Abstract
Introduction/Objectives
Pyoderma gangrenosum (PG) is a rare, rapidly progressive neutrophilic dermatosis commonly associated with systemic inflammatory diseases. We aimed to characterize the association of PG and inflammatory arthritis, as little is known outside of case reports and small cohort studies.
Method
We performed a systematic review in PubMed, EMBASE, and Scopus from inception to present using the terms arthritis and pyoderma gangrenosum. Patient demographics, clinical presentation, and treatment outcomes were recorded. Descriptive statistics and stratified analysis were used to compare factors of interest by type of arthritis.
Results
A total of 1399 articles were screened, and 129 patients with inflammatory arthritis and PG were included in the review. The most common types of arthritis were rheumatoid arthritis (RA) (50.4%), inflammatory bowel disease (IBD)–associated arthritis (10.9%), and psoriatic arthritis (8.5%). In the vast majority of cases, joint symptoms preceded PG, by a median of 10 years (inter-quartile range [IQR] 5–16). Corticosteroid monotherapy and biologic therapies, used alone or in combination, resulted in improvement or complete resolution of ulcers 71.4% and 67.3% of the time, respectively. Within the latter, infliximab, adalimumab, and anakinra were most successful in inducing remission overall. RA and non-RA did not differ significantly in treatment success or healing time.
Conclusions
This study shows that PG is frequently preceded by inflammatory arthritis, most commonly RA. Clinicians used a wide variety of treatment regimens with variable outcomes. While larger studies are needed to standardize the treatment of inflammatory arthritis-associated PG, this study suggests that in addition to systemic corticosteroids, biologic medications can be effective treatment options for these patients.
Key Points. • Inflammatory arthritis, most commonly rheumatoid arthritis, often precedes rather than follows pyoderma gangrenosum. • Other forms of arthritis associated with PG included IBD-associated arthritis and psoriatic arthritis. • Biologic therapies, such as infliximab, adalimumab, and anakinra, were largely successful in treating arthritis-associated pyoderma gangrenosum and may play an important role in corticosteroid-sparing therapy or in a maintenance regimen for this subset of patients. • The type of inflammatory arthritis associated with pyoderma gangrenosum may not be a helpful treatment guide as it was not significantly associated with treatment outcomes or healing time. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Classic pyoderma gangrenosum (PG) is a rare neutrophilic dermatosis characterized by skin ulcerations with violaceous borders and undermined edges often affecting the lower extremities. A common feature is pathergy, or lesions forming at sites of trauma. PG predominantly affects middle-aged women [1]. PG is thought to be an autoinflammatory process, but the pathophysiology and etiology of PG are not well understood [2]. There are no targeted or universally successful therapies, and no guidelines for treatment have been established. Reducing inflammation is a cornerstone of treatment, and systemic treatments are often used, including immunosuppressives and biologics [3].
PG may occur in isolation, as an inherited inflammatory syndrome, or in association with systemic disease and/or with extracutaneous manifestations [4]. A recent meta-analysis reported that 57% of PG patients have an associated systemic disease, most commonly inflammatory bowel disease (IBD), inflammatory arthritis, and hematologic malignancies. Approximately 13% of patients with PG have inflammatory arthritis, most commonly rheumatoid arthritis (RA) [5]. The association between PG and RA was investigated in a recent population-based case–control study which revealed that the lifetime prevalence of RA was higher among patients with PG than control subjects, and RA increases the odds of developing PG by more than threefold. It is advised that clinicians be aware of the increased likelihood of PG, and patients with RA should avoid predisposing factors of PG [6]. In addition to RA, PG has been reported in patients with spondyloarthritides, such as psoriatic arthritis, ankylosing spondylitis, and IBD-associated arthritis [7, 8]. While excluded from this study, PG may occur in inherited inflammatory syndromes such as PAPA (pyogenic arthritis, PG, and acne), PASH (PG, acne, and hidradenitis suppurativa), and PAPASH (pyogenic arthritis, PG, acne, and hidradenitis suppurativa).
Despite growing research, case reports and small cohort studies dominate the literature of arthritis-associated PG. Studies are lacking in characterizing this subset of patients and identifying any factors that affect the course of PG. Better understanding of arthritis-associated PG may have prognostic significance as successful treatment of the coexisting disease may lead to better PG outcomes [9]. It has also been suggested that arthritis-associated PG represents a refractory subset of patients [10]. The purpose of this study is to characterize the association of PG with inflammatory arthritis.
Materials and methods
We performed a systematic review of published articles in PubMed/MEDLINE, EMBASE, and Scopus (from inception to March 2021) to identify eligible studies. Articles were searched using the terms “pyoderma gangrenosum” and “arthritis.” Publications with titles and abstracts written in English were eligible for inclusion. Two authors independently screened a total of 1399 articles for relevance and critically reviewed the full text of 265 articles for eligibility. Figure 1 illustrates the Preferred Reporting Items for Systematic Review and Meta-Analyses flow diagram. Reference lists of selected articles were further screened for additional eligible articles. We included all case reports and case series describing patients of any age who were clinically diagnosed with both PG and inflammatory arthritis defined by the authors. Arthralgia without adequate workup, infectious arthritis, and syndromic forms of PG, such as PAPA, PASH, and PAPASH, were excluded. Articles that lacked data on clinical characteristics and treatment type for individual patients, such as large cohort studies, were excluded as well.
The following information were recorded for each patient: age, gender, arthritis type, rheumatologic workup, site of PG, histologic findings, time from onset to diagnosis of PG, time between PG diagnosis and arthritis onset, type of treatment used to treat PG, treatment outcome, and healing time. Treatment success was defined as improvement or resolution of ulcers reported by the clinician based on the follow-up reviewed in the articles. Healing time was defined as complete resolution of the PG ulcer. Inflammatory arthritis was classified based on underlying disease. Patients with spondyloarthritis, sacroiliitis, or spondyloarthropathy were grouped together as spondyloarthritis. Psoriatic and IBD-associated arthritis were categorized separately. Unspecified inflammatory arthritis was defined as monoarthritis, oligoarthritis, or polyarthritis lacking a diagnosis or description of underlying disease.
Descriptive statistics were used to characterize the demographic and clinical characteristics of the reported cases that were collected for this study. Group comparisons by type of arthritis were made using Wilcoxon rank-sum test for continuous data due to their skewed distribution and Fisher’s exact test for all categorical variables. Statistical analysis was performed using R: a language and environment for statistical computing. A p-value of less than 0.05 was considered to be statistically significant.
Results
A total of 129 cases of arthritis-associated PG from 110 articles published between 1966 and March 2021 were included in the study (Fig. 1). The median patient age was 49 years (inter-quartile range [IQR] 34–61). Ninety-nine (76.7%) patients were female. The median time of PG symptom onset to diagnosis was 4.5 months (IQR 1.7–12). PG was present on the lower legs in 87 (67.4%) patients. There were various types of arthritis that presented with PG. Sixty-five (50.4%) patients had RA, 19 (14.7%) had unspecified inflammatory arthritis, 14 (10.9%) had IBD-associated arthritis, and 11 (8.5%) had psoriatic arthritis. Of those with unspecified inflammatory arthritis, 3 patients had monoarthritis, none oligoarthritis, 13 polyarthritis, and 3 undescribed. Arthritis presented prior to PG in 98 (76.0%) patients by a median of 10 years (IQR 5–16). Sixteen (12.4%) cases reported worsened arthritic symptoms during PG presentation (Table 1).
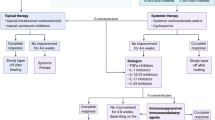
Ultimately, 123 (95.3%) patients with PG were successfully treated with systemic therapies; however, in 62 (48.1%) patients, the initial treatment was deemed ineffective by the clinician. Nineteen (14.7%) patients were initially treated solely with antibiotics despite further workup indicating no infection cause. A multitude of treatment regimens were used for PG ulcers (Table 2), with therapies used alone and in combination. Systemic corticosteroids were included in 118 treatment regimens, but steroids were used as monotherapy in only 49 regimens, of which improvement or resolution of PG ulcers was seen in 71.4% (35/49) within 13 months. Among regimens that included biologics, alone or in combination, success was seen in 67.3% (37/55). Infliximab and etanercept were the most frequently used biologics (24/55 and 8/55, respectively). Notably, adalimumab (8/55) was found to be successful in 87.5% (7/8) of treatment regimens. The most frequently used systemic therapies were grouped into categories, including corticosteroids alone, biologics alone, and combination therapies (Table 3). This analysis excluded antibiotics given that it is not a standard treatment for PG. When used alone, biologics had a success rate of 91.3% (21/23) and when used in combination with corticosteroids, a success rate of 77.8% (7/9). Of the 43 (33.3%) cases that reported healing time, 21 (48.8%) had complete resolution of the ulcer within 3 months of onset and 35 (81.4%) had complete resolution within 6 months of onset. The median healing time was 3 months (IQR 2.5–5).
Age, time of PG onset to diagnosis, and time of PG onset relative to arthritis were the only significant differences between RA and non-RA groups (Table 4). Patients with RA were found to be older when diagnosed with PG than patients with non-RA (p < 0.001). PG presented after arthritis in 57 (87.7%) patients with RA, compared to 41 (64.1%) patients with non-RA (p = 0.001). Treatment outcomes and healing time did not differ significantly between RA and non-RA groups.
Discussion
Neither the relationship between PG and arthritis nor the treatment of arthritis-associated PG is well understood. Treatment is often tailored to the individual patient, with little guidance from large-scale studies or clinical trials. One objective of this study was to describe treatment success and its associated factors. While treatment regimens varied widely in terms of combination of medications, dosage, and duration of treatment, regimens containing systemic corticosteroids, biologics, and cyclosporine were successful in the majority of cases. The type of arthritis associated with PG was not significantly associated with treatment outcomes or healing time.
The association between PG and arthritis has been well established [11,12,13] and the incidence of arthritis in PG patients has been found to range from 12.8% to 37% [1, 5, 8, 14]. Our review revealed that PG patients present with many types of arthritis, although RA is the most common. These results are consistent with studies in the literature [5, 15, 16]. The second most common form of PG-associated arthritis was IBD-associated arthritis, which may be explained by the fact that IBD is the leading underlying comorbidity in patients with PG [8]. Our study was limited by cases of inflammatory arthritis labeled as unspecified by the authors (14.7%). Knowledge of the predominant types of arthritis coexisting with PG can aid in guiding workup for a patient presenting with joint symptoms and ulcerative skin lesions.
Over two-thirds of our cases of arthritis-associated PG presented on the lower legs (67.4%). Notably, a high number (13.2%) of head and neck ulcers were reported, which has also been found in PG associated with hematologic malignancies [17]. In most of our cases, PG was diagnosed several years after the associated arthritis, particularly in RA-associated PG. Clinically, it is uncommon for PG to be a herald of RA. A similar latency period has been demonstrated in a large case–control study in which the vast majority of patients developed PG after at least 4 years from being diagnosed with RA [6]. A minority of patients demonstrated worsening arthritic activity with PG onset. This is consistent with the observation that PG activity is unrelated to the severity of arthritis, and the clinical course of PG does not mirror the clinical course of the associated inflammatory disease [16, 18].
Standardized treatment guidelines for PG do not exist, and lesions are often refractory, requiring multiple trials or combinations of medications. An effective treatment strategy typically includes both wound care and topical and/or systemic therapy [11]. Corticosteroids have been the mainstay treatment for PG and have been historically recommended as first-line systemic therapy [9]. Their utility has been demonstrated in a single-blind randomized trial and several retrospective case series [1, 19]. In this review, the majority (71.4%) of corticosteroid monotherapy treatment regimens were successful; however, many ulcers were refractory.
Steroid-sparing therapies for PG include anti-neutrophil therapies, immunosuppressants, and biologic agents. We found the majority (91.3%) of treatment regimens that included a biologic without other systemic therapies resulted in ulcer improvement or healing. It has been suggested that tumor necrosis factor (TNF) inhibitors may be the preferred treatment for arthritis-associated PG [20], and a randomized controlled trial found that infliximab was successful in treating patients with PG over placebo [21]. Infliximab was the most commonly used biologic in our review. While the pathogenesis of PG is unclear, IL-1β, IL-8, IL-17, TNF-α, and several chemokines and metalloproteinases have been implicated in skin lesions of PG [2, 22, 23]. PG and inflammatory joint disorders share pathophysiologic features in terms of cytokine overexpression [24]. This may explain the success with TNF inhibitors.
It has been suggested that ulcers in arthritis-associated PG have a worse prognosis than PG alone and present with slower healing time and longer treatment duration [10]. To explore the refractory nature of arthritis-associated PG, we compared our results to a recent multicenter single-blind randomized controlled trial that found 47% of PG ulcers treated with prednisolone or cyclosporine healed within 6 months [19]. While our data shows that 81.4% of PG ulcers with associated arthritis healed within the same timeframe, only 33.3% of articles reported complete resolution of ulcers, with the majority reporting significant improvement in ulcer healing as their marker of success. Thus, our results are most likely skewed due to the lack of long-term patient follow-up provided in the articles. In addition, when arthritis types were grouped to determine the effect on clinical outcomes, the presence of RA did not influence treatment success or how quickly an ulcer resolves. Again, these results are difficult to interpret given the high number of articles that did not provide details on healing time.
This study provides insight into specific clinical characteristics of arthritis-associated PG cases. The heterogeneity in patient details and the retrospective nature of this review are limitations. Articles were not consistent in reporting the distribution of arthritis, such as polyarthritis, oligoarthritis, and monoarthritis, nor the clinical course, such as acute, subacute, and chronic arthritis. We could not determine the frequency of distribution or clinical course of arthritis. Furthermore, our study was limited due to the fact that the vast majority of articles provided very little information on rheumatology laboratory or radiographic studies. In addition, publication bias may have skewed our results. It was necessary for our inclusion criteria of inflammatory arthritis to be broad in order to capture all of the cases in the literature, but it also lent to the inclusion of undifferentiated arthritis. With appropriate workup, these cases may have fallen under specific arthritis categories and impacted our results.
Conclusion
PG was frequently preceded by inflammatory arthritis, most commonly RA. More than 10% of these patients were solely treated with antibiotics and most likely misdiagnosed as having a skin and soft-tissue infection. While corticosteroids are the mainstay treatment for PG, biologics such as TNF inhibitors seem to play a larger role as a corticosteroid-sparing therapy and ultimately for a maintenance regimen in this subset of patients with PG-associated inflammatory arthritis. Corticosteroids and biologics are used to treat arthritis, but our review indicates that the type of arthritis associated with PG is not a useful treatment guide. Prospective studies are needed to further understand the association between PG and arthritis and the role of arthritis type in treatment and outcomes.
Data availability
Available.
References
Binus AM, Qureshi AA, Li VW, Winterfield LS (2011) Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol 165(6):1244–1250. https://doi.org/10.1111/j.1365-2133.2011.10565.x
Marzano AV, Cugno M, Trevisan V et al (2010) Role of inflammatory cells, cytokines and matrix metalloproteinases in neutrophil-mediated skin diseases. Clin Exp Immunol 162(1):100–107. https://doi.org/10.1111/j.1365-2249.2010.04201.x
Teagle A, Hargest R (2014) Management of pyoderma gangrenosum. J R Soc Med 107(6):228–236. https://doi.org/10.1177/0141076814534407
Borda LJ, Wong LL, Marzano AV, Ortega-Loayza AG (2019) Extracutaneous involvement of pyoderma gangrenosum. Arch Dermatol Res 311(6):425–434. https://doi.org/10.1007/s00403-019-01912-1
Kridin K, Cohen AD, Amber KT (2018) Underlying systemic diseases in pyoderma gangrenosum: a systematic review and meta-analysis. Am J Clin Dermatol 19(4):479–487. https://doi.org/10.1007/s40257-018-0356-7
Kridin K, Damiani G, Cohen AD (2021) Rheumatoid arthritis and pyoderma gangrenosum: a population-based case-control study. Clin Rheumatol 40(2):521–528. https://doi.org/10.1007/s10067-020-05253-7
Vavricka SR, Brun L, Ballabeni P, Pittet V, PrinzVavricka BM, Zeitz J, Rogler G, Schoepfer AM (2011) Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol 106(1):110–119. https://doi.org/10.1038/ajg.2010.343
Ashchyan HJ, Butler DC, Nelson CA et al (2018) JAMA dermatology original investigation the association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol 154(4):409–413. https://doi.org/10.1001/jamadermatol.2017.5978
Reichrath J, Bens G, Bonowitz A, Tilgen W (2005) Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol 53(2):273–283. https://doi.org/10.1016/j.jaad.2004.10.006
Charles CA, Bialy TL, Falabella AF, Eaglstein WH, Kerdel FA, Kirsner RS (2004) Poor prognosis of arthritis-associated pyoderma gangrenosum. Arch Dermatol 140(7):861–864. https://doi.org/10.1001/archderm.140.7.861
Ahronowitz I, Harp J, Shinkai K (2012) Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol 13(3):191–211. https://doi.org/10.2340/00015555-2008
Bhat RM, Nandakishore B, Sequeira FF et al (2011) Pyoderma gangrenosum: an Indian perspective. Clin Exp Dermatol 36(3):242–247. https://doi.org/10.1111/j.1365-2230.2010.03941.x
Holt PJ, Davies MG, Saunder KC, Nuki G (1980) Pyoderma gangrenosum: clinical and laboratory findings in 15 patients with special reference to polyarthritis. Medicine (Baltimore) 59(2):114–133
Powell FC, Schroeter AL, Su WP, Perry HO (1985) Pyoderma gangrenosum: a review of 86 patients. Q J Med 55(217):173–186
Langan SM, Groves RW, Card TR, Gulliford MC (2012) Incidence, mortality, and disease associations of pyoderma gangrenosum in the United Kingdom: a retrospective cohort study. J Invest Dermatol 132(9):2166–2170. https://doi.org/10.1038/jid.2012.130
Sayah A, English JC (2005) Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol 53(2):191–209. https://doi.org/10.1016/j.jaad.2004.07.023
Gupta AS, Ortega-Loayza AG (2019) Pyoderma gangrenosum: a too often overlooked facultative paraneoplastic disease. Ann Hematol 98(9):2247–2248. https://doi.org/10.1007/s00277-019-03732-9
Von den Driesch P (1997) Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol 137(6):1000–1005
Ormerod AD, Thomas KS, Craig FE et al (2015) Comparison of the two most commonly used treatments for pyoderma gangrenosum: results of the STOP GAP randomised controlled trial. BMJ 350:h2958. https://doi.org/10.1136/bmj.h2958
Maalouf D, Battistella M, Bouaziz JD (2015) Neutrophilic dermatosis: disease mechanism and treatment. Curr Opin Hematol 22(1):23–29. https://doi.org/10.1097/MOH.0000000000000100
Brooklyn TN, Dunnill MGS, Shetty A et al (2006) Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut 55(4):505–509. https://doi.org/10.1136/gut.2005.074815
Marzano AV, Fanoni D, Antiga E et al (2014) Expression of cytokines, chemokines and other effector molecules in two prototypic autoinflammatory skin diseases, pyoderma gangrenosum and sweet’s syndrome. Clin Exp Immunol 178(1):48–56. https://doi.org/10.1111/cei.12394
Wollina U, Tchernev G (2014) Pyoderma gangrenosum: pathogenetic oriented treatment approaches. Wien Med Wochenschr 164(13–14):263–273. https://doi.org/10.1007/s10354-014-0285-x
Cugno M, Gualtierotti R, Meroni PL, Marzano AV (2018) Inflammatory joint disorders and neutrophilic dermatoses: a comprehensive review. Clin Rev Allergy Immunol 54(2):269–281. https://doi.org/10.1007/s12016-017-8629-0
Funding
Dr. Marcia Friedman.
3T32HL094294-08S1
KL2TR002370
DoM Wheels Up Early Physician Scientist Career Development Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sawka, E., Zhou, A., Latour, E. et al. Inflammatory arthritis-associated pyoderma gangrenosum: a systematic review. Clin Rheumatol 40, 3963–3969 (2021). https://doi.org/10.1007/s10067-021-05768-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-021-05768-7