Abstract
Previous studies have shown that low-level laser therapy (LLLT) promotes posttraumatic nerve regeneration. The objective of the present study was to assess the efficacy of 685-nm LLLT at the dosage of 3 J/cm2 in the functional recovery of the sciatic nerve in rats following crushing injury. The left sciatic nerves of 20 male Wistar rats were subjected to controlled crush injury by a hemostatic tweezers, and the rats were randomly allocated into two experimental groups as follows: control group and laser group. Laser irradiation (685 nm wavelength; 15 mW, CW, 3 J/cm2, spot of 0.028 cm2) was started on the postsurgical first day, above the site of injury, and was continued for 21 consecutive days. Functional recovery was evaluated at 3 weeks postoperatively by measuring the sciatic functional index (SFI) and sciatic static index (SSI) at weekly intervals. The treated rats showed improvement in motion pattern. The SFI and SSI results were significant when comparing two groups on the 14th and 21st postoperative days (p < 0.05). There were intra-group differences detected in laser group in different periods (p < 0.05). Low-level laser irradiation, with the parameters used in the present study, accelerated and improved sciatic nerve function in rats after crushing injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peripheral nerve injury results in partial or total sensory, motor, and functional loss, and alterations such as diseases, tumors, and traumatic damage are common. Many studies were conducted in search of improvement or acceleration of the recovery of injured peripheral nerves. Physical agents, such as electricity, electromagnetism, therapeutic ultrasound, and low-level laser (LLL) have been employed to stimulate the regeneration of the peripheral nerves [1–3]. The treatment methods studied include LLL, whose use has increased in the last decade [3, 4]. A review of the literature on phototherapy for peripheral nerve repair found that the use of laser was based on several wavelengths (632–904 nm), lesion types (crushing, neurorrhaphy, and tubulation), sample types, the duration and manner of the emission, and the measurement types (such as electrophysiological, morphometric, and functional) [4]. Several authors have concluded that this treatment promotes a positive influence on the regeneration of traumatic injuries [3, 5–12]. However, other authors did not observe any beneficial action [13, 14]. The laser affects biological tissues differently according to the usage parameters, such as dose, wavelength, continuous or pulsed mode, treatment duration, and application site [3, 7, 15–17]. Thus, finding the optimal conditions for laser irradiation is essential in the application of laser in medicine.
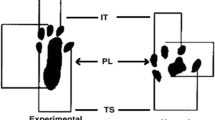
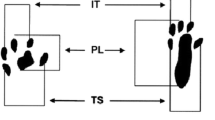
Functional assessment has become one of the methods for studying peripheral nerve regeneration in animal models since the description of the sciatic functional index (SFI) by De Medinaceli et al. [18, 19] that was later modified by Bain et al. [20]. The method uses a platform where the footprints are printed on a strip of paper for analysis of parameters such as print length, toe spread, and intermediate toe spread [7]. SFI result is ranging from zero to −100, which is a negative indicator of nerve function, with zero meaning absence of dysfunction and −100 meaning complete dysfunction [21].
The sciatic static index (SSI), a time-saving digitized static footprint analysis, is another way of assessing recovery of function after sciatic injury in animal models. It uses the footprints, acquired when the animal is on a static position, and minimizes bias related to gait’s velocity. Also, the SSI improves the acquisition of footprints and is more repeatable and accurate than the SFI [22]. In Smit et al.’s study, there were good correlations between the traditional SFI and the newly developed SSI. Despite promising results, static footprint analysis is still not widely used [23].
These methods have the advantage of not being invasive, besides showing close correlation with the degree of histological regeneration, and can be employed as a replacement for invasive methods [24]. Therefore, it might be better to use functional assessment rather than simply the electrophysiological and histological bases for axon growth and muscular innervation, provided the main interest of the survey is functional outcome [25].
The aim of this study, therefore, was to evaluate the effect of low-level laser therapy (LLLT) at a 685-nm wavelength on the healing processes of the sciatic nerve following crushing lesion. The investigation of an experimental model related to the analysis of functional recovery could provide relevant data as the basis of future clinical applications in the treatment of nerve injuries.
Methods
All experimental procedures were performed according to the guidelines for the ethical treatment of experimental animals and approved by Islamic Azad University, Faculty of Veterinary Sciences, and Animal Care and Use Local Ethics Committee.
Twenty healthy adult male Wistar rats, with an average weight of 300 g, from the Pasteur Institute of Iran, were utilized in this study. Two rats were housed per cage in a temperature- and humidity-controlled room with 12 h/12 h light/dark cycle and were allowed normal cage activities under standard laboratory conditions. The animals were fed with standard chow and filtered tap water ad libitum.
The animals were randomly assigned for two experimental groups of ten rats each, according to the procedure to be carried out:
-
Control group: animals submitted to crushing lesion of the sciatic nerve, without laser irradiation (the equipment remained turned off throughout the application).
-
Laser group: animals submitted to crushing lesion of the sciatic nerve, with subsequent irradiation in the region of the injury, for 21 consecutive days.
Anesthesia
After being weighed, each rat was given anesthetic medication consisting of ketamine hydrochloride10% associated with xylazine hydrochloride 2 % (50 and 10 mg/kg, respectively), in a single dose intramuscularly.
Operating procedure
After verifying the state of consciousness, each rat was positioned in ventral recumbency while the fore and rear paws fixed in abduction. A 3-cm long incision was made on the skin through a gluteal approach, and the left sciatic nerve was exposed by a layer-by-layer dissection. A hemostatic tweezers, exerting a force of 54 N [26, 27], was used for a period of 30 s to create a 3-mm-long crush injury, 10 mm above the bifurcation, to achieve good reproducibility of the lesion [27]. The sciatic nerve was kept moist with 37 °C sterile saline solution throughout the surgical procedure. After the lesion had been produced, the incision was sutured with simple stitches of nylon 3/0 thread.
Postoperative management
Following the surgical procedure, each rat was given a single dose of penicillin-procaine (400.000 IU) for prophylaxis of infections. Nalbuphine hydrochloride (2 mg/kg, SC) was also administered for analgesic. The administration of the analgesic was maintained every 12 h, over two consecutive days. The skin sutures were removed after 2 weeks following the operation.
Laser irradiation
A 685-nm InGaAlP (15 mW, CW, 3 J/cm2, spot of 0.028 cm2, Teralaser; DMC® São Carlos, SP, Brazil) was used in the present study. The mean power of the equipment was measured before the experiment, with a power-measuring device. Treatments were made through the contact technique, directly above the site of injury with a time of 10 s per point. Irradiation was initiated on the first day after the operation and was continued for 21 consecutive days, always at the same times (10 a.m). During the treatments, animals were sedated (xylazine 2 %, at the dose of 12 mg/kg) and they were kept immobile by two assistances. In the control group, the equipment remained turned off throughout the application, while in the laser group, it remained turned on since the beginning so that the only effect they both had in common was tissue massaging.
Assessment of nerve function recovery
The assessment of sciatic nerve function recovery was carried out by calculating the SFI and SSI. The animals’ footprints were obtained before the operation and 7, 14, and 21 days after surgery, by means of strips of paper marked out in millimeters, on a walkway constructed in accordance with the proposal by Bain et al. [20]. After the rats had undergone initial training for walking (5 min), their paws were coated with nankeen ink so that we could record the footprints for analysis of the SFI. The collected measurements of the footprints were the intermediate toe (IT) spread is the space between the second and fourth toes, the toe spread (TS) is the space between the first and fifth toes, and the print length (PL) is the space between the heel and the third toe. All measurements were taken from the normal (N) and experimental (E) sides. The SFI was calculated according to the following equation:
The SSI is a time-saving and easy technique for accurate functional assessment of peripheral nerve regeneration in rats and is calculated using the static factors, not considering the PL, according to the equation:
Like SFI, an index score of 0 was considered normal and an index of −100 indicated total impairment. When no footprints were measurable, the index score of −100 was given.
Statistical analysis
All data were reviewed by the university’s statistical department. Data were analyzed by using Microsoft Office Excel and SPSS statistical software package. The SFI and SSI data were submitted to ANOVA analyses with posttests of Bonferroni between groups, with significance level of 5 % (p < 0.05).
Results
The surgical procedure and the laser application were well tolerated by all rats, and no animal died during the experiment. The study was conducted with a total of 80 footprint images in the different periods, preoperative, 7th, 14th, and 21st postoperative days. They were evaluated by the SFI and SSI formulas. The results obtained with the SFI of the two groups on average were in the preoperative period, control −5.73 and the laser group −6.23. The mean values obtained on the 7th, 14th, and 21st postoperative days, were, respectively, control group −93.41, −85.09, and −72.03 and for the laser group −81.93, −54.39, and −31.11 (Fig. 1).
The SSI of the control group was, on average, −4.29 of the data collected prior to the injury; −69.05 on the 7th postoperative day; −56.65 on the 14th day; and −51.43 on the 21st day after surgery. For the laser group, the mean values were −4.70 before injury, −58.34 on the 7th day, −41.67 on the 14th day, and −19.01 on the 21st day after surgery (Fig. 2).
The SFI and SSI results were significant when comparing two groups on the 14th and 21st postoperative day (p < 0.05). Statistical analysis of the control group, for the different days of the SFI and SSI collection, showed that only the pre-injury values were statistically significant (p < 0.05) when compared with the values from the other days. For the laser group, there were statistical differences between the 7th and 14th days and between the 14th and 21st days (p < 0.05).
Discussion
Injuries of the peripheral nerves cause important dysfunctions and can give rise to lifelong sequelae, depending on the nerve injury grade. Today, LLL, one of the types of biostimulants, is used most often to treat a variety of pathological conditions of peripheral nerves. The fact that laser irradiation somehow interferes with a nerve function seems to be well accepted, according to the demonstration that it reduces latency time and increases nervous conveyance speed [28, 29]. Also, despite of evidences on the contrary [5], there are experimental evidences that the laser has a positive effect on injured nerves’ regeneration [30–32], which encouraged its use in human beings.
Lasers using other wavelengths are being developed and researched, such as those emitting in the range of 650–830 nm [6, 33–38] and 904 nm [13, 39]. Many of these studies did not describe all the parameters necessary, such as the dose, power of the apparatus, duration of the application, and manner of application. This leads to difficulty in understanding their methodologies and, hence, in reproducing their results and also difficulties in making comparisons between studies. However, the conclusions of such studies seem to be incomplete and controversial, which has encouraged us to develop this research in order to assess if indeed LLLT (685 nm) can stimulate functional recovery of the sciatic nerve, using an injury model by crushing in rats.
The rat was used as the animal model for its ease of acquisition, handling, low operating costs, and also the similarities to the human distribution of nerve trunks [40].
The way in which the crushing lesions were produced has also been utilized in a variety of other studies. The duration of the compression varied, but 30 s was the length of time most utilized among the abovementioned studies [9, 41, 42], with the exceptions of that by Khullar et al. [33], who utilized 10 min, and Anders et al. [3], who utilized 90 s. Compared to section followed by neurorrhaphy, the crushing injury produced in this study has the advantage of preserving, at least partially, the nerve support structure, thus, favoring regeneration [19, 22, 24, 43]. This is an appropriate lesion for the purposes of the present research, with results to be assessed in the short time. Indeed, the differences between treated and untreated sciatic nerves are easier to detect in an early phase of recovery (3 weeks postoperatively) because the spontaneous recovery usually seen in rats tends to make any comparison more difficult in longer periods of time [15].
The effects of LLL irradiation on nerve regeneration are well known. Irradiation ranging from 632 to 901 nm with LLL light modulated, dose-dependently, the proliferation of Schwann cells in in vitro rats enhanced nervous cell differentiation, increased axonal growth and myelination, and improved morphological recovery in experimental sciatic nervous lesions [44–47]. In most experimental studies, regeneration of the peripheral nerve was usually assessed through histological, morphometric, and electrophysiological methods, which did not anticipate any information about functional recovery, which would be of best interests. On the other hand, functional assessment in animals is often challenging, but the SFI and SSI enable the accurate quantitative evaluations of the functional recovery in rats’ sciatic nerve.
Although SFI is a quantitative method, it is dependent on the pressure exerted by the foot on the floor, and it is restricted to a point in time, which limits the information obtained. Automutilation and inversion or eversion deformations often limit the functional assessment with the use of SFI [48], despite this limitation, it is a widely used parameter because of its reliability [49]. Bervar described an alternative analysis of static footprint (SSI) to assess functional loss following injury to the rat sciatic nerve, during animal standing or periodic rest. SSI was developed based on the premise that the recovery of muscle tone after nerve injury is a constituent part of integral nerve and muscle functional recovery and forces acting on the body, i.e., body weight and postural muscle tone during standing influenced footprint parameters [22]. The main difference between SFI and SSI was that the distance between the tip of the third toe and the posterior margin of the sole discolored area, defined as the print length parameter, was not considered.
In the present study, the rats were irradiated for 21 days, based on previous findings [50]. The values obtained in this study, related to the SFI and SSI, showed that after crush injury in sciatic nerve, there was a functional loss in both experimental groups on the 7th postoperative day; however, in the control group, the functional index had decreased without significant difference on the 14th and 21st days. In the same period, the laser group showed significant functional improvement when it was compared with that on the 7th day. The statistical analysis showed significant results between groups. The results obtained in this survey showed that the LLL (685 nm) was effective in stimulating acceleration of functional recovery in the animals studied.
Conclusion
This study, considering the parameter analyzed, suggested that the use of LLL (685 nm, at the dosage of 3 J/cm2) was effective in the acceleration of functional recovery in the first 3 weeks, after crush injury in rat sciatic nerve.
References
Medonça AC, Barbieri CH, Mazzer N (2003) Directly applied low intensity direct electric currents enhances peripheral nerve regeneration in rats. J Neurosci Methods 129:183–190
Raso MVV, Barbieri CH, Mazzer N, Fazan VS (2005) Can therapeutic ultrasound influence the regeneration of peripheral nerves? J Neurosci Methods 142:185–192
Anders JJ, Geuma S, Rochkind S (2004) Phototherapy promotes regeneration and functional recovery of injured peripheral nerve. Neurol Res 26:233–239
Gigo-Benato D, Geuna S, Rochkind S (2005) Phototherapy for enhancing peripheral nerve repair: a review of the literature. Muscle Nerve 31(6):694–701
Chen YS, Hsu SF, Chiu CW, Lin JG, Chen CT, Yao CH (2005) Effect of low-power pulsed laser on peripheral nerve regeneration in rats. Microsurgery 25(1):83–89
Gigo-Benato D, Geuna S, de Castro RA, Tos P, Fornaro M, Boux E, Battiston B, Giacobini-Robecchi MG (2004) Low-power laser biostimulation enhances nerve repair after end-to-side neurorrhaphy: a double-blind randomized study in the rat median nerve model. Lasers Med Sci 19(1):57–65
Marcolino AM, Barbosa RI, Neves LMS, Vinas TS, Duarte DTB, Mazzer N, Fonseca MCR (2010) Low intensity laser (830 nm) functional to recovery of the sciatic nerve in rats. Acta Ortop Bras 18(4):207–211
Rochkind S, Barrnea L, Razon N, Bartal A, Schwartz M (1987) Stimulatory effect of He-Ne low dose laser on injured sciatic nerves of rats. Neurosurgery 20(6):843–847
Rochkind S, Nissan M, Alon M, Shamir M, Salame K (2001) Effects of laser irradiation on the spinal cord for the regeneration of crushed peripheral nerve in rats. Lasers Surg Med 28(3):216–219
Rochkind S (2006) Photoengineering of neural tissue repair processes in peripheral nerves and the spinal cord: research development with clinical applications. Photomed Laser Surg 24(2):151–157
Rochkind S, Drory V, Alon M, Nissan M, Ouaknine GE (2007) Laser phototherapy (780 nm), a new modality in treatment of long-term incomplete peripheral nerve injury: a randomized double-blind placebo-controlled study. Photomed Laser Surg 25(5):436–442
Santos AP, Suaid CA, Xavier M, Yamane F (2012) Functional and morphometric differences between the early and delayed use of phototherapy in crushed median nerves of rats. Lasers Med Sci 27(2):479–486
Bagis S, Comelekoglu U, Coskun B, Milcan A, Buyukakilli B, Sahin G, Ozisik S, Erdogan C (2003) No effect of GA-AS (904 nm) laser irradiation on the intact skin of the injured rat sciatic nerve. Lasers Med Sci 18(2):83–88
Cömelekoğlu U, Bagiş S, Büyükakilli B, Sahin G, Erdoğan C, Kanik A (2002) Acute electrophysiological effect of pulsed gallium-arsenide low-energy laser irradiation on isolated frog sciatic nerve. Lasers Med Sci 17(1):62–67
Endo C, Barbieri CH, Mazzer N, Fazan VS (2008) A laserterapia de baixa intensidade acelera a regeneração de nervos periféricos. Acta Ortop Bras 16(5):305–310
dos Reis FA, Belchior AC, de Carvalho PT, da Silva BA, Pereira DM, Silva IS, Nicolau RA (2009) Effect of laser therapy (660 nm) on recovery of the sciatic nerve in rats after injury through neurotmesis followed by epineural anastomosis. Lasers Med Sci 24(5):741–747
Rochkind S, Rousso M, Nissan M, Villarreal M, Barr-Nea L, Rees DG (1989) Systemic effects of low-power laser irradiation on the peripheral and central nervous system, cutaneous wounds, and burns. Lasers Surg Med 9(2):174–182
de Medinaceli L, Freed WJ, Wyatt RJ (1982) An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol 77(3):634–643
de Medinaceli L, de Renzo E, Wyatt RJ (1984) Rat sciatic functional index data management system with digitized input. Comput Biomed Res 17(2):185–192
Bain JR, Mackinnon SE, Hunter DA (1989) Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg 83(1):129–138
Andraus RAC, Barbieri CH, Mazzer N (2010) Local low power laser irradiation accelerates the regeneration of the fibular nerve in rats. Acta Ortop Bras 18(3):152–157
Bervar M (2000) Video analysis of standing—an alternative footprint analysis to assess functional loss following injury to the rat sciatic nerve. J Neurosci Methods 102:109–116
Smit X, van Neck JW, Ebeli MJ, Hovius SER (2004) Static footprint analysis: a time-saving functional evaluation of nerve repair in rats. Scand J Plast Reconstr Surg Hand Surg 38(6):321–325
Oliveira EF, Mazzer N, Barbieri CH, Selli M (2001) Correlation between functional index and morphometry to evaluate recovery of the rat sciatic nerve following crush injury: experimental study. J Reconstr Microsurg 17(1):69–75
Varejão ASP, Meek MF, Ferreira AJA, Patrício JAB, Cabrita AMS (2001) Functional evaluation of peripheral nerve regeneration in the rat: walking track analysis. J Neurosci Methods 108:1–9
Beer GM, Steurer J, Meyer VE (2001) Standardizing nerve crushes with a non-serrated clamp. J Reconstr Microsurg 17:531–534
Varejaõ AS, Cabrita AM, Meek MF, Bulas-Cruz J, Melo-Pinto P, Raimondo S, Geuna S, Giacobini-Robecchi MG (2004) Functional and morphological assessment of a standardized rat sciatic nerve crush injury with a non-serrated clamp. J Neurotrauma 21:1652–1670
Nissan M, Rochkind S, Razon N, Bartal A (1986) HeNe laser irradiation delivered transcutaneously: its effect on the sciatic nerve of rats. Lasers Surg Med 6:435–438
Lowe AS, Baxter GD, Walsh DM, Allen JM (1994) Effect of low intensity laser (830 nm) irradiation on skin temperature and antidromic conduction latencies in the human median nerve: relevance of radiant exposure. Lasers Surg Med 14:40–46
Assia E, Rosner M, Belkin M, Solomon A, Schwarts M (1989) Temporal parameters of low energy laser irradiation for optimal delay of post-traumatic degeneration of rat optic nerve. Brain Res 476:205–212
Breugel HHFI, Bär PR (1993) He-Ne laser irradiation affects proliferation of cultured rat Schwann cells in a dose-dependent manner. J Neurocytol 22:185–190
Hamilton GF, Robinson TK, Ray RH (1992) The effects of helium-neon laser upon regeneration of the crushed peroneal nerve. J Orthop Sport Phys Ther 15:209–214
Khullar SM, Brodin P, Messelt EB, Haanaes HR (1995) The effects of low level laser treatment on recovery of nerve conduction and motor function after compression injury in the rat sciatic nerve. Eur J Oral Sci 103:299–305. doi:10.1111/j.1600-0722.1995.tb00030.x
Khullar SM, Brodin P, Fristad I, Kvinnsland IH (1999) Enhanced sensory reinnervation of dental target tissues in rats following low level laser (LLL) irradiation. Lasers Med Sci 14:177–184. doi:10.1007/s101030050082
Walsh DM, Baxter GD, Allen JM (2000) Lack of effect of pulsed low-intensity infrared (820 nm) laser irradiation on nerve conduction in the human superficial radial nerve. Lasers Surg Med 26:485–490. doi:10.1002/1096-9101(2000)26:5<485::AID-LSM8>3.0.CO;2-6
Shamir MH, Rochkind S, Sandbank J, Alon M (2001) Doubleblind randomized study evaluating regeneration of the rat transected sciatic nerve after suturing and postoperative low-power laser treatment. J Reconstr Microsurg 17:133–137. doi:10.1055/ s-2001-12702
Nicolau RA, Martinez MS, Rigau J, Tomàs J (2004) Effect of power 655 nm diode laser irradiation on the neuromuscular junctions of the mouse diaphragm. Lasers Surg Med 34:277–284. doi:10.1002/lsm.20006
Byrnes KR, Waynant RW, Ilev IK, Wu X, Barna L, Smith K, Heckert R, Gerst H, Anders JJ (2005) Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers Surg Med 36:171–185. doi:10.1002/lsm.20143
Reis FA, Belchior ACG, Nicolau RA, Fonseca TS, Carvalho PTC (2008) Effect of gallium-aluminum-arsenide laser therapy (660 Nm) on recovery of the sciatic nerve in rats following neurotmesis lesion and epineural anastomosis: functional analysis. Rev Bras Fisioter 12(3):215–221
Bagis S, Comelekoglu U, Sahin G, Buyukakilli B, Erdogan C, Kanik A (2002) Acute electrophysiologic effect of pulsed gallium-arsenide low energy laser irradiation on configuration of compound nerve action potential and nerve excitability. Lasers Surg Med 30:376–380. doi:10.1002/lsm.10057
Rochkind S, Nissan M, Lubart R, Avram J, Bartal A (1988) The in-vivo nerve response to direct low-energy-laser irradiation. Acta Neurochir (Wien) 94:74–77. doi:10.1007/BF01406620
Rochkind S, Vogler I, Barr-Nea L (1990) Spinal cord response to laser treatment of injured peripheral nerve. Spine 15:6–10. doi:10.1097/00007632-199001000-00003
Bridge PM, Ball DJ, Mackinnon SE, Nakao Y, Brandt K, Hunter DA, Hertl C (1994) Nerve crush injuries: a model for axonotmesis. Exp Neurol 127:284–290
Belchior ACG, Dos Reis FA, Nicolau RA, Silva IS, Perreira DM, De Carvalho PTC (2009) Influence of laser (660 nm) on functional recovery of the sciatic nerve in rats following crushing lesion. Lasers Med Sci 24:893–899
Barbosa RI, Marcolino AM, de Jesus Guirro RR, Mazzer N, Barbieri CH, de Cássia Registro Fonseca M (2010) Comparative effects of wavelengths of low-power laser in regeneration of sciatic nerve in rats following crushing lesion. Lasers Med Sci 25(3):423–430
Mohammed IF, Kaka LN (2007) Promotion of regenerative processes in injured peripheral nerve induced by low level laser therapy. Photomed Laser Surg 25(2):107–111
Shin DH, Lee E, Hyun JK, Lee SJ, Chang PY, Kim JW, Choid YS, Bum SK (2003) Growth-associated protein-43 is elevated in the injured rat sciatic nerve after low power laser irradiation. Neurosci Lett 344:71–74
Yu P, Matloub HS, Sanger JR, Narini P (2001) Gait analysis in rats with peripheral nerve injury. Muscle Nerve 24(2):231–239
Meek MF, Den Dunnen WF, Schakenraad JM, Robinson PH (1999) Long-term evaluation of functional nerve recovery after reconstruction with a thin-walled biodegradable poly (DL-lactide-epsilon-caprolactone) nerve guide, using walking track analysis and electrostimulation tests. Microsurgery 19(5):247–253
Monte-Raso VV, Barbieri CH, Mazzer N, Yamasita AC, Barbieri G (2008) Is the Sciatic Functional Index always reliable and reproducible? J Neurosci Methods 170:255–261
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takhtfooladi, M.A., Jahanbakhsh, F., Takhtfooladi, H.A. et al. Effect of low-level laser therapy (685 nm, 3 J/cm2) on functional recovery of the sciatic nerve in rats following crushing lesion. Lasers Med Sci 30, 1047–1052 (2015). https://doi.org/10.1007/s10103-015-1709-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-015-1709-6