Abstract
The purpose of this study was to investigate the prevalence of plasmid-mediated AmpC β-lactamases in Escherichia coli and Klebsiella pneumoniae from five children’s hospitals in China. A total of 494 E. coli and 637 K. pneumoniae isolates were collected from five children’s hospitals in China from 2005 to 2006. The isolates with decreased susceptibility to cefoxitin were subjected to confirmation test with 3-aminophenyl boronic acid. Polymerase chain reaction (PCR) amplification of the blaAmpC, blaTEM, blaCTXM, and blaSHV genes and their gene sequencing were performed. Transconjugants were achieved by conjugation experiments. Plasmid-mediated AmpC β-lactamases were found in 10.1% of K. pneumoniae (64/637) and in 2.0% of E. coli (10/494) strains. The proportion of plasmid-mediated AmpC-producing strains significantly increased from 2005 (2.6%) to 2006 (9.3%) (p<0.001). The DHA-1-producing isolates were the most prevalent type (93.2%, 69/74). The sequences of blaDHA-1 genes were all identical to those from the GenBank. Strains of blaCMY-2 were isolated from five isolates (6.8%), which were all from E. coli. One sequence of blaCMY-2 differs from blaCMY-2 in the GenBank. Eighteen of the 74 (24.3%) AmpC-producing K. pneumoniae and E. coli isolates coproduced an extended-spectrum β-lactamase (ESBL). Cefoxitin resistance was transferred to 15 of the 74 positive strains (20.3%). Our study has demonstrated the occurrence of plasmid-mediated AmpC β-lactamases in E. coli and K. pneumoniae in Chinese pediatric patients and DHA-1 type AmpC enzymes had the highest prevalent rate. The CMY-2 AmpC β-lactamases from the children’s hospitals in China in this study are the first reported. Hence, continuous surveillance of the prevalence and evolution of AmpC β-lactamase is important.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antimicrobial resistance in Gram-negative isolates recovered from pediatric populations is a growing problem worldwide. Especially, the production of Ambler class C beta-lactamases (AmpC β-lactamases) is one of the prevalent mechanisms of β-lactam resistance [1]. Plasmid-mediated AmpC β-lactamases were first detected in 1989 [2], and various AmpC enzymes have been subsequently found, particularly in Escherichia coli and Klebsiella pneumoniae [3], which are the most commonly isolated species of the Enterobacteriaceae family in the clinical laboratory. The most frequent plasmid-mediated AmpC enzymes, including CMY-2, DHA, ACT-1, and CMY-2, were first reported in Germany [4] and Taiwan. CMY-2 AmpC β-lactamase was initially identified in E. coli in 2000 [5]. DHA-1 was identified in Saudi Arabia and France [6], while DHA-2 was identified in K. pneumoniae in France [7].
The increasing prevalence of plasmid-mediated AmpC β-lactamases in E. coli and K. pneumoniae is becoming a serious worldwide problem. To make this problem worse, many laboratories are having difficulties in detecting these enzymes using a unified criteria in clinical isolates [8, 9]. A report has shown that, in the United States, among 752 K. pneumoniae and E. coli strains from 70 sites in 25 states, 7 to 8.5% of the K. pneumoniae and 4% of the E. coli strains contain plasmid-mediated AmpC-type enzymes [10]. As the prevalence of E. coli- and K. pneumoniae-producing plasmid-mediated AmpC β-lactamases arise in tandem with the pervasive use of the broad-spectrum cephalosporins [11], it is getting difficult to select antibiotics to treat patients, especially pediatric patients. In addition, plasmid-mediated AmpC β-lactamases confer transmissible cephalosporin resistances to pathogens [12], which may pose an important problem to public health.
Cases of TEM-, SHV-, and CTX-M-type extended-spectrum β-lactamases (ESBLs), and DHA-type AmpC β-lactamases in E. coli and K. pneumoniae isolates have been reported in China [13, 14]. However, the true rate of occurrence of plasmid-mediated AmpC β-lactamases in K. pneumoniae and E. coli among the Chinese pediatric population remains unknown.
We investigate the prevalence and genotypic distributions of plasmid-mediated AmpC β-lactamases in clinical isolates of K. pneumoniae and E. coli from 2005 to 2006 at five children’s hospitals in China, and report isolates that coproduce AmpC and ESBLs. In addition, the first identification of the CMY-2 AmpC β-lactamase from the children’s hospitals in China is also described.
Materials and methods
Screening of plasmid-mediated AmpC β-lactamase isolates
Clinical non-duplicate isolates of 637 (321, 316) K. pneumoniae and 494 (251, 243) E. coli were collected between January 2005 and December 2006 from five children’s hospitals in China, which included the following: Beijing Children’s Hospital, representing the northern part of China; Chongqing Children’s Hospital, representing the western part of China; Guangzhou Children’s Hospital, representing southern China; Shanghai Children’s Hospital and Fudan Children’s Hospital of Fudan University representing the eastern part of China (Fig. 1). Each isolate came from an individual patient. K. pneumoniae strains were isolated mainly from patients in the neonatology wards and the intensive care unit (ICU), and they were cultured from sputum specimens (69.7%). E. coli strains were isolated mainly from patients in the respiratory medicine wards and neonatology wards (Table 1). They were cultured mainly from sputum (49.4%) and urine specimens (32.7%). The remaining specimens were obtained from blood and pleural and bronchoalveolar lavage fluid.
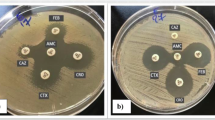
All isolates were identified by the Vitek and the API 20E systems (bioMerieux Vitek, Hazelwood, MO). In accordance with the 2005 CLSI criteria [15], isolates with resistance or with decreased susceptibility to cefoxitin (FOX) were selected for further study. The positive isolates which have an inhibition zone diameter ≤18 mm were forwarded to our laboratory for further confirmation using the double-disk synergy (DDS) test. Phenotypic confirmatory tests for plasmid-mediated AmpC β-lactamases were performed with 3-aminophenyl boronic acid (APB, Sigma-Aldrich, Milwaukee, WI), as described by Yagi et al. [16], with the following modifications: for the disk potentiation test, 300 μg of APB was added to a 30-μg cefoxitin disk (Oxoid, Basingstoke, UK) and the zone was compared with that obtained without APB. A zone diameter ≥5 mm was interpreted as a positive result in the disk potentiation test in the DDS test. In addition, the negative isolates were then regarded as negative for AmpC production by DDS tests with APB. E. coli DH5α2919, which produces plasmid-mediated AmpC β-lactamase, was tested as the positive control, and K. pneumoniae ATCC700603 was used as the negative control.
For the screening of ESBL-positive isolates, cefotaxime and ceftazidime were used as indicator antibiotics [17]. In the case that isolates were resistant to ceftazidime and/or cefotaxime, then the screening results were confirmed by the use of the same indicator antibiotics in both the presence and absence of clavulanic acid according to the 2005 CLSI recommendations.
Antimicrobial susceptibility testing
Minimum inhibitory concentration (MIC) determination was carried out by the agar dilution method with Mueller Hinton (MH) agar according to the 2005 CLSI recommendations, including nine β-lactams (ampicillin, aztreonam, ceftazidime, cefotaxime, cefepime, cefoxitin, cefoperazone, imipenem, and amoxicillin/clavulanic acid) and three non-β-lactam antibiotics (gentamicin, amikacin, and ciprofloxacin). E. coli ATCC 25922 was used as a quality control strain.
Detection of AmpC genes by PCR
Preparation of template DNA and multiplex PCR
A single colony of each organism was inoculated in 5 ml of Luria-Bertani (LB) broth (Oxoid, Basingstoke, UK) and suspended in 0.5 ml of sterile water, which was heated at 95°C for 10 min. After centrifugation at 17,000g for 5 min at 4°C, the DNA-containing supernatant was extracted and used as the source of template for further amplification [18].
AmpC multiplex polymerase chain reaction (PCR) was performed on FOX-resistant isolates using the method of Péréz-Péréz and Hanson [18]. In doing this, 1.25 U of Taq DNA polymerase (Takara Biotechnology (DALIAN) Co. Ltd.) was contained in each reaction. Five-microliter aliquots of PCR product were analyzed by gel electrophoresis with 2% agarose. The gels were stained with ethidium bromide at 10 μg/ml and visualized by UV transillumination. A 100 bp DNA ladder from New England Biolabs was used as a marker.
Sequence analysis of DHA-1- and CMY-2-like full-length PCR amplicon
The full-length PCR amplicon used for the sequence analysis was generated with primers designed to flank the entire gene for DHA-1 and CMY-2. The PCR program was performed as described by Liebana et al. [19]. The PCR products were processed using the Qiagen (Hilden, Germany) PCR purification kit. DNA sequence analysis was carried out using the direct sequencing of both strands with an autosequencer (ABI 3730XL, Perkin-Elmer, Foster City, CA). Amplified products were sequenced at least twice. The sequence analysis of AmpC was performed by computer-generated nucleic acid analysis using the BLAST program (http://www.ncbi.nlm.nih.gov/BLAST/).
Characterization of ESBL genes by PCR and sequencing
The selected isolates with positive screen tests were subjected to a molecular screening for β-lactamases using PCR tests as previously described for TEM [20], SHV [21], and CTX-M [22]. In each reaction, 1.25 U of Taq DNA polymerase was incorporated as previously, and subsequent sequencings of the PCR products were then performed.
Conjugation
Conjugation experiments were carried out in LB broth with E. coli J53AzR as the recipient. Cultures of donor and recipient cells in the logarithmic phase (0.5 ml each) were added to 4 ml of fresh LB broth and incubated overnight without shaking. Transconjugants were selected on trypticase soy agar (TSA) plates containing 100 μg/ml of sodium azide (Sigma Chemical Co., St. Louis, MO) in order to identify the plasmid-mediated cefoxitin resistance. To determine if cefoxitin resistance was transferred, colonies were replica-plated onto a TSA with and without cefoxitin (20 μg/ml). MICs for the donors, recipient, and transconjugants were measured using the MH agar dilution, with reference to the guidelines of the CLSI. The AmpC genes of transconjugants were confirmed by PCR.
Results
The occurrence rates of AmpC producers in K. pneumoniae and E. coli
Among 494 E. coli and 637 K. pneumoniae isolates from five children’s hospitals, 128 of the 207 FOX-insusceptible clinical isolates (inhibition zone ≤18 mm) yielded positive AmpC DDS tests. The occurrence rate of AmpC producers in K. pneumoniae and E. coli was 11.4% (128/1,131), including 86 in K. pneumoniae and 42 in E. coli. Seventy-four of the 128 positive isolates (57.8%) were confirmed to be plasmid-mediated AmpC β-lactamase producers from multiplex PCR. The prevalence of plasmid-mediated AmpC-producing strains was 6.5% (74/1131) in K. pneumoniae and E. coli isolates. The occurrence rate of plasmid-mediated AmpC-producing strains in K. pneumoniae (10.1%, 64/637) was higher than that of the E. coli (2.0%, 10/494) strains.
The plasmid-mediated AmpC-producing strains was only 2.6% (12 of 464 isolates) in 2005, but the proportion of plasmid-mediated AmpC-producing strains significantly increased (p<0.001) to 9.3% (62 of 667 isolates) in 2006.
The distribution of genotypes in plasmid-mediated AmpC β-lactamase isolates
Among the 74 plasmid-mediated AmpC-producing strains, the DHA-1 β-lactamase was harbored by 69 (93.2%) of the isolates. One hundred percent (64/64) of K. pneumonia and 50% (5/10) of E. coli plasmid-mediated AmpC-producing strains were with DHA-1. Strains of blaCMY-2 were detected in five isolates (6.8%), which were all from E. coli. Four strains of blaCMY-2 were isolated from Chongqing in a different year. Eighteen of the 74 AmpC-producing K. pneumoniae and E. coli isolates (24.3%) co-carried with ESBL genes. In addition, TEM, SHV, CTX-M-9, and CTX-M-1 were found in 12, three, five, and one isolates of that AmpC-producing K. pneumoniae, respectively. The DHA-1(+) plus TEM(+) strain was the most predominant (accounting for 33.3% (6/18) of co-carrying strains), with the next common being the DHA-1(+) plus TEM(+) and CTX-M-9(+) strain (22.2%, 4/18). Only three plasmid-mediated AmpC-producing strains coproduced ESBL enzymes in E. coli, and all of them were co-carried with CTX-M and TEM.
The sequences of blaDHA-1 genes were all identical to those of blaDHA-1 from the GenBank. The sequences of blaCMY-2 in the four strains were all identical to those from the GenBank, and one strain differed from blaCMY-2 in the GenBank by a G-to-A change at position 262 of the structural gene, leading to an amino acid (Gly to Ser) substitution, which had been submitted to the GenBank (GenBank accession no. EU162133).
The characteristics of antibiotic resistance in plasmid-mediated AmpC-producing strains
In our study, all 74 plasmid-mediated AmpC-producing organisms showed multiple antibiotic resistances. The resistance rates of the 74 AmpC-producing isolates to the antibiotics are as follows: ampicillin, 94.6%; cefoperazone, 98.7%; cefoxitin, 100%; amoxicillin/clavulanic acid, 98.7%; cefotaxime, 96.2%; ceftazidime, 98.7%; aztreonam, 94.9%; and cefepime, 83.3%. The MIC50/MIC90 of the 74 plasmid-mediated AmpC-producing isolates are 16/32, 256/>512, 128/512, 256/512, 32/128, 256/512, 512/>512, and 64/64 μg/ml, respectively. In addition, the resistance rate of AmpC producers to gentamicin is 74.4%; to amikacin, 38.5%; and to ciprofloxacin, 75.6%. The MIC50/MIC90 are 256/>512, 4/>512, and 2/32, respectively. All of the 74 AmpC-producing isolates are susceptible to imipenem. The high-level resistances to cefoxitin, cefotaxime, and ceftazidime, which were frequently used in pediatric patients, are shown in Table 2.
Transfer of AmpC β-lactamases and antimicrobial resistance in transconjugants
Cefoxitin resistance can be transferred by conjugation in 20.3% (15 of 74) of AmpC-positive donors. The presence of transferred AmpC β-lactamases genes in the transconjugants is confirmed through multiplex PCR. The type of all transconjugants is DHA-1. For all 15 transconjugants, the sequence of the DHA-1 gene is identical to that originally reported. The MIC of cefoxitin against the transconjugants is 128 to 256 μg/ml, representing a 16- to 32-fold increase relative to that of the recipient E. coli J53AzR (8 μg/ml). The MIC of cefoxitin is greater than the corresponding MICs for the recipient strain. All transconjugants have elevated MICs for ampicillin, aztreonam, ceftazidime, cefotaxime, cefepime, cefoxitin, cefoperazone, gentamicin, and ciprofloxacin. The MIC of the transconjugants is similar to that of their donors.
Discussion
Plasmid-mediated AmpC β-lactamase-producing E. coli and K. pneumoniae are being increasingly found in many parts of the world [8, 9], but reports from China are relatively rare, especially from children’s hospitals. We report that the prevalence of plasmid-mediated AmpC β-lactamase-producing K. pneumoniae isolated from the Chinese pediatric patients is 10.1%. Although our results suggest that plasmid-mediated AmpC β-lactamases currently have lower occurrence rates as compared to the 11% previously reported by Morland et al. in K. pneumoniae [23], it was higher than the value noted by Alvarez et al. [10]. The incidence and prevalence rates of plasmid-mediated AmpC β-lactamases are higher in 2006 than that in 2005.
Since the first report of an AmpC gene in 2001 [24], DHA-1-producing K. pneumoniae and E. coli strains have been present in China [14]. CMY-2 is the most prevalent of the plasmid-mediated AmpC enzymes, which is most widely distributed geographically [8]. Nonetheless, report of CMY-2-producing K. pneumoniae and E. coli is rare in China, except for isolates from animals that were recently reported by Liu et al. in Southern China [25]. To the best of our knowledge, and as supported by the absence of reports in PubMed, the initial identification of the CMY-2 AmpC β-lactamase isolated from children in China is described for the first time in our study. Five isolates of the CMY-2 AmpC β-lactamase include one isolate (point mutation) from the Shanghai Children’s Hospital (eastern China) and four other isolates from the Chongqing Children’s Hospital (western China) in different years, all obtained from the sputum of five children who had pneumonia.
Cephalosporin resistance among K. pneumoniae and E. coli has increased worldwide [26], as shown in our antimicrobial susceptibility data, but the rates of resistance to cephalosporins, including that to cefepime, are high. However, all AmpC-producing isolates remain susceptible to imipenem. Multi-resistant organisms should be treated with antibiotic regimens other than cephalosporins. Continuous or frequent use of cephalosporins probably leads to higher resistance rates of AmpC-producing isolates of enterobacters, especially in pediatric populations; cephalosporins are very commonly used in the children’s hospitals. Therefore, it would be wise to perform surveillance cultures to monitor resistance levels in the different wards [27]. In addition, our findings may have important implications in the control of AmpC β-lactamase-producing K. pneumoniae and E. coli strains, which are likely to be overlooked in the children’s hospitals. In order to prevent the spread of resistant hospital flora, we suggest that the restriction of the prescription of broad-spectrum antimicrobial agents is necessary [28].
According to the result of the transfer of resistance, cefoxitin resistance is transferred to 20% of all AmpC producers. However, transferred resistance is detected in 59% of the K. pneumoniae and 44% of the E. coli isolates according to Alvarez et al. [10]. We do not know the reason for the contrasting results. The presence of transferred relevant AmpC genes in transconjugants is confirmed by PCR; this suggests that these plasmids of AmpC β-lactamases may be spreading horizontally. Based on our study, the plasmid AmpC-producing isolates are genotypically different. Thus, transfer can play an important role in the widespread resistance in K. pneumoniae and E. coli. This may create serious problems when treating pediatric patients.
Pathogens producing ESBLs and plasmid-mediated AmpC β-lactamases pose a serious threat to patient treatment. It was reported that the association of plasmid-mediated AmpC β-lactamases with ESBLs may pose diagnostic challenges [29], since the presence of an ESBL can be masked by the expression of an AmpC, and the exact detection of plasmid-mediated AmpC β-lactamases in isolates that produce both ESBLs and plasmid-mediated AmpC β-lactamases is important from the clinical and public health aspects. CTX-M-15 has been associated, in many cases, with OXA-30, which is capable of hydrolyzing cefepime, for example, but we have sequenced the PCR product to confirm that CTX-M-15 was not found in these isolates. The bla genes coding for several β-lactamases may be found in different plasmids, but, often, they coexist on the same plasmid [8]. Dissemination of these AmpC β-lactamase-encoding plasmids is thought to facilitate the spread of resistance against a wide range of antibiotics among K. pneumoniae and E. coli. In Korea, 8.7% of plasmid-mediated AmpC β-lactamases producers also produce ESBLs [30]. Based on our study, 18 of the 74 (24.3%) plasmid-mediated AmpC β-lactamase-producing organisms coproduce an ESBL in which TEM-ESBL was the major type, followed by SHV-ESBL. The extended spectrum was caused by point-mutations in the TEM and SHV genes. The spread of plasmid-mediated AmpC β-lactamase-producing strains with ESBL genes is a concern, as it causes limitations in the selection of antibiotics for the optimal treatment of patients. For example, the use of penicillins and cephalosporins is excluded.
Plasmids containing genes that encode for AmpC and/or ESBLs often contain resistance determinants for other classes of antimicrobial agents and are readily transmissible from strain to strain and among different species of Enterobacteriaceae, as well as aminoglycosides, in many cases due to the location of these genes on one and the same plasmid. A close relationship between AmpC and/or ESBL production and ciprofloxacin resistance in K. pneumoniae has been reported to be existent worldwide. Our other study also indicates that the plasmid-mediated quinolone resistance qnr gene is associated with the bla gene Enterobacteriacea for TEM, SHV, CTX-M-1, CTX-M-9, or DHA-1 (data not published).
In conclusion, this study demonstrates the occurrence and increasing prevalence of plasmid-mediated AmpC enzymes in E. coli and K. pneumoniae isolated from five children’s hospitals in China. The DHA-1 genotype is the predominant plasmid-mediated AmpC β-lactamase. The presence of CMY-2 AmpC β-lactamases from the Chinese pediatric patients in this study is the first reported. Therefore, continuous surveillance of the prevalence and evolution of AmpC β-lactamase is important.
References
Verdet C, Benzerara Y, Gautier V, Adam O, Ould-Hocine Z, Arlet G (2006) Emergence of DHA-1-producing Klebsiella spp. in the Parisian region: genetic organization of the ampC and ampR genes originating from Morganella morganii. Antimicrob Agents Chemother 50(2):607–617
Bauernfeind A, Chong Y, Schweighart S (1989) Extended broad spectrum beta-lactamase in Klebsiella pneumoniae including resistance to cephamycins. Infection 17(5):316–321
Hanson ND (2003) AmpC beta-lactamases: what do we need to know for the future? J Antimicrob Chemother 52(1):2–4
Bauernfeind A, Jungwirth R, Schweighart S, Theopold M (1990) Antibacterial activity and beta-lactamase stability of eleven oral cephalosporins. Infection 18(Suppl 3):S155–S167
Yan JJ, Ko WC, Tsai SH, Wu HM, Jin YT, Wu JJ (2000) Dissemination of CTX-M-3 and CMY-2 beta-lactamases among clinical isolates of Escherichia coli in southern Taiwan. J Clin Microbiol 38(12):4320–4325
Gaillot O, Clément C, Simonet M, Philippon A (1997) Novel transferable beta-lactam resistance with cephalosporinase characteristics in Salmonella enteritidis. J Antimicrob Chemother 39(1):85–87
Fortineau N, Poirel L, Nordmann P (2001) Plasmid-mediated and inducible cephalosporinase DHA-2 from Klebsiella pneumoniae. J Antimicrob Chemother 47(2):207–210
Philippon A, Arlet G, Jacoby GA (2002) Plasmid-determined AmpC-type beta-lactamases. Antimicrob Agents Chemother 46(1):1–11
Song W, Jeong SH, Kim JS, Kim HS, Shin DH, Roh KH, Lee KM (2007) Use of boronic acid disk methods to detect the combined expression of plasmid-mediated AmpC beta-lactamases and extended-spectrum beta-lactamases in clinical isolates of Klebsiella spp., Salmonella spp., and Proteus mirabilis. Diagn Microbiol Infect Dis 57(3):315–318
Alvarez M, Tran JH, Chow N, Jacoby GA (2004) Epidemiology of conjugative plasmid-mediated AmpC beta-lactamases in the United States. Antimicrob Agents Chemother 48(2):533–537
Moland ES, Hong SG, Thomson KS, Larone DH, Hanson ND (2007) Klebsiella pneumoniae isolate producing at least eight different beta-lactamases, including AmpC and KPC beta-lactamases. Antimicrob Agents Chemother 51(2):800–801
Paterson DL (2006) Resistance in gram-negative bacteria: enterobacteriaceae. Am J Med 119(6 Suppl 1):S20–S28; discussion S62–S70
Xiong Z, Zhu D, Wang F, Zhang Y, Okamoto R, Inoue M (2002) Investigation of extended-spectrum beta-lactamase in Klebsiellae pneumoniae and Escherichia coli from China. Diagn Microbiol Infect Dis 44(2):195–200
Wei ZQ, Chen YG, Yu YS, Lu WX, Li LJ (2005) Nosocomial spread of multi-resistant Klebsiella pneumoniae containing a plasmid encoding multiple beta-lactamases. J Med Microbiol 54(Pt 9):885–888
Clinical and Laboratory Standards Institute (CLSI) (2005) Performance standards for antimicrobial susceptibility testing; fifteenth informational supplement. CLSI, Wayne, PA
Yagi T, Wachino J, Kurokawa H, Suzuki S, Yamane K, Doi Y, Shibata N, Kato H, Shibayama K, Arakawa Y (2005) Practical methods using boronic acid compounds for identification of class C beta-lactamase-producing Klebsiella pneumoniae and Escherichia coli. J Clin Microbiol 43(6):2551–2558
National Committee for Clinical Laboratory Standards (NCCLS) (2003) Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A8, 8th edn. NCCLS, Wayne, PA
Pérez-Pérez FJ, Hanson ND (2002) Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol 40(6):2153–2162
Liebana E, Gibbs M, Clouting C, Barker L, Clifton-Hadley FA, Pleydell E, Abdalhamid B, Hanson ND, Martin L, Poppe C, Davies RH (2004) Characterization of beta-lactamases responsible for resistance to extended-spectrum cephalosporins in Escherichia coli and Salmonella enterica strains from food-producing animals in the United Kingdom. Microbial Drug Resist 10(1):1–9
Hanson ND, Thomson KS, Moland ES, Sanders CC, Berthold G, Penn RG (1999) Molecular characterization of a multiply resistant Klebsiella pneumoniae encoding ESBLs and a plasmid-mediated AmpC. J Antimicrob Chemother 44(3):377–380
Rasheed JK, Jay C, Metchock B, Berkowitz F, Weigel L, Crellin J, Steward C, Hill B, Medeiros AA, Tenover FC (1997) Evolution of extended-spectrum beta-lactam resistance (SHV-8) in a strain of Escherichia coli during multiple episodes of bacteremia. Antimicrob Agents Chemother 41(3):647–653
Pitout JD, Hossain A, Hanson ND (2004) Phenotypic and molecular detection of CTX-M-beta-lactamases produced by Escherichia coli and Klebsiella spp. J Clin Microbiol 42(12):5715–5721
Moland ES, Black JA, Ourada J, Reisbig MD, Hanson ND, Thomson KS (2002) Occurrence of newer beta-lactamases in Klebsiella pneumoniae isolates from 24 U.S. hospitals. Antimicrob Agents Chemother 46(12):3837–3842
Zhang YL, Li JT, Zhao MW (2001) Detection of amp C in Enterobacter cloacae in China. Int J Antimicrob Agents 18(4):365–371
Liu JH, Wei SY, Ma JY, Zeng ZL, Lu DH, Yang GX, Chen ZL (2007) Detection and characterisation of CTX-M and CMY-2 beta-lactamases among Escherichia coli isolates from farm animals in Guangdong Province of China. Int J Antimicrob Agents 29(5):576–581
Tan TY, Ng LS, Teo L, Koh Y, Teok CH (2007) Detection of plasmid-mediated ampc in Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis. J Clin Pathol [Epub ahead of print]
Peterson LR (2005) Squeezing the antibiotic balloon: the impact of antimicrobial classes on emerging resistance. Clin Microbiol Infect 11(Suppl 5):4–16
Paterson DL (2004) “Collateral damage” from cephalosporin or quinolone antibiotic therapy. Clin Infect Dis 38(Suppl 4):S341–S345
Yan JJ, Ko WC, Wu HM, Tsai SH, Chuang CL, Wu JJ (2004) Complexity of Klebsiella pneumoniae isolates resistant to both cephamycins and extended-spectrum cephalosporins at a teaching hospital in Taiwan. J Clin Microbiol 42(11):5337–5340
Song W, Kim JS, Kim HS, Yong D, Jeong SH, Park MJ, Lee KM (2006) Increasing trend in the prevalence of plasmid-mediated AmpC beta-lactamases in Enterobacteriaceae lacking chromosomal ampC gene at a Korean university hospital from 2002 to 2004. Diagn Microbiol Infect Dis 55(3):219–224
Acknowledgments
This work was supported by a grant (no. 2004BA720A09–01) from the Health Ministry of China. We would like to thank all of the participating hospitals for their support. We also thank Dingxia Shen, the 301 Hospital of Chinese People Library Army, for graciously supplying the strain of E. coli J53AzR and the control strains, and Lin Yuan, for his assistance in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, H., Yang, Y., Lu, Q. et al. The prevalence of plasmid-mediated AmpC β-lactamases among clinical isolates of Escherichia coli and Klebsiella pneumoniae from five children’s hospitals in China. Eur J Clin Microbiol Infect Dis 27, 915–921 (2008). https://doi.org/10.1007/s10096-008-0532-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-008-0532-4