in the postnatal developing rat: a longitudinal and lateral symmetry study. J Anat 206:93–99
Rushton WAH (1951) A theory of the effects of fibre size in medullated nerve. J Physiol 115:101–102
Smith RS, Koles ZJ (1970) Myelinated nerve fibers: computed effects of myelin thickness on conduction velocity. Am J Physiol 219:1256–1258
Jin G, Prabhakaran MP, Liao S, Ramakrishna S (2010) Photosensitive materials and potential of photocurrent mediated tissue regeneration. J Photochem Photobiol B 102:93–101
Gigo-Benato D, Russo TL, Tanaka EH, Assis L, Salvini TF, Parizotto NA (2010) Effects of 660- and 780-nm low-level laser therapy on neuromuscular recovery after crush injury in rat sciatic nerve. Lasers Surg Med 42:673–682
Bagis S, Comelekoglu U, Coskun B, Milcan A, Buyukakilli B, Sahin G, Ozisik S, Erdogan C (2003) No effect of Ga-As (904 nm) laser irradiation on the intact skin of the injured rat sciatic nerve. Lasers Med Sci 18:83–88
Câmara CNS, Brito MVH, Silveira EL, Silva DSG, Simões VRF, Pontes RWF (2011) Histological analysis of low-intensity laser therapy effects in peripheral nerve regeneration in Wistar rats. Acta Cir Bras 26:12–18
Anders JJ, Geuna S, Rochkind S (2004) Phototherapy promotes regeneration and functional recovery of injured peripheral nerve. Neurol Res 26:233–239
Wollman Y, Rochkind S, Simantov R (1996) Low-power laser irradiation enhances migration and neurite sprouting of cultured rat embryonal brain cells. Neurol Res 18:467–470
Rochkind S, Barr-Nea L, Volger I (1990) Spinal cord response to laser treatment of injured peripheral nerve. Spine 15:6–10
Leung MC, Lo SC, Siu FK, So KF (2002) Treatment of experimentally induced transient cerebral ischemia with low-energy laser inhibits nitric oxide synthase activity and up-regulates the expression of transforming growth factor-beta 1. Lasers Surg Med 31:283–288
Van Breugel HHFI, Bar PR (1993) He-Ne laser irradiation affects proliferation of cultured rat Schwann cells in a dose-dependent manner. J Neurocytol 22:185–190
Polosukhin VV (1997) Ultrastructure of alveolar macrophages under endobronchial laser therapy of acute and chronic inflammatory lung disease. Immunol Invest 26:297–311
Kreisler M, Christoffers AB, Willershausen B, d'Hoedt B (2003) Effect of low-level GaAlAs laser irradiation on the proliferation rate of human periodontal ligament fibroblasts: an in vitro study. J Clin Periodontol 30:353–358
Bontioti EN, Kanje M, Dahlin LB (2003) Regeneration and functional recovery in the upper extremity of rats after various types of nerve injuries. J Peripher Nerv Syst 8:159–168
Tos P, Calcagni M, Gigo-Benato D, Boux E, Geuna S, Battiston B (2004) Use of muscle-vein-combined y-chambers for repair of multiple nerve lesions: experimental results. Microsurgery 24:459–464
Santos AP, Suaid CA, Fazan VPS, Barreira AA (2007) Microscopic anatomy of brachial plexus branches in Wistar rats. Anat Rec 290:477–485
Geuna S, Tos P, Guglielmone R, Battiston B, Giacobini-Robecchi MG (2001) Methodological issues in size estimation of myelinated nerve fibers in peripheral nerves. Anat Embryol 204:1–10
Holland GR (1982) The effect of buffer molarity on the size, shape and sheath thickness of peripheral myelinated nerve fibres. J Anat 135:183–190
Dyck PJ, Gutrecht JA, Bastron JA, Karnes WE, Dale AJD (1968) Histologic and teased-fiber measurements of sural nerve in disorders of lower motor and primary sensory neurons. Mayo Clin Proc 43:81–123
Gutrecht JA, Dyck PJ (1970) Quantitative teased-fiber and histologic studies of human sural nerve during postnatal development. J Comp Neurol 138:117–129
Auer RN (1994) Automated nerve fibre size and myelin sheath measurement using microcomputer-based digital image analysis: theory, method and results. J Neurosci Methods 51:229–238
Belchior ACG, Reis FA, Nicolau RA, Silva IS, Perreira DM, Carvalho PTC (2009) Influence of laser (660 nm) on functional recovery of the sciatic nerve in rats following crushing lesion. Lasers Med Sci 24:893–899
Acknowledgements
The authors thank Prof. Amilton Antunes Barreira, Department of Neurology, and Prof. Haylton Jorge Suaid, Department of Surgery and Anatomy, University of São Paulo, Ribeirão Preto for permission to use the laboratory of facilities and equipment, Mr. Antonio Renato Meirelles e Silva for technical assistance, and Mr. Geraldo Cássio dos Reis for the statistical analysis. This study was supported by CAPES, FAPESP, and FAEPA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santos, A.P., Suaid, C.A., Xavier, M. et al. Functional and morphometric differences between the early and delayed use of phototherapy in crushed median nerves of rats. Lasers Med Sci 27, 479–486 (2012). https://doi.org/10.1007/s10103-011-0972-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-011-0972-4