Abstract
With the aim of accelerating the regenerative processes, the objective was to study the influence of gallium–aluminum–arsenide (GaAlAs) laser (660 nm) on functional and histomorphological recovery of the sciatic nerve in rats. The sciatic nerves of 12 Wistar rats were crushed divided into two groups: control and laser therapy. For the latter, GaAlAs laser was utilized (660 nm, 4 J/cm2, 26.3 mW and 0.63 cm2 beam), at three equidistant points on the lesion, for 20 days. Comparison of the sciatic functional index (SFI) showed that there was a significant difference only between the pre-lesion value of the laser therapy group and that after the 21st day in the control group. It was concluded that the parameters and methods utilized demonstrated positive results regarding the SFI over the time period evaluated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The treatment of peripheral nerve lesions constitutes an important functional problem. Although there is some recovery in most such lesions, it occurs slowly and incompletely [1]. Peripheral nerves are highly vulnerable to traumas such as crushing and sectioning. It is estimated that the incidence of traumatic lesions is more than 500,000 new patients per year in some countries [2] and that 2.8% of these patients acquire lifelong incapacities [3]. This justifies the continuous development of therapies that allow reductions in the levels of injury and incapacity [4, 5]. The degree of lesion depends on the specific nerve involved, the magnitude and type of pressure exerted, and the duration of the compression [6]. The results of the injury commonly include: axonal degeneration and retrograde degeneration of the corresponding neurons in the spinal medulla, followed by very slow regeneration [7]. Lesions of the nerve structure result in loss of or diminished sensitivity and/or motricity in the innervated territory, and this adversely affects the daily activities of patients with such lesions. These consequences make it imperative that objectives be established to obtain early recovery from such lesions [8].
It is common practice in physiotherapy to use electrotherapy for regenerative purposes. Electrical [4], ultrasound [5] and low-power laser (LPL) stimulation have been utilized for accelerating the regenerative processes, with the aim of restoring patients’ functional capacities. Laser applied in the process of functional regeneration and recovery from peripheral nerve lesions started to be investigated in the 1970s, and there have been several divergences in the results obtained so far [9].
A review of the literature on phototherapy for peripheral nerve repair found that the use of laser was based on several wavelengths (632–904 nm), lesion types (crushing, neurorrhaphy and tubulation), sample types, the duration and manner of the emission and the measurement types (such as electrophysiological, morphometric and functional) [10].
Our work was undertaken in the light of the need for studies on the use of diode laser light in the red region of the electromagnetic spectrum for the neuron repair process. This is our justification for conducting this study on the influence of gallium–aluminum–arsenide LPL (660 nm) on functional recovery of the sciatic nerve in rats following crushing lesion (axonotmesis).
Materials and methods
Animals
We used 12 adult male rats of Wistar lineage, with body weights between 300 g and 350, from the central vivarium of the University for State and Pantanal Region Development (UNIDERP). They were kept in two cages (six animals per cage) under controlled lighting and temperature, with standard food and water available ad libitum.
All the experimental procedures were carried out in accordance with the norms of the Brazilian College for Animal Experimentation (COBEA). The project was approved by the Research Ethics Committee of the University of the Paraíba Valley (UNIVAP), under number L184/2005/CEP.
The animals were divided into two experimental groups, according to the procedure to be carried out.
-
Control group (n = 6): animals that were subjected to crushing lesion of the sciatic nerve, unilaterally, without undergoing irradiation.
-
Laser therapy group (n = 6): animals that were subjected to crushing lesion of the sciatic nerve, unilaterally, with subsequent irradiation in the region of the injury, for 20 consecutive days.
General surgical method
After being weighed, each animal was given pre-anesthetic medication consisting of butorphanol (Turbogesic®, 2 mg/kg) [11] together with acepromazine (Acepran®, 1 mg/kg), in a single dose intramuscularly. Fifteen minutes later, zolazepam and tiletamine (Zoletil® 50, 40 mg/kg) were administered [12]. Once anesthetized, the animals were positioned in ventral decubitus, while the front and hind paws were kept in abduction. Antisepsis with iodated alcohol was followed by trichotomy and an incision in the lateral face of the right thigh, from the level of the greater trochanter to the knee. The sciatic nerve was exposed and, with the aid of a magnifying glass and hemostatic tweezers (6.3 MPa), we produced a lesion by crushing the nerve approximately 3 mm distally from its emergence. The compression was maintained for 30 s.
After the lesion had been produced, the soft tissue was sutured with simple stitches of monofilament nylon thread (Mononylon 5/0, Ethicon®). Following the surgical procedure, each animal was given a dose of Fentanil® intraperitoneally (0.032 mg/kg) [13] for prophylaxis of infections and promotion of analgesia. The administration of the analgesic was maintained over two consecutive days, every 12 h after creation of the lesion.
Laser therapy
A gallium aluminum arsenide (GaAlAs) laser emitter made by KLD® (Endophoton model) was utilized, with wavelength of 660 nm, power of 26.3 mW and beam area of 0.63 cm2, in continuous mode. The application method was transcutaneous, at specific points and in contact with the skin. The energy density was 4 J/cm2, the power density was 0.0413 W/cm2 and the length of application was 96.7 s. The average power of the equipment was measured before the experiment, with a power-measuring device (2-Watt Broadband Power/Energy Meter, Model 13 PEM 001/J, Holland). Three points of the surgical incision were irradiated: one point at each extremity and another at the midpoint. The laser therapy was started on the first day after the operation and was continued for 20 consecutive days. The animals in the control group were subjected to the same procedure, but with the laser switched off.
Functional analysis
The animals’ footprints were obtained before the operation and 7 days, 14 days and 21 days after, by means of strips of paper marked out in millimeters, on a walkway constructed in accordance with the proposal by Dijkstra et al. [14]. After the rats had undergone initial training for walking (5 min), their paws were coated with nankeen ink so that we could record the footprints for analysis of the sciatic functional index (SFI).
Data analysis
After the data had been, they were subjected to the Shapiro–Wilk normality test because of the small size of the sample. Thus, if the sample presented a normal distribution (parametric), the analysis of variance (ANOVA) statistical test was applied with the Tukey post-test, so that comparative analysis could be performed between the groups. In the case of non-parametric distribution, the Kruskal–Wallis test would be adopted. For these procedures, BioEstat® 3.0 statistical software was utilized, with a significance level (P) of 5%, i.e., if P ≥ 0.05, the hypothesis of normality of the variables would not be rejected or comparison between the groups would not present a statistical difference, respectively.
Results
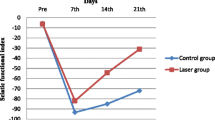
The mean SFI for the control group was −3.5 ± 17.9 before the lesion had been created, −116.7 ± 27.9 on the 7th day after the operation, −124.0 ± 27.1 on the 14th day and −117.7 ± 15.7 on the 21st day. In the laser therapy group, the mean SFI was −90.6 ± 54.0 on the 7th day after the operation, −83.1 ± 57.5 on the 14th day and −57.8 ± 57.4 on the 21st day. In the Shapiro–Wilk normality test, it was observed that all the groups presented data with normal distribution (P ≥ 0.05) (Table 1).
Comparative statistical analysis (ANOVA) on the control group, between the different collection days for the SFI, showed that only the pre-lesion values were statistically significant in relation to the other days (P < 0.001). In the laser therapy group, comparisons between the pre-lesion values and the values on the 7th and 14th days after the operation presented statistically significant differences (P < 0.01 and P < 0.05), but there was no statistical difference between the pre-lesion values and those on the 21st day (P > 0.05).(Fig. 1) There were also statistical differences between the 7th and 14th days and between the 14th and 21st days (P < 0.01).
Comparison between the control and laser therapy groups showed that there was a significant difference only between the pre-lesion value for the laser therapy group and that for the 21st day after the operation for the control group (P < 0.001).
Discussion
The objective of this study was to analyze the influence of the GaAlAs laser (660 nm) on the regeneration of the sciatic nerve in rats following crushing lesion, with evaluation of the results by means of the sciatic functional index.
Preliminary studies had demonstrated that laser therapy would have action on both the function and regeneration of the peripheral nerve. Thus, the study was devised and its methodology was subsequently investigated so that viable data could be generated.
Rats were chosen as the experimentation animal because of the ease of acquiring and handling them. Moreover, rodents (and particularly mice and rats) have become the most frequently utilized models because of their low maintenance costs and the distribution of their nerve trunks, which is similar to the distribution in humans [2]. Although lesions of the sciatic nerve are rare in humans, this model provides a test bench for lesions that involve plurifascicular nerves with axons of different sizes and types that compete within the reach of distal endoneural tubes and are targets for re-innervation [2].
A crushing lesion was chosen, because the regeneration takes place within a favorable setting provided by reactive Schwann cells and the preservation of endoneural tubule continuity [15–17]. Both of these increase axon prolongation and facilitate adequate re-innervation of the target. After a short latent period (1 or 2 days), axons regenerate linearly [18].
Crushing lesions were appropriate for investigating cellular and molecular events that intervene in peripheral nerve regeneration and for giving access to factors such as drugs that could increase the regeneration speed and the effectiveness of the re-innervation [2].
The way in which the crushing lesions were produced (with hemostatic tweezers) has also been utilized in a variety of other studies [7, 19–24]. The duration of the compression varied, but 30 s was the length of time most utilized among the above-mentioned studies, with the exceptions of that by Khullar et al. [22], who utilized 10 min, and Anders et al. [21], who utilized 90 s.
Helium–neon (HeNe) laser (632.8 nm) in the red emission region of the electromagnetic spectrum is the wavelength that has been studied most with regard to biomodulation of the biological response in repair processes [7, 19–21, 25–28]. Today, lasers using other wavelengths are being developed and researched, such as those emitting in the range of 650–830 nm (gallium–aluminum–arsenide) [22, 24, 29–35] and 904 nm (gallium–arsenide) [23, 36]. GaAlAs laser (660 nm) was selected because it has low power and a wavelength that is common in clinical practice. Furthermore, there have not been many previous studies investigating this wavelength [24].
In clinical practice, low-power laser therapy utilizes doses of 1–4 J/cm2, together with output powers of between 10 mW and 90 mW, and it has been widely utilized in a variety of musculoskeletal studies, and also in relation to painful and inflammatory processes [9]. On this basis, a density of 4 J/cm2 was justifiable in our study. It is important to stress that this parameter is extremely variable in laser therapy studies relating to nerve regeneration.
The utilization of laser as a beneficial biomodulating instrument in nerve regeneration events is still controversial. Some studies have produced positive evidence [7, 8, 19, 21, 22, 24–29, 31, 32, 35, 37], while others have not [20, 23, 30, 34, 36, 38]. Many of these studies did not describe all the parameters necessary, such as the dose, average power of the apparatus, duration of the application and manner of application. This leads to difficulty in understanding their methodologies and, hence, in reproducing their results, and also difficulties in making comparisons between studies.
Walkways are an evaluation method that has been greatly used [14, 39–41], and they are readily applicable because they are easy to apply and inexpensive. Some studies have sought to modernize this data collection method with digital cameras, thereby making dynamic evaluations possible [14, 39].
The results obtained from our study, with regard to the SFI, demonstrate that, after the crush lesion had been created, there was severe functional loss in both groups on the 7th day after the operation. However, while the SFI for the control group became even worse on the 14th day, the laser therapy group presented functional improvement in comparison with the 7th day. The explanation for the low SFI in the irradiated group on the 7th day is probably that, during the first hours after the lesion had been created in the axon, the cell bodies had started to undergo a series of alterations called chromatolysis that are characterized histologically by ingurgitation of cells, degeneration of the Nissl substance (the rugose endoplasmic reticulum of the neuron) and migration of the nucleus from the center to the periphery. These alterations have the aim of producing proteins (actin and tubulin) related to the regeneration of the cytoskeleton of the axons, to the detriment of neurotransmitter production, and are related to intracellular transportation and movement of the growth cone [16–18, 42, 43]. The first 7 days after creation of the lesion were probably marked by these events, but the use of laser therapy within 24 h of creation of the lesion reduced the immediate functional loss. This demonstrates that the therapy should be started during this initial period and corroborates the affirmations of Dahlin [16]. Fourteen days after the operation, both the control and laser therapy groups showed a positive trend regarding improving functional capacity, but this was more accentuated, with better values, in the treated group.
In the functional analysis of the control group, the comparisons between the 7th and 14th days and between the 14th and 21st days did not demonstrate significant differences, although all the evaluation days after the lesion had been created presented a difference in relation to the pre-lesion value.
With regard to the laser therapy group, the comparative analyses of the SFI between the different evaluation days demonstrated significant differences from the pre-lesion value, except for the 21st day. This suggested that the laser had had a beneficial effect on the functional improvement.
The molecular basis that would explain the effectiveness of laser therapy on nerve regeneration is still unclear. Karu [44] found that irradiation of isolated mitochondria induced positive alterations in cell homeostasis. The author suggested that some components of the respiratory chain (cytochromes, flavins and dehydrogenase) would be primary photoreceptors or chromophores, i.e., capable of absorbing light of certain wavelengths, thereby activating the respiratory chain. Thus, this would result in increased synthesis of adenosine triphosphate (ATP), which would affect the hydrogen levels in the cells, activate other transported ions such as sodium and potassium, and also alter the calcium flow between the mitochondria and cytoplasm. This energy level favored by laser therapy might have had an influence on the process studied.
Manteifel and Karu [45] reported that cytochrome-c-oxidase, the terminal enzyme of the respiratory chain, is a photoreceptor of visible light in the red region of the spectrum, i.e., the same wavelength region as utilized in our study. The absorption of the light by this enzyme would accelerate the transportation of electrons in the respiratory chain, which would lead to increased transmembrane electrical potential in the mitochondria, thereby activating the synthesis of ATP and, consequently, the cell metabolism.
Rochkind and Quaknine [1] reported that the effects of low-power laser are dependent on the light dose, since low doses cause regulation of oxidation–reduction in the cell metabolism, while high doses lead to photodynamic damage. This affirmation was possible after they had analyzed different wavelengths and energy doses applied to fibroblast cells. They found that at 630 nm a greater number of mitoses was obtained than at 360 nm and 780 nm. The peak was attained with a dose of 15 J/cm2, but at doses of more than 60 J/cm2 there was a reduction in cell reproduction. On the basis of these results, the use of a low density of 4 J/cm2 in our study was justified, thereby avoiding photodynamic damage and suggesting that increased numbers of mitoses on the fibroblasts of our sample would be found in the formative layers of the peripheral nerve (epineuron, perineuron and endoneuron), as in the studies by Dahlin [16] and Rummler et al. [46].
Other events might explain the functional improvement, such as: (a) improvement in the transportation of energy and propagation of calcium ions (Ca2+) in the cytoplasm [46], which would generate increased cellular functional potential [47]; (b) acceleration of wound repair and increased resistance of the scar tissue [48]; and (c) anti-inflammatory action. Furthermore, the accelerated tissue regeneration and improved blood circulation were due to the following effects: (1) increased activity of some cells, such as leukocytes and phagocytes, and increased calcium in the cell cytoplasm; (2) accelerated cell division and growth; (3) activation of protein and cytokine synthesis; and (4) relaxation of the vessel walls (vasodilatation) by photolysis of complexes such as nitric oxide (NO). Consequently, it has been postulated that these reactions would lead to indirect effects such as bactericide, regeneration and vasodilatation [49]. Karu et al. [50] stated that NO is also associated with energy production and stimulation of mitochondrion biogenesis and apoptosis, and is involved with analgesic effects and increased microcirculation.
Schwartz et al. [27] found that, after irradiation of muscle cell cultures (632 nm, 3 J/cm2, 20 mW), there was a rise in the levels of nerve growth factor (NGF), which is a neurotropic factor secreted by skeletal muscles that influences the survival and regeneration of sympathetic and sensitive neurons in the peripheral nervous system. Other neurotropic growth factors are also biostimulated by laser therapy, such as growth-associated protein 43 (GAP-43) [24] and fibroblast growth factor [51]. The improvement in the SFI might be due to these events, but histocytochemical studies will need to be undertaken.
It is believed that the functional improvement is also not due to the release of neurotransmitters at the neuromuscular junction, as a result of the study by Nicolau et al. [34], because of the similarity in wavelength (655 nm) and energy density utilized (1–12 J/cm2).
Despite the beneficial action of laser therapy on the SFI, the mean values obtained on the 21st day after the lesion had been created were neither the same as nor greater than the pre-lesion values. This suggests that the period of 21 days was still insufficient for complete nerve regeneration to take place.
One important fact to be mentioned is the pre-lesion SFI. It was noted that there was no statistically significant difference between the two groups at this evaluation time, thus demonstrating that the study sample was homogeneous.
Sciatic nerve injury implies functional losses with regard to walking patterns, because of the direct relationship between the distribution and contribution of the spinal nerve fibers that form the sciatic nerve [52].
The improvement in the SFI by laser therapy in our study corroborates the findings of Shin et al. [24]. Those authors utilized an index associated with immunocytochemistry to analyze the therapeutic effect of laser therapy (650 nm, 5 mW) on sciatic nerve lesions of axonotmesic type, with only four consecutive days of treatment. After the fifth week, there was no statistically significant difference between the treated group and the control group. It is interesting to note the similarity between that study and ours, with regard to wavelength, animal, lesion type and time taken to achieve a positive effect (3 weeks). Nonetheless, further studies are necessary, with the aim of making observations over periods of treatment longer than 21 days, or even longer than 5 weeks.
Others studies found satisfactory results with different wavelengths, such as 632.8 nm [26–28], 780 nm [31], 808 nm [33] and 820 nm [32]. However, in an evaluation of the effect of GaAlAs laser (820 nm) on sciatic nerves of rats subjected to axonotmesis, Khullar et al. [22] found that there was beneficial action on functional and electrophysiological evaluations, while histology did not demonstrate any significant result, despite the differences in wavelength, power and dose.
Beau and colleagues [15] stated that myelinization of the regenerated fibers took place between 14 days and 21 days after creation of a lesion. However, the lesion type utilized in that study was neurotmesic. From this, it can be seen that, although the lesion in our study (of axonotmesic type) was less severe, the 21 laser applications were insufficient. In any event, neither morphometry nor electrophysiology can measure the most important factor, i.e., the SFI, probably because of the proportion of regenerated fibers that appropriately reach their target. Thus, morphometric analysis on experimental traumatic lesions provides a reliable image of the trophic conditions in the regenerated nerves, but it does not assist in our understanding the function [53].
Conclusion
The utilization of low-power gallium–aluminum–arsenide laser (660 nm) showed positive results with regard to functional recovery in the sciatic nerve of rats following crushing lesion.
References
Rochkind S, Quaknine GE (1992) New trend in neuroscience: low-power laser effect on peripheral and central nervous system (basic science, preclinical and clinical studies). Neurol Res 14:2–11
Rodrígues FJ, Valero-Cabré A, Navarro X (2004) Regeneration and functional recovery following peripheral nerve injury. Drug Discov Today Dis Models 1:177–185. doi:10.1016/j.ddmod.2004.09.008
Noble J, Munro CA, Prasad VSSV (1998) Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma 45:116–122. doi:10.1097/00005373-199807000-00025
Mendonça AC, Barbieri CH, Mazzer N (2003) Directly applied low intensity direct electric current enhances peripheral nerve regeneration in rats. J Neurosci Methods 129:183–190. doi:10.1016/S0165-0270(03)00207-3
Raso VVM, Barbieri CH, Mazzer N, Fasan VS (2005) Can therapeutic ultrasound influence the regeneration of the peripheral nerves? J Neurosci Methods 142:185–192. doi:10.1016/j.jneumeth.2004.08.016
Inserra MM, Bloch DA, Terris DJ (1998) Functional indices for sciatic, peronal, and posterior tibial nerve lesions in the mouse. Microsurgery 18:119–124 doi:10.1002/(SICI)1098-2752(1998)18:2<119::AID-MICR10>3.0.CO;2-0
Rochkind S, Nissan M, Alon M, Shamir M, Salame K (2001) Effects of laser irradiation on the spinal cord for the regeneration of crushed peripheral nerve in rats. Lasers Surg Med 28:216–219. doi:10.1002/lsm.1041
Oliveira LS, Sobral LL, Takeda SY, Betini J, Guirro RR, Somazz MC, Teodori RM (2008) Electrical stimulation and swimming in the acute phase of axonotmesis: their influence on nerve regeneration and functional recovery. Rev Neurol 47:11–15
Basford JR (1995) Low intensity laser therapy: still not an established clinical tool. Lasers Surg Med 16:331–342. doi:10.1002/lsm.1900160404
Gigo-Benato D, Geuna S, Rochkind S (2005) Phototherapy for enhancing peripheral nerve repair: a review of the literature. Muscle Nerve 31:694–701. doi:10.1002/mus.20305
Kozhakhmetov AN (1993) The evaluation of the adequacy of anesthesia during delivery (in Russian). Akush Ginekol (Mosk) 2:36–39
Almeida EMP, Nunes N, Fantinatti AP, Santos PSP, Bolzan AA, Rezende ML (2000) Efeitos cardiorrespiratórios da associação de tiletamina/zolazepam em cães (Canis familiaris) pré-tratados ou não pela acepromazina. Braz J Vet Anim Sci 37:45–51
Privado MS, Sakata RK, Issy AM (2004) Estudo comparativo entre fentanil por vias peridural e venosa para analgesia de operações ortopédicas. Rev Bras Anestesiol 54:634–639. doi:10.1590/S0034-70942004000500003
Dijkstra JR, Meek MF, Robinson PH, Gramsbergen A (2000) Methods to evaluate functional nerve recovery in adult rats: walking track analysis, video analysis and the withdrawal reflex. J Neurosci Methods 96:89–96. doi:10.1016/S0165-0270(99)00174-0
Beau JML, Ellisman MH, Powell HC (1988) Ultrastructural and morphometric analysis of long-term peripheral nerve regeneration through silicone tubes. J Neurocytol 17:161–172. doi:10.1007/BF01674203
Dahlin LB (2004) The biology of nerve injury and repair. J Am Soc Surg Hand 4:143–155. doi:10.1016/j.jassh.2004.06.006
Johnson EO, Zoubos AB, Soucacos PN (2005) Regeneration and repair of peripheral nerves. Injury 36:S24–S29. doi:10.1016/j.injury.2005.10.012
Lent R (2004) Cem bilhões de neurônios: conceitos fundamentais da neurociência, 3rd edn. Atheneu, São Paulo, p 135
Rochkind S, Nissan M, Lubart R, Avram J, Bartal A (1988) The in-vivo nerve response to direct low-energy-laser irradiation. Acta Neurochir (Wien) 94:74–77. doi:10.1007/BF01406620
Rochkind S, Vogler I, Barr-Nea L (1990) Spinal cord response to laser treatment of injured peripheral nerve. Spine 15:6–10. doi:10.1097/00007632-199001000-00003
Anders JJ, Borke RC, Woolery SK, Merwe WPV (1993) Low power laser irradiation alters the rate of regeneration of the facial nerve. Lasers Surg Med 13:72–182. doi:10.1002/lsm.1900130113
Khullar SM, Brodin P, Messelt EB, Haanaes HR (1995) The effects of low level laser treatment on recovery of nerve conduction and motor function after compression injury in the rat sciatic nerve. Eur J Oral Sci 103:299–305. doi:10.1111/j.1600-0722.1995.tb00030.x
Bagis S, Comelekoglu U, Sahin G, Buyukakilli B, Erdogan C, Kanik A (2002) Acute electrophysiologic effect of pulsed gallium-arsenide low energy laser irradiation on configuration of compound nerve action potential and nerve excitability. Lasers Surg Med 30:376–380. doi:10.1002/lsm.10057
Shin DH, Lee E, Hyun J, Lee SJ, Chang YP, Kim J, Choi YS, Kwon BS (2003) Growth-associated protein-43 is elevated in the injured rat sciatic nerve after low power irradiation. Neurosci Lett 344:71–74. doi:10.1016/S0304-3940(03)00354-9
Rochkind S, Nissan M, Razon N, Schwartz M, Bartal A (1986) Electrophysiological effect of HeNe laser on normal and injured sciatic nerve in the rat. Acta Neurochir (Wien) 83:125–130. doi:10.1007/BF01402391
Sotelo PR, Sosa VMR, Martínez RT, Barry HG (1996) El laser helio-neon en la regeneración del nervio ciático seccionado y suturado. Rev Cubana Cir 35:00
Schwartz F, Brodie C, Appel E, Kazimirsky G, Shainberg A (2002) Effect of helium/neon laser irradiation on nerve growth factor synthesis and secretion in skeletal muscle cultures. J Photochem Photobiol B 66:195–200. doi:10.1016/S1011-1344(02)00267-1
Snyder SK, Byrnes KR, Borke RC, Sanches A, Anders JJ (2002) Quantitation of calcitonin gene-related peptide mRNA and neuronal cell death in facial motor nuclei following axotomy and 633 nm low power laser. Lasers Surg Med 31:216–222. doi:10.1002/lsm.10098
Khullar SM, Brodin P, Fristad I, Kvinnsland IH (1999) Enhanced sensory reinnervation of dental target tissues in rats following low level laser (LLL) irradiation. Lasers Med Sci 14:177–184. doi:10.1007/s101030050082
Walsh DM, Baxter GD, Allen JM (2000) Lack of effect of pulsed low-intensity infrared (820 nm) laser irradiation on nerve conduction in the human superficial radial nerve. Lasers Surg Med 26:485–490 doi:10.1002/1096-9101(2000)26:5<485::AID-LSM8>3.0.CO;2-6
Shamir MH, Rochkind S, Sandbank J, Alon M (2001) Double-blind randomized study evaluating regeneration of the rat transected sciatic nerve after suturing and postoperative low-power laser treatment. J Reconstr Microsurg 17:133–137. doi:10.1055/s-2001-12702
Miloro M, Halkias LE, Mallery S, Travers S, Rashid RG, Neb O (2002) Low-level laser effect on neural regeneration in Gore-Tex tubes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 93:27–34. doi:10.1067/moe.2002.119518
Gigo-Benato D, Geuna S, Rodrigues AC, Fornaro PTM, Boux E, Battiston B, Giacobini-Robecchi MG (2004) Low-power laser biostimulation enhances nerve repair after end-to-side neurorrhaphy: a double-blind randomized study in the rat median nerve model. Lasers Med Sci 19:57–65. doi:10.1007/s10103-004-0300-3
Nicolau RA, Martinez MS, Rigau J, Tomàs J (2004) Effect of power 655 nm diode laser irradiation on the neuromuscular junctions of the mouse diaphragm. Lasers Surg Med 34:277−284. doi:10.1002/lsm.20006
Byrnes KR, Waynant RW, Ilev IK, Wu X, Barna L, Smith K, Heckert R, Gerst H, Anders JJ (2005) Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers Surg Med 36:171−185. doi:10.1002/lsm.20143
Bagis S, Comelekoglu U, Coskun B, Milcan A, Buyukakilli B, Sahin G, Ozisik S, Erdogan C (2003) No effect of GA-AS (904 nm) laser irradiation on the intact skin of the injured rat sciatic nerve. Lasers Med Sci 18:83–88. doi:10.1007/s10103-003-0258-6
Rochkind S, Barr-Nea L, Bartal A, Nissan M, Lubart R, Razon N (1988) New methods of treatment of severely injured sciatic nerve and spinal cord: an experimental study. Acta Neurochir (Wien) 43:91–93 b
Chelyshev IA, Kubitskii AA, Plakseichuk A (1996) Regeneratsiia nervnykh volokon pri obluchenii nizkointensivnymi lazerami. Morfologiia 110:47–50
Bervar M (2000) Video analysis of standing—an alternative footprint analysis to assess functional loss following injury to the rat sciatic nerve. J Neurosci Methods 102:109–116. doi:10.1016/S0165-0270(00)00281-8
Koka R, Hadlock TA (2001) Quantification of functional recovery following rat sciatic nerve transaction. Exp Neurol 168:192–195. doi:10.1006/exnr.2000.7600
Varejão ASP, Cabrita AM, Geuna S, Melo-Pinto P, Filipe VM, Gramsbergen A, Meek MF (2003) Toe out angle: a functional index for the evaluation of the sciatic nerve recovery in the rat model. Exp Neurol 183:695–699. doi:10.1016/S0014-4886(03)00208-5
Lubiatowski P, Unsal FM, Nair D, Ozer K, Siemionow M (2008) The epineural sleeve technique for nerve graft reconstruction enhances nerve recovery. Microsurgery 28:160–167. doi:10.1002/micr.20472
Monte-Raso VV, Barbieri CH, Mazzer N, Yamasita AC, Barbieri G (2008) Is the sciatic functional index always reliable and reproducible? J Neurosci Methods 170:255–261. doi:10.1016/j.jneumeth.2008.01.022
Karu TI (1988) Molecular mechanisms of the therapeutic effect of low-intensity laser irradiation. Lasers Life Sci 2:53–74
Manteifel VM, Karu TI (2005) Structure of mitochondria and activity of their respiratory chain in successive generations of yeast cells exposed to He-Ne laser light. Izv Akad Nauk Ser Biol 32:556–566
Rummler LS, Paul TD, Gupta R (2004) The anatomy and biochemistry of myelin and myelination. Oper Tech Orthop 14:146–152. doi:10.1053/j.oto.2004.06.005
Klebanov GI, Kreinina MV, Poltanov EA, Khristoforova TV, Vladimirov YA (2001) Mechanism of therapeutics effect of low-intensity infrared laser irradiation. Bull Exp Biol Med 131:239–241. doi:10.1023/A:1017643230376
Woodruff LD, Bounkeo JM, Brannon WM, Dawes KS, Barham CD, Waddell DL, Enwemeka CS (2004) The efficacy of laser therapy in wound repair: a meta-analysis of the literature. Photomed Laser Surg 22:241–247. doi:10.1089/1549541041438623
Vladimirov YA, Osipov AN, Klebanov GI (2004) Photobiological principles of therapeutic applications of laser radiation. Biochemistry 69:89–90
Karu TI, Pyatibrat LV, Afanasyeva NI (2004) A novel mitochondrial signaling pathway activated by visible-to-near infrared radiation. Photochem Photobiol 80:366–372. doi:10.1562/2004-03-25-RA-123.1
Ihsan FRM (2005) Low-level laser therapy accelerates collateral circulation and enhances microcirculation. Photomed Laser Surg 23:289–294. doi:10.1089/pho.2005.23.289
Montoya GJV, Ariza J, Sutachán JJ, Hurtado H (2002) Relationship between functional deficiencies and the contribution of myelin nerve fibers derived from L-4, L-5, and L-6 spinolumbar branches in adult rat sciatic nerve. Exp Neurol 173:266–274. doi:10.1006/exnr.2001.7806
de Medinaceli L (1995) Interpreting nerve morphometry data after experimental traumatic lesions. J Neurosci Methods 58:29–37. doi:10.1016/0165-0270(94)00156-B
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Belchior, A.C.G., dos Reis, F.A., Nicolau, R.A. et al. Influence of laser (660 nm) on functional recovery of the sciatic nerve in rats following crushing lesion. Lasers Med Sci 24, 893–899 (2009). https://doi.org/10.1007/s10103-008-0642-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-008-0642-3