Abstract
Success in sandwich technique procedures can be achieved through an acceptable bond between the materials. The aim of this study was to compare the effect of 35% phosphoric acid and Er,Cr:YSGG laser on shear bond strength of conventional glass-ionomer cement (GIC) and resin-modified glass-ionomer cement (RMGIC) to composite resin in sandwich technique. Sixty-six specimens were prepared from each type of glass-ionomer cements and divided into three treatment groups as follows: without pretreatment, acid etching by 35% phosphoric acid for 15 s, and 1-W Er,Cr:YSGG laser treatment for 15 s with a 600-μm-diameter tip aligned perpendicular to the target area at a distance of 1 mm from the surface. Energy density of laser irradiation was 17.7 J/cm2. Two specimens in each group were prepared for evaluation under a scanning electron microscope (SEM) after surface treatment and the remainder underwent bonding procedure with a bonding agent and composite resin. Then the shear bond strength was measured at a crosshead speed of 0.5 mm/min. Two-factor analysis of variance and post-hoc Tukey test showed that the cement type, surface treatment method, and the interaction of these two factors significantly affect the shear bond strength between glass-ionomer cements and composite resin (p < 0.05). Surface treatment with phosphoric acid or Er,Cr:YSGG laser increased the shear bond strength of GIC to composite resin; however, in RMGIC only laser etching resulted in significantly higher bond strength. These findings were supported by SEM results. The fracture mode was evaluated under a stereomicroscope at ×20.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The “sandwich” technique is one of the most recommended dental composite restorative procedures in which two different restorative materials are used to yield a restoration with the best physical-mechanical and esthetic properties of each material [1]. The first layer is a liner of flowable composite, conventional glass-ionomer cement (GIC) or resin-modified glass-ionomer cement (RMGIC), which is covered with a laminate of restorative composite resin. In class II and V cavities it is recommended to use glass-ionomer liner at the gingival floor, especially when gingival margin is extended to the root surface. This material provides better retention and seal due to the chemical bonding to tooth structure, reducing microleakage and marginal gap in non-enamel margins. Glass-ionomer provides long-term fluoride release, thereby decreasing the possibility of recurrent caries. The disadvantages of these materials, such as low physical-mechanical properties and esthetics, can be compensated by the overlaying composite resin [2, 3].
For the success of this technique, there should be a reasonable bond between the two materials [4]. The bond strength values between glass-ionomer cement and composite resin have been reported in different studies to be highly dependent on the materials used and the methods of handling [5]. In general, the bond is acceptable when fracture occurs inside each material rather than in the bonded interface (i.e., cohesive rather than adhesive) [6].
The common method for GIC use is acid etching of the cement surface, application of the bonding agent and finally adding composite resin. Some studies have demonstrated that acid etching enhances the bond strength due to increases in mechanical retention [7–10]; however, this has not been confirmed by other studies [6, 11, 12].
High technique sensitivity, low strength of GIC, and lack of chemical bonding between GIC and composite resin due to differences in their setting reactions, have led to the introduction of RMGIC. This new material has better physical-mechanical properties and its resin components can be chemically bonded with composite resin [3]. According to previous studies, acid etching of this cement has no effect on bond strength [13], and it even decreases shear bond strength of this cement to composite resin [14].
Different methods have been suggested for surface treatment other than acid etching. One of these methods is laser etching. A new generation of erbium lasers, Er,Cr:YSGG laser, can be used for surface treatment. Er,Cr:YSGG pulsed laser uses a combination of laser energy, water, and air to ablate enamel, dentin, bone, and soft tissues. The wavelength of this laser (2,780 nm) has an affinity for water. Ablation is accomplished by hydrokinetic energy that prevents temperature rise [15–21]. Initial observations of enamel and dentin surfaces treated with erbium lasers have shown the similarity between these surfaces and acid-etched ones; therefore, these results prompted clinicians to use laser as an alternative to chemical etching [18]. It has been demonstrated that Er,Cr:YSGG laser application can be an appropriate alternative technique to etching by 37% phosphoric acid in removing the smear layer and preparing dentin [22]. Türkmen et al. showed that acid etching of GIC does not have a significant effect on surface roughness under SEM (scanning electron microscopy), but surface treatment using Nd-YAG laser extensively roughened the surface [23]. Therefore, it seems that laser treatment of glass-ionomer cements may result in greater mechanical retention. Since no studies have so far been carried out in this regard, the present study was designed in an attempt to compare the effects of phosphoric acid and Er,Cr:YSGG laser treatment on the surface of GIC and RMGIC in sandwich technique and their influence on shear bond strength of these cements to composite resins.
Materials and methods
In this in vitro study, 66 specimens were prepared from each of GIC (GC Fuji II; GC Corporation, Tokyo, Japan) and RMGIC (Fuji II LC; GC Corporation, Tokyo, Japan). In each of the cements, the powder and liquid were mixed according to the manufacturer’s instructions. The prepared mixture was packed into a cylindrical plastic mold (a diameter of 5 mm and a height of 4 mm) and placed on a glass slab. Another glass slab was used on the other side of the mold to make the free surface of the cement smooth. In GIC specimens, there was a 6-min interval from the start of mixing to complete curing of the cement, but in RMGIC the specimens were light-cured for 20 s at 400 mW/cm2 through each of the glass slabs. The tip of the light-curing unit (Astralis 7; Ivoclar Vivadent, Amherst, NY, USA) was placed 1 mm above the surface of the cement.
Then the specimens of each cement type were divided into three treatment groups (n = 22). In the control groups, no surface pretreatment was carried out before bonding. In the acid-etched groups, the surface of glass-ionomer was etched with 35% phosphoric acid gel (Scotchbond Etchant; 3M ESPE, St. Paul, MN, USA) for 15 s. Then the acid was rinsed and the excess water was dried using a moist cotton pellet to prevent dehydration of the specimens before bonding. In the laser-treated groups, the surface of glass-ionomer was treated with Er,Cr:YSGG laser (Waterlase YSGG; Biolase Europe GmbH, Germany) at a pulse energy of 1 W (10% water, 11% air) for 15 s with a 600-μm-diameter G-type tip. The laser tip was aligned perpendicular to the target area at a distance of 1 mm from the surface. The beam spot size was 0.282 mm2 and the energy density of the laser beam was 17.7 J/cm2. In this system, the wavelength of emitted photons is 2,780 nm, pulse duration is 140-200 μs and repetition rate is 20 Hz. After laser treatment, a brown superficial layer of tiny flakes was seen on the surface of each specimen. Since it has been reported that this layer may interfere with the bonding process [24], it was removed using a moist cotton pellet and then the specimens were rinsed with water before the adhesion procedure.
Subsequent to surface treatment, two specimens of each group were randomly selected for SEM analysis and the other ones underwent the bonding procedure immediately. First, bonding agent (Adper Single Bond; 3M ESPE, St. Paul, MN, USA) was applied to glass-ionomer surface in all the groups according to manufacturer’s instructions and light-cured for 10 s at 400 mW/cm2. Then the second plastic mold (a diameter of 2.5 mm and a height of 2 mm) was placed on glass-ionomer specimen, filled with composite resin (Filtek Z250; 3 M ESPE, St. Paul, MN, USA) and light-cured for 40 s as previously described.
Subsequent to removal of the plastic mold, GIC specimens were kept in a humidity chamber at 37°C for 1 h before being immersed in distilled water at 37°C for the next 23 h. RMGIC specimens were immersed in distilled water at 37°C for 24 h immediately after curing composite resin [25]. In the next stage, subsequent to mounting of the specimens in acrylic block, shear bond strength was measured using Hounsfield Test Equipment (H5K-S model; Salfords, Redhill, Surrey, England). The force was applied to the bonding interface using a chisel-like loading head at a crosshead speed of 0.5 mm/min until fracture occurred and the maximum breaking loads were recorded in Newtons.
After mechanical failure, the fracture modes in all the specimens were evaluated under a stereomicroscope (Nikon; Japan) at ×20. Fracture patterns were classified as cohesive (inside the glass-ionomer cement or composite resin), adhesive (in the bonding interface) or a combination of both cohesive and adhesive failures [6].
In order to prepare specimens for SEM analysis, they were gold-sputtered and exposed to high vacuum. Then the specimens were examined under a SEM (TESCAN VEGA; USA) at ×1500.
Shear bond strength data were expressed as mean ± standard deviation (SD). After checking data for normality (Kolmogorov-Smirnov test) and the groups for homogeneity of variances (Levene test), data were analyzed using the two-factor analysis of variance in which bond strength was the dependent variable and the cement type and the method of surface treatment were the test factors. A post-hoc Tukey test was used for two-by-two comparison of the groups. In addition, the significance of relationship between bond strength and surface treatment methods in each cement group was assessed with one-way analysis of variance and a post-hoc Tukey test. Statistical significance was defined at p < 0.05.
Results
Table 1 shows descriptive statistics and statistically significant differences for all the groups of each cement type. Two-factor analysis of variance indicated that the mean of shear bond strength was significantly influenced by both the cement type (F1,114 = 58.61, p < 0.001) and the type of surface treatment (F2,114 = 24.94, p < 0.001). Significant differences between the groups were revealed by Tukey test regarding the two factors (Table 1). On the other hand, the interaction between the two factors of cement type and surface treatment methods was significant (F2, 114 = 5.27, p = 0.006).
Comparison of the groups in each cement type, using one-way analysis of variance and a post-hoc Tukey test, indicated that in GIC the bond strengths in acid- or laser-etched groups were significantly higher than those in the control group (p < 0.05); however, the difference between these two groups was not significant (p = 0.94). Nevertheless, in the RMGIC, the bond strength value was significantly higher only in the laser-treated group (p < 0.05), and the difference between the acid-etched and control groups was not significant (p = 0.07). Figures 1 and 2 are the error bar charts of bond strength values by the factors analyzed. In Table 2, the fracture mode has been presented in each group.
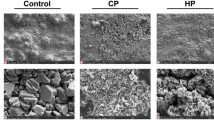
The SEM observation revealed the topographic specifications of glass-ionomer surfaces in different treatment groups (Fig. 3a-f).
Discussion
Sometimes there are cases in which a restorative procedure based on sandwich technique, using GIC or RMGIC layered with composite resin, is preferred. In these situations, not only the bonding of glass-ionomer to dental substrates is an important factor in the success of restoration but also there should be an acceptable bond between glass-ionomer and composite resin [4]. In the present study, the effect of surface treatment with 35% phosphoric acid or Er,Cr:YSGG laser on shear bond strength of GIC and RMGIC to composite resin was investigated.
According to the results of this study, the mean of shear bond strength of RMGIC to composite resin was higher than GIC (p < 0.001), which is consistent with the results of some previous studies [25–27]. The setting mechanism of GIC is only based on an acid-base reaction between the powder and liquid components whereas in RMGIC, in addition to this reaction, a resin polymerization phase is present as well. Polymerization reaction may be light-initiated, chemical, or dual depending on the type of the product. This way the presence of resinous components in RMGIC structure, similar to the resinous components of the composite resin, can be effective in increasing bond strength of this cement to composite resin [14, 23, 25–28]. In the surface of set RMGIC there is a superficial catalyst rich in air-inhibited layer, which can copolymerize with resin composite [26]. Also, the residual unreacted methacrylate groups on the polyacid chain within the polymerized RMGIC may form strong covalent chemical bonds with the resin bonding agent [27].
On the other hand, the mean of shear bond strengths of GIC to composite resin was significantly higher in acid- or laser-etched groups than the control group, without any pretreatment (p < 0.01); however, there was no significant difference between acid and laser treatment methods (p = 1). Consistent with the results of the present study, some of the previous investigations have reported that acid etching of GIC increases bond strength to composite resin [7, 8–10]. In the process of acid etching, phosphoric acid preferentially attacks the matrix of the hardened GIC, resulting in a rough and porous surface which is a retentive surface to increase the adhesion to composite resin [10]. However, in contrast with the results of our study there are some reports that acid etching of GIC has no effect on bond strength to composite resin [6, 11], or even decreases it [12]. According to these studies, dissolution of GIC matrix by acid forms a weak zone with cracks in the surface of GIC, which can be partially reinforced with the bonding agent but during shear bond testing failure occurs at this weakened region [11, 12, 29, 30]. In our study, laser treatment of GIC increased bond strength as well. Regarding the wavelength of Er,Cr:YSGG laser, it is absorbed maximally by water molecules. It may also target the hydroxyl groups, with subsequent cutting or alteration of the surface of materials [16]. Considering the structure of GIC, which is composed of a glass component (usually flouroaluminosilicate glass) and water-soluble polymeric acids, and also regarding the necessity of water for acid-base reaction and setting of the cement [3, 14, 31], the dynamics of the setting reaction of GIC and its incomplete initial setting [31], it is expected that Er,Cr:YSGG laser will produce some porosity and micro-irregularities in the cement matrix, increasing the mechanical retention with the bonding agent and composite resin. This way, as it is seen in Table 2, fracture mode has shifted from adhesive in the control group to cohesive in the acid- or laser-etched groups, which demonstrates the positive effect of these surface treatments in improving the adhesion between GIC and composite resin. Cohesive failure in glass-ionomer cements might be attributed to numerous air inclusions present in the cement. These air inclusions can act as stress points, giving rise to the increased likelihood of cohesive failure. The same phenomenon can also occur in resin-based systems, but the number of defects within the resin is much less than that in glass-ionomer cements [32].
SEM views confirmed increase in surface roughness of GIC with acid or laser etching while in the control group the surface was completely smooth (Fig. 3a-c). The microcracks on the GIC surface are thought to be due to the application of vacuum during preparation of specimens for SEM analysis, as this material is very brittle [23].
In RMGIC, only laser treatment significantly increased the shear bond strength (p < 0.001); however, both acid and laser etching improved fracture pattern (Tables 1 and 2). Similar to the results of the present study, certain studies have reported that acid etching of RMGIC has no effect on bond strength to composite resin [1, 6, 13]. It seems that RMGICs are not influenced by acid etching due to their high resin content [1, 13]. In another study, it has been reported that acid etching of one type of RMGIC can result in lower shear bond strength as it may partially remove the HEMA and decrease the availability of oxygen-inhibited functional methacrylate groups which contribute to the adhesion to composite resin [27]. However, this fact does not necessarily apply to all RMGICs [14]. In explaining the effect of laser on RMGIC, the chemical structure of this cement should be considered. Some of the water content of the GIC is replaced by a water/HEMA mixture in RMGIC. More complex materials have been developed by modification of the polyacid with side chains which can polymerize by light-curing mechanisms. The initial set of these materials is due to the formation of a polymerization matrix while the acid-base reaction hardens and strengthens the matrix formed slowly. The set cement will have two inter-penetrating matrices, i.e., the ionic matrix from the acid-base reaction and the polymerization matrix from the free-radical reaction [3, 14]. Therefore, it seems that although cement is not permeable to acid after initial setting due to higher resin content, Er,Cr:YSGG laser can still penetrate into the ionic matrix and adsorb to water or hydroxyl groups of the material’s structure and induce micro-irregularities in the surface through its hydrokinetic effect [16]. Therefore, bond strength increased significantly in the laser-etched group rather than the acid-etched one. These findings were confirmed in SEM views which indicated an increase in the surface roughness in the RMGIC after laser treatment while there was not any significant surface topographic alteration in the acid-etched group in comparison with the control group (Fig. 3d-f).
In the present study, macroscopic observation of laser-treated surfaces of GIC and RMGIC specimens showed a brown flakey superficial layer. These flakes cover the surface and reduce bond strength; therefore, in this study we mechanically removed this layer. In a recent study, Hibst reported the formation of a layer of tiny flakes after tooth preparation with a laser beam. He suggested that this layer should be removed before application of the filling material [24]. To overcome this problem, mechanical or chemical removal of this layer has been suggested. Gutknecht et al. and Carvalho et al. have suggested acid etching of the laser-prepared cavity [33, 34]. This suggestion can be considered in future studies on the subject.
Conclusions
Considering the limitations of the present study, it can be concluded that surface treatment of GIC using 35% phosphoric acid or Er,Cr:YSGG laser may increase the shear bond strength of this cement to composite resin and improve fracture mode while in RMGIC the highest bond strength can be achieved by laser treatment; however, both laser- and acid-etching procedures may improve fracture pattern.
References
Taher NM, Ateyah NZ (2007) Shear bond strength of resin modified glass ionomer cement bonded to different tooth-colored restorative materials. J Contemp Dent Pract 8:25–34
Roberson TM (2006) Fundamentals in tooth preparation. In: Roberson TM, Heymann HO, Swift EJ Jr (eds) Sturdevant’s art and science of operative dentistry, 5th edn. Mosby, Inc, St. Louis, pp 281–321
Hilton TJ, Broome JC (2006) Direct posterior esthetic restorations. In: Summitt JB, Robbins JW, Hilton TJ, Schwartz RS (eds) Fundamentals of operative dentistry, a contemporary approach, 3rd edn. Quintessence Publishing Co, Inc, Chicago, pp 289–339
Hinoura K, Suzuki H, Onose H (1991) Factors influencing bond strengths between unetched glass ionomers and resins. Oper Dent 16:90–95
Bowen RL, Marjenhoff WA (1992) Dental composites/glass ionomers: the materials. Adv Dent Res 6:44–49
Zanata RL, Navarro MF, Ishikiriama A, da Silva e Souza Junior MH, Delazari RC (1997) Bond strength between resin composite and etched and non-etched glass ionomer. Braz Dent J 8:73-78
Suliman AA, Schulein TM, Boyer DB, Kohout FJ (1989) Effects of etching and rinsing times and salivary contamination on etched glass-ionomer cement bonded to resin composites. Dent Mater 5:171–175
Sneed WD, Looper SW (1985) Shear bond strength of a composite resin to an etched glass ionomer. Dent Mater 1:127–128
Hinoura K, Moore BK, Phillips RW (1987) Tensile bond strength between glass ionomer cements and composite resin. J Am Dent Assoc 114:167–172
Subrata G, Davidson CL (1989) The effect of various surface treatments on the shear strength between composite resin and glass-ionomer cement. J Dent 17:28–32
Sheth JJ, Jensen ME, Sheth PJ, Versteeg J (1989) Effect of etching glass-ionomer cements on bond strength to composite resin. J Dent Res 68:1082–1087
Taggart SE, Pearson GJ (1991) The effect of etching on glass polyalkenoate cements. J Oral Rehabil 18:31–42
Tate WH, Friedl KH, Powers JM (1996) Bond strength of composites to hybrid ionomers. Oper Dent 21:147–152
Sidhu SK, Watson TF (1995) Resin-modified glass ionomer materials. Am J Dent 8:59–67
Waterlase, Biolase, WWW.Biolase.com
Hadley J, Young DA, Eversole LR, Gornbein JA (2000) A laser-powered hydrokinetic system for caries removal and cavity preparation. J Am Dent Assoc 131:777–785
Obeidi A, Liu PR, Ramp LC, Beck P, Gutknecht N (2009) Acid-etch interval and shear bond strength of Er,Cr:YSGG laser-prepared enamel and dentin. Lasers Med Sci. doi:10.1007/s10103-009-0652-9
Botta SB, da Ana PA, Zezell DM, Powers JM, Matos AB (2009) Adhesion after erbium, chromium:yttrium-scandium-gallium-garnet laser application at three different irradiation conditions. Lasers Med Sci 24:67–73. doi:10.1007/s10103-007-0521-3
Hossain M, Nakamura Y, Tamaki Y, Yamada Y, Murakami Y, Matsumoto K (2003) Atomic analysis and Knoop hardness measurement of the cavity floor prepared by Er, Cr:YSGG laser irradiation in vitro. J Oral Rehabil 30:515–521
Huang HH, Chuang YC, Chen ZH, Lee TL, Chen CC (2007) Improving the initial biocompatibility of a titanium surface using an Er, Cr:YSGG laser-powered hydrokinetic system. Dent Mater 23:410–414
Wang X, Zhang C, Matsumoto K (2005) In vivo study of the healing processes that occur in the jaws of rabbits following perforation by an Er,Cr:YSGG laser. Lasers Med Sci 20:21–27
Hossain M, Nakamura Y, Yamada Y, Suzuki N, Murakami Y, Matsumoto K (2001) Analysis of surface roughness of enamel and dentin after Er, Cr:YSGG laser irradiation. J Clin Laser Med Surg 19:297–303
Turkmen C, Sazak H, Gunday M (2006) Effects of the Nd:YAG laser, air-abrasion and acid-etchant on filling materials. J Oral Rehabil 33:64–69
Hibst R (2002) Lasers for caries removal and cavity preparation: State of the art and future directions. J Oral Laser Applications 2:203–212
Li J, Liu Ya, Liu Yu, Soremark R, Sundstrom F (1996) Flexure strength of resin-modified glass ionimer cements and their bond strength to dental composites. Acta Odontol Scand 54:55–58
Burgess JO, Barghi N, Chan DC, Hummert T (1993) A comparative study of three glass ionomer base materials. Am J Dent 6:137–141
Kerby RE, Knobloch L (1992) The relative shear bond strength of visible light-curing and chemically curing glass ionomer cement to composite resin. Quintessence Int 23:641–644
Farah CS, Orton VG, Collard SM (1998) Shear bond strength of chemical and light-cured glass ionomer cements bonded to resin composites. Aust Dent J 43:81–86
Wexler G, Beech DR (1988) Bonding of a composite restorative material to etched glass ionomer cement. Aust Dent J 33:313–318
Papagiannoulis P, Eliades G, Lekka M (1990) Etched glass ionomer liners: surface properties and interfacial profile with composite resin. J Oral Rehabil 17:25–36
Powers JM, Sakaguchi RL (2006) Craig's restorative dental materials. Mosby Elsevier Inc, Missouri
Burrow MF, Nopnakeepong U, Phrukkanon S (2002) A comparison of microtensile bond strengths of several dentin bonding systems to primary and permanent dentin. Dent Mater 18:239–245
de Carvalho RC, de Freitas PM, Otsuki M, de Eduardo CP, Tagami J (2008) Micro-shear bond strength of Er:YAG-laser-treated dentin. Lasers Med Sci 23:117–124. doi:10.1007/s10103-006-0434-6
Gutknecht N, Apel C, Schafer C, Lampert F (2001) Microleakage of composite fillings in Er, Cr:YSGG laser-prepared class II cavities. Lasers Surg Med 28:371–374. doi:10.1002/lsm.1064
Acknowledgments
We thank Dr. Majid Abdolrahimi for the revision of the English manuscript. This project was carried out by the financial support from the Deputy Dean of Research at Tabriz University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Navimipour, E.J., Oskoee, S.S., Oskoee, P.A. et al. Effect of acid and laser etching on shear bond strength of conventional and resin-modified glass-ionomer cements to composite resin. Lasers Med Sci 27, 305–311 (2012). https://doi.org/10.1007/s10103-010-0868-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-010-0868-8