Abstract
This study investigates the healing process that takes place in the bone and soft tissue of the maxilla and the mandible after perforation by an Er,Cr:YSGG laser device. The jaws of New Zealand white rabbits were irradiated with an Er,Cr:YSGG laser, forming wounds 0.4 mm in diameter. Irradiation parameters were as follows: repetition rate was 20 pulse/s, pulse duration was 140–200 μs, power was 2 W, exposure time was 10 s, energy density was 80 J/cm2. After sacrifice at 0–56 days post-surgery, gross observations and histological examinations were performed. Effective hemostasis was achieved after Er,Cr:YSGG laser surgery. There was a minimal delay before the healing began. After 56 days all of the bone defects had been completely replaced by new bone. In conclusion, the Er,Cr:YSGG laser allows precise surgical ablation with minimal thermal damage to adjacent tissues in vivo. The overall subsequent healing was favorable. This laser may potentially be used in minor oral surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Using a laser as a surgical tool allows a surgeon to operate on tissues more precisely and to ablate tissues in areas where access is limited. In bone-cutting, it is possible to use a laser to make some desired shapes and to minimize the mechanical damage in the surrounding tissues [1, 2]. Furthermore, as conventional rotary bone-cutting instruments have thermal necrosis and cause particle release to the atmosphere, development of a laser technique for bone surgery offers an attractive alternative [3]. Previously-published studies of lasers and bone cutting have largely focused on the use of the CO2 laser, in both the continuous-wave and pulsed modes, to perform osteotomies. However, compared with conventional mechanical methods, CO2 laser osteotomies were demonstrated to induce a significant delay in the healing process, which was attributed to the thermal necrosis or the carbonization generated adjacent to the irradiated area, and the foreign body reactions to charred material [4–6].
With the recent development of solid-state lasers, a new kind of erbium laser, the Er,Cr:YSGG laser, has been developed, with an emission wavelength of 2.78 μm. Energy from this laser is absorbed effectively by tissue and water, with minimal collateral thermal damage and tissue charring [7, 8]. Preliminary investigations have shown that it can provide efficient and precise ablation in both hard and soft tissues [9]. However, to date, the healing process that takes place in jaw tissue after an operation by Er,Cr:YSGG laser has not been investigated.
The present study was performed in rabbits’ jaws in order to observe the healing processes that take place in bone and soft tissue of the maxilla and the mandible after perforation by Er,Cr:YSGG laser in vivo.
Materials and methods
Laser devices
In the present study, an Er,Cr:YSGG laser (Millennium, Biolase, San Clemente, CA, USA), emitting at 2.78 μm, pulsed with a duration of 140–200 μs and a repetition rate of 20 Hz, was employed. The power output of the laser can be varied from 0 to 6 W. The delivery system consisted of a fiber-optic tube terminating in a handpiece with a sapphire crystal tip bathed in an adjustable air–water spray. The tip used in the present study was 400 μm in diameter and 8.0 mm in length. The beam spot size at the tip was 1.26×10−3 mm 2.
Animals used in the experiment
The present study was carried out after being permitted by the Committee of Animal Experimentation of Showa University, Japan. Twelve New Zealand white rabbits, all 1–2 years old, male, weighing from 4.02 to 4.65 kg, were kept in individual metal cages at room temperature, with 12 h of light per day and 50% relative humidity. They received a standard pelleted laboratory diet and water ad libitum.
Surgical procedure
The twelve rabbits were anesthetized with intravenously-administered 6% sodium pentobarbital given slowly as a dose of 30 mg/kg. After anesthesia, the intraoral surgical field together with the handpiece and fiber of the laser device were sterilized with chloroxedine and physical saline. Laser ablation sites were chosen in both the maxilla and mandible: on the central lines of the jaws and 1.0 cm under the central gingival papilla. Then ablation was performed in a contact mode, with the sapphire tip being kept perpendicular to the irradiated bone surface and slightly touching the mucosa, forming a wound 0.4 mm in diameter and 3 mm in depth. The jaws were irradiated for 10 s. During irradiation, the Er,Cr:YSGG laser was employed at 2.0 W with a 50% water and 50% air spray. Energy density was 80 J/cmm 2. All of the laser ablations were performed by the same operator. After the operations, the wounds were compressed with moist gauze for 1 min to gain complete hemostasis. The rabbits were then returned to their cages without activity limitations.
Histological observation
The rabbits were sacrificed by means of an overdose of pentobarbital at intervals of 0, 3, 7, 14, 28, 56 days post-surgery, respectively. Following sacrifice, the mandibular and maxillary jaws were removed and fixed in 10% neutral buffered formalin. Then the samples were decalcified with Plank-Rychlo solution and embedded in celloidin. The serial histological sections (20 μm) were stained with hematoxylin and eosin using standard procedures, and then examined by light microscopy.
Results
Immediately after laser surgery
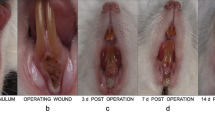
The laser-treated surgical regions were circular lesions with lost surface mucosa. The remaining mucosa immediately around the laser-induced wounds appeared as pale whitish foci. There was minor bleeding from the wounds, which was stopped by gauze compression within a very short time (Fig. 1A).
The gross appearance of the rabbit’s maxilla after laser perforation. A Immediately after surgery. Hemostasis was achieved completely. B Three days after surgery. The wound formed an ulcer-like defect in the mucosa (arrow). Localized slightly erythematous tissue was noted around the defect. C Seven days after surgery. The mucosa at the surgical area had healed completely. There were no signs of swelling or erythematous tissue
Histological observation showed that laser ablation formed an opening through the soft tissue into the bone that was filled with extravasated erythrocyte, leukocyte, and amorphous proteinaceous fibrinous material. Slight carbonization and necrosis can be observed on the surface of the opening in soft tissue, underneath which there was evidence of hyalinization and vacuolization, representing a thermally damaged layer. The necrotic and damaged zones in soft tissue totaled 10–40 μm in diameter (Fig. 2A). Compared with the soft tissue, the bone seemed to be more severely damaged: beneath a layer of slight carbonization and a zone of coagulation necrosis characterized by an increased affinity for hematoxylin and loss of distinctive microscopic features, there was a thermally damaged layer characterized by numerous empty osteocytic lacunae; vessels within the Haversian and Volkmann canals contained thrombi; the necrotic and damaged zones added up to 70–90 μm. However, significant damage to marrow cells could not be observed (Fig. 2B, Table. 1).
Histological microscopy of wounds immediately after ablation. A Slight carbonization and necrosis can be observed on the surface of the entry into the soft tissue, which was filled with extravasated blood cells and amorphous proteinaceous fibrinous material (H-E stain, bar=60 μm). B Bone wound: under a layer of slight carbonization and a zone of coagulation necrosis (CN), there was a thermally damaged layer (TD) characterized by numerous empty osteocytic lacunae (H-E stain, bar=10 μm)
Three days after laser surgery
All of the rabbits tolerated laser surgery without postoperative difficulties. The wound formed an ulcer-like defect in the mucosa at the treated area. There was no sign of swelling or exudate. Localized slightly erythematous tissue was noted around the defect (Fig. 1B).
An epithelium bridge was observed to form, covering the ulcered surface. Under the ulcer bed, the granulated tissue that had formed exhibited a mixed leukocytic infiltration with fibrin. There was evidence of the beginning of organization. However, in the wound in the bone tissue, there remained an infiltration of entrapped leukocytes, mainly neutrophils. The infiltration of cells from the wound into the bone tissue was significantly blocked by the carbonized char. The underlying necrotic tissue had been absorbed, forming a void between the char and the bone tissue (Fig. 3).
Seven days after laser surgery
After seven days the mucosa in the surgical area had healed completely. There were no signs of swelling or erythematous tissue (Fig. 1C). Histological examination showed that epithelial generation was evident, with varying degrees of hyperparakeratosis. Fibroblasts and mononuclear inflammatory cells were noted in the submucosa, and the infiltration of neutrophil leukocytes had decreased (Fig. 4A). Different healing processes could be observed in the maxillary and mandibular bone: in the maxilla, carbonized char was partially absorbed and the gap between char and bone tissue had diminished; the granulated tissue was characterized by intensive fibroblasts (Fig. 4B); in the mandible, only minimal changes in the carbonized char were observed; the granulated tissue was still characterized with an infiltration of inflammation leukocytes and fibrin, and organization was evident.
One week after laser surgery. A In soft tissue, epithelial generation was evident with varying degrees of hyperparakeratosis. Infiltration of neutrophil leukocytes had decreased (H-E stain, bar=50 μm). B Wound in the bone tissue: carbonized char was partially absorbed and the gap between char and bone tissue had diminished; the granulation tissue was characterized with intensive fibroblasts and an infiltration of leukocytes remained (H-E stain, bar=40 μm)
14 days after laser surgery
After 14 days, the epithelium at the surgical area had differentiated well, and the density of inflammation cells in the submucosa had decreased. The carbonized particles in the soft tissue had been totally absorbed. A horizontal orientation of fibroblasts was noted. In the maxillary bone, the periosteum began to bridge, and large amounts of trabecular bone were observed to form. Prominent osteoid formation was noted in the defects, especially in the margins of the defects. There was significant basophilic tutorialization near the periosteam, showing active osteoblastic behavior. At this point in time, the wound in the mandibular bone was characterized by an intensive infiltration of fibroblasts; the proliferation of new bone also began (Fig. 5).
Two weeks after laser surgery. In the maxillary bone, the periosteum began to bridge, and large amounts of trabecular bone were observed to form. Prominent osteoid formation was noted in the defects, especially in the margins of the defects.A Low magnification, H-E stain, bar=40 μm. B High magnification, H-E stain, bar=8 μm
28 days after laser surgery
After 28 days, in the soft tissue, the histological features of the area exposed to the laser were now virtually indistinguishable from the adjacent areas, except that there were still some remaining fibroblasts, showing that the reconstruction of collagen fiber was not yet complete. The laser-induced defects in the bone had largely decreased, being replaced by a large amount of lamellar, maturing bone. The lamellar pattern of the new bone was similar to that of the normal cortex. A few particles of carbonized bone still remained in the defects, which were surrounded by the fibroblasts. There were no significant differences between the maxilla and the mandible.
56 days after laser surgery
Complete bridging of the bone defects by mature lamellar bone was finished by 56 days after surgery. Small particles of carbonized bone had become encapsulated by cortical bone, forming foreign bodies. All of the bone defects had disappeared, totally replaced by the new bone. According to the lamellar pattern, no difference could be found between the new bone and the peripheral normal cortex. However, remodeling of the inferior aspect of the cortical bone tissue was ongoing (Fig. 6).
Discussion
The ideal laser for surgical use is one that can precisely ablate bone and soft tissue and coagulate vessels while minimizing thermal damage [3]. Some previous work has suggested that the Er,Cr:YSGG laser is able to ablate bone and soft tissue conveniently and precisely [10]. The present study was designed to observe the immediate damage and subsequent healing after laser surgery in the jaws of rabbits. Rabbits have been used to evaluate the postoperative outcome of bone surgery by lasers in many previous studies [4, 11, 12, 19]. Therefore, they were also adopted in this investigation.
Results indicate that the Er,Cr:YSGG laser ablated both soft tissues and bone tissues effectively, and caused only minimal thermal damage to adjacent tissues. The Er,Cr:YSGG laser incised the rabbits’ jaws easily and produced a defect (0.4 mm in diameter and 3 mm in depth) in less than 10 s. Histological sections showed that the immediate adjacent thermal damage was less than 100 μm, which is consistent with previous reports [13–15]. Subsequent healing progressed in an orderly fashion from acute infiltration of inflammation cells to early proliferation of connective tissue, and then to differentiation to trabecular bone. Ample osteoid formation was readily discerned. By 14 days, soft tissue in the surgical area was well differentiated and large amounts of trabecular bone were observed to form in the bone defects. By four weeks, trabecular bone had been replaced by lamellar bone and the lamellar pattern of the new bone was similar to that of the normal cortex. The most rapid bone healing occurred along the margins of the defect, showing that the central area of the defect underwent more thermal damage from the laser beam. When the experiment was terminated at 56 days, all of the defects had been replaced by lamellar bone, although the remodeling was ongoing.
Many previous studies have demonstrated that bone surgery performed with lasers may result in a delay before subsequent healing. Buchelt et al [16] reported that osteotomy with a Hol:YAG laser resulted in pseudoarthrosis for 12 weeks. McDavid et al [6] reported that 63 days after laser osteotomies with a CO2 or Nd:YAG laser, little evidence could be found that there was bone in contact with the residual char layer within the ablation defect. Compared with those lasers, our Er,Cr:YSGG laser ablation produced a minimal delayed response after bone ablation. One possible reason for this minimal delay can be attributed to the minimal adjacent thermal damage to surrounding tissue during laser surgery. The Er,Cr:YSGG laser emits in the mid-infrared portion of the spectrum, at 2.78 μm. This emission is heavily absorbed by water, which has an absorption coefficient of 7,700 cm−1. Moreover, the organic matrix and inorganic salts, the major components of the bone, also have very high absorption coefficients for mid-infrared lasers [17, 18]. During laser irradiation, the photon energy is intensely absorbed by bone, so that the penetration depth is only a few microns [19]. Large amounts of photons are absorbed in a small volume of tissue, producing a rapid rise in the temperature of the substrate. Then the water in the substrate boils and expands, resulting in a microexplosion effect. Plenty of air/water spray helps to protect surrounding tissues from excessive thermal damage. A similar phenomenon was also observed when another erbium laser, an Er:YAG laser, was used in the ablation of bone [20]. The similar emission wavelength (2.94 μm) of this laser to the Er,Cr:YSGG laser used here may explain the analogous minimal thermal damage to adjacent tissues.
Some conventional tools, such as mechanical rotary burs, have been used in osteotomy for a long time. Because burs cannot be used directly into soft tissue, such a control was not used in the present study. Comparison with previous reports indicates that the biggest differences in the subsequent healing were found during the first week, when there is no char formation after osteotomy with burs [15, 20]. However, char formation was obvious after Er,Cr:YSGG laser surgery, especially in bone. At three days post-treatment, although the necrotic tissue was absorbed or resolved, residual char restrained inflammatory cells’ infiltrations, forming a void between the granulation and the damaged bone. At seven days post-treatment, the gap diminished but still existed. By two weeks, little difference could be found between the effects of Er,Cr:YSGG laser surgery and bur osteotomy, except that the wounds caused by the burs showed more osteoid tissue formation. Some researchers have also reported a retarding effect of char after bone surgery by CO2 laser or Nd:YAG laser, which seems to exist as long as several weeks post-treatment [6].
A summary of the whole healing process in this study leads to the following conclusions about Er,Cr:YSGG laser surgery. (1) The bone heals more slowly than the soft tissue, probably because bone tissue is more susceptible to thermal damage. Eriksson and Albrektsson [21] demonstrated that the threshold for bone survival was 47 °C for 1 min, which represents an ~10 °C increase in temperature and a duration time of 1 min. Reconstruction of hard tissue also takes more time. (2) Maxillar bone heals faster than mandibular bone, probably mainly because maxillar bone has better blood circulation. On the other hand, as the mandibular bone has more density, more tissue is required to regenerate during healing. (3) The major proliferation of bone occurs from the endosteal surface. Before the periosteum bridges, osteoid tissue appears near the endosteal surface. During the course of the maturation of the bone, remnants of the necrotic bone get incarcerated in the newly-forming bone; some of the fragments of this were still present eight weeks after surgery.
Hemorrhage was effectively controlled by the Er,Cr:YSGG laser, as reported by other researchers [13, 14]. However, strict hemostasis could not be achieved by the Er,Cr:YSGG laser, because lasers operated in pulsed mode have inherently limited hemostatic properties due to mechanical shock associated with the laser-tissue interaction that blows the “seal” of the thin layer of blood and tissue produced by coagulation off [22]. The copious air/water spray also has this blow-off effect. When strict hemostasis is required, using minor air/water spray and pressing the wound after surgery may solve this problem.
The CO2 and Nd:YAG lasers have been effectively used for oral surgical procedures. Based on the previous findings and the study herein, the Er,Cr:YSGG laser was able to provide efficient, precise and safe ablation to both soft and hard tissues. This laser is thus expected to have widespread application in the oral surgical area. When validated, this experimental surgical technique may prove useful and unique in its ability to produce a pathway into bone. Therefore, some minor operations, such as fenestration, drainage of periapical abscesses, and apicoectomy may be possible with this laser.
Conclusion
The results of the present study lead us to conclude that the Er,Cr:YSGG laser facilitates precise surgical ablation with minimal thermal damage to adjacent tissues in vivo. Although there is a minimal delay before the subsequent healing process begins, the overall postoperational healing is favorable. Hence the Er,Cr:YSGG laser is an instrument with significant potential for use in minor oral surgery, especially where hard tissue is concerned.
References
Dibartolomeo JR (1981) Argon and CO2 lasers in otolaryngology: which one, when and why? Laryngoscope 26(Suppl):5–6
Li ZZ, Reinisch L, Merwe WPV (1992) Bone ablation with Er:YAG laser CO2 laser: study of thermal and acoustic effects. Lasers Surg Med 12:79–85
Lewandrowski KU, Lorente C, Schomacker KT, Flotte TJ, Wilkes JW, Deutsch TF (1996) Use of the Er:YAG laser for improved planting in maxillofacial surgery. Lasers Surg Med 19:40–45
Clayman L, Fuller T, Beckman H (1978) Healing of continuous-wave and rapid superpulsed carbon dioxide laser induced bone defects. J Oral Surg 36:932–937
Fisher SE, Frame JW (1984) The effects of the carbon dioxide surgical laser on oral tissues. Br J Oral Maxillofac Surg 22:414–425
McDavid VG, Cobb CM, Rapley JW, Glaros AG, Spencer P (2001) Laser irradiation of bone: III. Long-term healing following treatment by CO2 and Nd:YAG lasers. J Periodontol 72:174–182
Walsh JT, Deutsh TF (1989) Er:YAG laser ablation of tissue: measurement of ablation rates. Lasers Surg Med 9:327–337
Nuss RC, Fabian RL, Sarkar R, Puliafito CA (1988) Infrared laser bone ablation. Lasers Surg Med 8:381–391
Pratisto H, Frenz M, Ith M et al (1994) Use of 3 μm laser irradiation in middle ear surgery. Proc SPIE 2323:179–184
Wang X, Ishizaki NT, Suzuki N, Kimura Y, Matsumoto K (2002) Morphological changes of bovine mandibular bone irradiated by Er,Cr:YSGG laser: an in vitro study. J Clin Laser Med Surg 20:245–250
Stein E, Sedlacek T, Fabian RL, Nishioka NS (1990) Acute and chronic effects of bone ablation with a pulsed holmium laser. Lasers Surg Med 10:384–388
Payne JT, Peavy GM, Reinisch L, Van Sickle DC (2001) Cortical bone healing following laser osteotomy using 6.1 μ wavelength. Lasers Surg Med 29:38–43
Rizoiu IM, Eversole LR, Kimmel AI (1996) Effects of erbium, chromium: yttrium, scandium, gallium, garnet laser on mucocutanous soft tissues. Oral Surg Oral Med Oral Pathol 82:386–395
Shah UK, Poe DS, Rebeiz EF, Perrault DF, Pankratov MM, Shapshay SM (1996) Erbium laser in middle ear surgery: in vitro and in vivo animal study. Laryngoscope 106:418–422
Friesen LR, Cobb CM, Rapley JW, Brokman LF, Spencer P (1999) Laser irradiation of bone: II. Healing response following treatment by CO2 and Nd:YAG lasers. J Periodontol 70:75–83
Buchelt M, Kutschera HP, Katterschafka T, Kiss H, Lang S, Beer R, Losert U (1994) Erb:YAG and Hol:YAG laser osteotomy: the effect of laser ablation on bone healing. Lasers Surg Med 15:373–381
Bayly JG, Kartha VB, Steven WH (1963) The absorption spectrum of liquid phase H2O, and D2O from 0.7 μm to 10 μm. Infrared Phys 14:211–223
Doyle DB, Bendit EG, Blout ER (1975) Infrared spectroscopy of collagen and collagen-like polypeptides. Biopolymers 14:937–957
Bonner RF, Smith PD, Leon M (1986) Quantification of tissue effect due to pulsed Erb:YAG laser at 2.9 μm with beam delivery in a wet field in zirconium fluoride fibers. SPIE 713:2–5
Nelson JS, Orenstein A, Liaw LH, Berns MW (1989) Mid-infrared erbium:YAG laser ablation of bone: the effect of laser osteotomy on bone healing. Lasers Surg Med 9:362–374
Eriksson AR, Albrektsson T (1983) Temperature threshold levels for heat-induced bone tissue injury: a vital-microscopic study in the rabbit. J Prosthet Dent 50:101–107
Herdman RCD, Charlton A, Hinton AE (1993) An in vitro comparison of the erbium:YAG laser and the carbon dioxide laser in laryngeal surgery. J Laryngol Otol 107:908–911
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Zhang, C. & Matsumoto, K. In vivo study of the healing processes that occur in the jaws of rabbits following perforation by an Er,Cr:YSGG laser. Lasers Med Sci 20, 21–27 (2005). https://doi.org/10.1007/s10103-005-0329-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-005-0329-y