Abstract
Background
The association between pyoderma gangrenosum (PG) and rheumatoid arthritis (RA) was not investigated in the setting of controlled studies. The risk of PG among patients with RA is not established.
Objective
The study aims to evaluate the magnitude of the association between RA and the subsequent development of PG. Additionally, we aimed to characterize patients with RA-associated PG relative to other patients with PG.
Methods
A population-based case-control study was conducted comparing PG patients (n = 302) with age-, sex-, and ethnicity-matched control subjects (n = 1497) with respect to the presence of RA. Logistic regression models were utilized for univariate and multivariate analyses.
Results
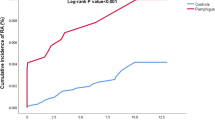
The prevalence of RA was greater in patients with PG than in control subjects (4.7% vs. 1.5%, respectively; P < 0.001). More than threefold increase in the odds of PG with RA (OR, 3.29; 95% CI, 1.66–6.50) was noted. This association retained its statistical significance following a sensitivity analysis excluding RA cases diagnosed up to 2 years prior to PG (OR, 2.72; 95% CI, 1.25–5.91) and after adjusting for confounding factors (adjusted OR, 2.80; 95% CI, 1.23–5.86). RA preceded the diagnosis of PG in the majority of patients by a mean (SD) latency of 9.2 (7.4) years. Patients with RA-associated PG were older relative to the remaining patients with PG (62.2 [15.0] vs. 53.4 [20.9] years, respectively; P = 0.006).
Conclusions
RA increases the odds of developing PG by more than threefold. Physicians managing patients with RA should be aware of this increased burden. Patients with RA may be advised to avoid additional precipitating factors of PG.
Key Points • The odds of developing PG are increased by more than threefold in patients with RA. • PG followed the diagnosis of RA in the majority of patients with these coexistent conditions by an average latency of 9.2 years. • Patients with RA-associated were older relative to other patients with PG at the onset of PG. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pyoderma gangrenosum (PG) is a rare, chronic, and cutaneous ulcerative disease with a distinctive morphologic presentation typified by painful ulcers with violaceous, undermined borders, typically on the lower extremities. While the pathogenesis of PG is yet to be fully elucidated, several lines of evidence suggest that neutrophil dysfunction and aberrant systemic inflammation play a vital role in the induction of PG lesions [1, 2]. A recent immunohistological study postulated that a T cell response resulting in destruction of pilosebaceous units is involved in the pathogenesis of PG [3]. More than half of patients with PG are associated with an underlying systemic disease at their presentation, with inflammatory bowel disease (IBD), inflammatory arthritis, and hematological malignancies being the most frequently encountered conditions [4].
RA is a chronic, usually symmetric, inflammatory autoimmune disease, primarily affecting peripheral joints [5]. Although the precise pathomechanism underlying RA is not fully comprehended, environmental factors such as smoking, in a genetically predisposed individual, are thought to arouse the emergence of RA [5]. Patients with RA may experience a wide array of cutaneous manifestations [6, 7]. These include, among others, rheumatoid nodules, accelerated rheumatoid nodulosis, rheumatoid vasculitis, palisaded neutrophilic and granulomatosis dermatitis, Felty syndrome, and pyoderma gangrenosum (PG) [6, 7].
Several case reports and case series had pointed to the coexistence of RA and PG in individual patients. A recent meta-analysis depicted that the pooled prevalence of RA among reported patients with PG was estimated at 8.7% (95% CI, 7.2–10.3) [4]. However, the current literature lacks controlled observational studies investigating and quantifying the association between PG and RA. The likelihood of the occurrence of PG among patients with RA was not evaluated.
Aiming to fill the aforementioned gap, we sought to perform a population-based case-control study to explore the association between RA and the subsequent development of PG. Moreover, we aimed to disclose the epidemiological features of patients with coexistent PG and RA relative to other patients with PG.
Methods
Study design and database
We conducted a retrospective population-based case-control study aiming to identify the risk of developing subsequent PG following the diagnosis of RA. For this design to be implemented, only patients in whom the exposure (RA) preceded the outcome (PG) were included in the analysis. In another cross-sectional sub-analysis, all patients with a dual diagnosis of RA and PG were included regardless of the sequence in which the investigated conditions appeared.
The current study was performed on the basis of the computerized database of Clalit Health Services (CHS). CHS is the largest healthcare organization in Israel, ensuring and providing healthcare services for approximately 4,927,000 enrollees as of October 2018 (57% of the general population of Israel based on the Central Bureau of Statistics). CHS provides a wide spectrum of both public and private healthcare services. The computerized database of CHS is grounded on persistent real-time data input from clinical, pharmaceutical, and administrative operating systems. This database was proven very effective in facilitating the performance of epidemiological studies.
The chronic disease registry of CHS extracts data from various sources, including hospital and primary care reports, purchase of medications, health services utilization, and laboratory and imaging data. These accumulated data is then manually cross-checked and validated by the primary care practitioner of each patient. This registry was shown to be of high validity and reproducibility [8].
The ethical committee of Ben-Gurion University approved the current study in accordance with the declaration of Helsinki.
Study population and covariates
Between the years 2000 and 2018, we identified all individuals given a diagnostic code consistent with the diagnosis of PG. Afterward, the inclusion of PG cases in this study was based on the following criteria: (i) a documented diagnosis of PG registered by a community board-certified dermatologist at least twice or (ii) documentation of the diagnosis of PG in hospital discharge letters from dermatological wards.
The diagnosis of RA was based on its documentation in the chronic registry of CHS. It is based on the documentation of board-certified rheumatologists, the purchase of RA-related drugs, and suggestive laboratory and imaging data and is eventually validated by the managing general healthcare provider.
The control group encompassed up to 5 controls per every patient, matched randomly by age, sex, and ethnicity. The date of diagnosis served as an index date for every case and for the corresponding matched control patients. Before their recruitment, controls were ascertained to be alive and to contribute longitudinal data for the CHS dataset.
Outcome measures were controlled for underlying comorbidities utilizing the Charlson comorbidity index, a validated epidemiological method of quantifying comorbidities. This index has been evidenced to be reliable in forecasting lethal outcomes [9]. Smoking status was classified as a current smoker or never/past smoker. The adjustment for smoking was performed owing to its predisposing role in RA [10] and owing to conflicting evidence regarding its role in triggering inflammatory bowel disease-associated PG [11,12,13,14].
A sensitivity analysis was performed to ensure that the observed association was not overestimated due to ascertainment bias. This analysis was based on reiterating the calculations with an exclusion period of 2 years prior to the index date (diagnosis of PG and recruitment of controls). Thus, all individuals given a diagnosis of RA in this period were omitted from calculations.
Statistical analysis
Baseline characteristics are described with means and standard deviations (SD) for continuous variables, whereas percentages are used to signify categorical values. A comparison of sociodemographic and clinical factors between patients with PG and controls was performed using the chi-square test and t test, as indicated.
Logistic regression was used to calculate odds ratios (ORs) and 95% CIs to compare cases and control with respect to the presence of RA. The association was calculated based on individuals who developed PG following the diagnosis of UC in accordance with the presence of a temporal relationship between exposure and outcome in case-control studies. In the cross-sectional sub-analysis, all patients with both diagnoses were included. Two-tailed P values less than 0.05 were considered as statistically significant. All statistical analyses were performed using SPSS software, version 25 (SPSS, Armonk, NY: IBM Corp).
Results
Characteristics of the study population
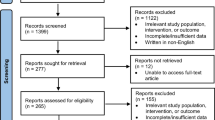
Three hundred two patients with PG and 1497 matched control individuals were included in the current study. The mean (SD) age at the diagnosis of patients and recruitment of control subjects was 54.0 (20.8) years. One hundred seventy-five (57.9%) patients with PG were females, and 255 (84.4%) were of Jewish ancestry. The two groups were comparable in terms of sex and ethnic composition, average body mass index (BMI), and the prevalence of smoking (Table 1). The mean (SD) Charlson comorbidity score was greater in cases than in controls (2.3 [2.7] vs. 1.3 [1.8], respectively; P < 0.001). Severe comorbidities were encountered more frequently among cases relative to controls (37.4% vs. 19.2%, respectively; P < 0.001). The features of the study population are outlined in Table 1. Selection of study population and main study outcomes are pictorially illustrated in Fig. 1.
Cross-sectional study design
The lifetime prevalence of RA was higher among patients with PG than among control subjects (5.6% vs. 1.7%, respectively; OR, 3.51; 95% CI, 1.87–6.59; P < 0.001). When a stratified analysis was held, the association between PG and RA was greater among younger (< 45 years; OR, 10.28; 95% CI, 1.86–56.68) and male (OR, 4.06; 95% CI, 1.08–15.33) patients. It is noteworthy that the association was still significant among patients older than 45 years of age, as well as among female patients (Table 2). When patients were divided according to the ethnic background and the smoking status, the association held significance only among Jewish (OR, 3.44; 95% CI, 1.72–6.86) and nonsmoker (OR, 4.22; 95% CI, 2.01–8.83) patients (Table 2).
Case-control study design
Among patients with coexistent PG and RA (n = 17), the diagnosis of PG followed that of RA in the majority of patients (82.4%; n = 14). In cases where PG followed the onset of RA, the mean latency between the conditions was 9.2 (7.4) years. Of these patients, 71.4% (n = 10) developed PG after at least 4 years from being diagnosed with RA. In 17.6% (n = 3) patients, the diagnosis of PG preceded that of RA by a mean (SD) latency of 6.2 (3.1) years.
We then performed a case-control study, including only cases where the exposure (RA) preceded the outcome (PG; Table 3). The prevalence of RA was greater among patients with PG than among control subjects (4.7% vs. 1.5%, respectively; P < 0.001). More than threefold increase in the odds of PG was observed with preceding RA (OR, 3.29; 95% CI, 1.06–6.50). When stratified by age, sex, and ethnicity, the association between RA and subsequent PG was more protruding among younger (< 54 years; OR, 10.28; 95% CI, 1.86–56.68), male (OR, 5.07; 95% CI, 1.01–25.43), and Jewish (OR, 3.15; 95% CI, 1.47–6.76) patients. The association was only of marginal statistical significance among patients of Arab ethnicity (OR, 3.90; 95% CI, 0.84–18.05). In a stratified analysis based on the smoking status, the association was only significant among nonsmokers (OR, 4.04; 95% CI, 1.83–8.95; Table 3).
A sensitivity analysis excluding patients diagnosed with RA up to 2 years before PG was carried out. The association of RA and later PG retained its statistical significance (OR, 2.72; 95% CI, 1.25–5.91). Moreover, we performed a multivariate analysis adjusting for confounding factors like comorbidities, smoking, and body mass index. The association was proven independently significant in this analysis (adjusted OR, 2.80; 95% CI, 1.23–5.86; P = 0.006).
The clinical characteristics of patients with RA-associated PG as compared with other patients with PG
We then addressed the epidemiological characteristics of patients with RA-associated PG (n = 17) as compared with the remaining patients with PG (n = 285). Patients with a dual diagnosis of PG and RA were significantly older at the onset of PG (62.2 [15.0] vs. 53.4 [20.9] years, respectively; P = 0.006) and had higher prevalence of ischemic heart diseases (41.2% vs. 20.4%, respectively; P = 0.043). The sex distribution, ethnic background, BMI, frequency of smoking, hyperlipidemia, hypertension, and diabetes mellitus were comparable between the two subgroups (Table 4).
Discussion
The current population-based study has revealed a more than threefold increase in the odds of developing PG in patients with RA. The association was found stronger among young, male, and Jewish patients and was robust to multivariate analysis adjusting for confounding factors. PG followed RA in the majority of cases by an average duration of 9.7 years. Compared with other patients with PG, patients with RA-associated PG were younger at the onset of the cutaneous disease.
A recent meta-analysis reviewed the literature and summarized the distribution and frequency of underlying diseases among patients with PG. This study synthesized data across 21 studies encompassing 2611 patients with PG and found that 57% of these patients have at least one underlying comorbid condition. The prevalence of inflammatory arthritis in these eligible studies ranged between 0.0% [15] and 32.6% [16], whereas its pooled prevalence was estimated at 12.8% (95% CI, 9.2–16.9), representing the second most common comorbidity [4]. Among these 21 eligible studies, 18 specified the precise prevalence of RA, which ranged between 0% [15, 17,18,19,20,21] and 19.2% [22], providing a pooled prevalence of 8.7% (95% CI, 7.2–10.3) [4].
Despite this evidence, the extent in which RA predisposes to the development of later PG is yet to be established. This gap stems from the lack of case-control studies comparing patients with PG and controls with regard to the presence of RA, as well as from lack of cohort studies tracking patients with RA for the occurrence of PG. This knowledge may be of great benefit for physicians managing patients with PG since early detection of the comorbid underlying disease may attenuate the overall disease course and lead to favorable outcomes in PG [23]. Additionally, awareness of this association is of help for physicians managing RA since it sheds light on the real-life likelihood of developing a devastating and hard-to-treat extra-articular manifestation.
Our study signifies that patients with a dual diagnosis of PG and RA were significantly older than the remaining patients with PG. This observation is attributable to the differential distribution of comorbidities in patients with PG across various age strata. The remaining patients with PG assumingly include a considerable portion of patients with underlying (IBD), the most common underlying condition in PG [4], which tends to present in the second to fourth decades of life [24]. This finding accords with the findings of Ashchyan et al. [23] showing that PG patients older than 65 years were more likely to have RA (13.3%) relative to those younger than 65 years of age (6.2%; P = 0.02). In our study, the absolute prevalence of RA was higher among patients older than 54 years, but the association of RA with PG was more prominent among younger patients in the age-stratified analysis. The latter finding probably reflects the lower prevalence of RA in the control group in this age category, increasing the OR of the association in younger patients.
Our findings denote that the likelihood of PG was higher among nonsmokers. Although the influence of smoking on the risk of RA-associated PG was not investigated, several studies in IBD-associated PG yielded inconclusive results. While several studies suggested that cigarette smoking does not impose a risk for cutaneous manifestations in IBD (including PG) [12,13,14], cigarette smoking emerged as a significant predictor of PG in a cohort of patients with ulcerative colitis [11]. Our observation, therefore, aligns with the conclusions postulated from the majority of studies examining the role of smoking in IBD-associated PG. Further research is required to recognize the role that smoking plays in the induction of PG. It is noteworthy that topical nicotine had been anecdotally utilized in the management of PG, resulting in favorable response [25, 26]. The latter may lend weight to the current observation.
The current study provides epidemiological evidence concerning the magnitude of the association between PG and RA. The population-based nature enabled the inclusion of patients originating from primary, secondary, and tertiary care settings, a feature that increases the generalizability and validity of the findings and lowers the risk of selection bias. In order to minimize the likelihood of ascertainment bias, a sensitivity analysis was performed. The case-control design enabled identification of the temporal relationship between diseases. This contributed to delineating the risk of having PG in patients with prior RA based on the assumption that OR approaches relative risk in case-control studies investigating rare diseases [27]. Our study, however, has several limitations to consider. The lack of data regarding the clinical characteristics, severity (as estimated by the acceptable severity scores like DAS28), and immunoserological profile (like seropositivity of anti-CCP and RF antibodies) of patients was the main of these drawbacks. Additionally, we were not able to retrieve data regarding important laboratory variables like acute phase reactant (C-reactive protein erythrocyte sedimentation rate).
In conclusion, we found that the presence of RA is associated with a more than threefold increase in the odds of having subsequent PG. This association was strongest among male, younger, Jewish, and nonsmoker patients. The findings were not altered substantially in a sensitivity analysis excluding diagnoses up to 2 years prior to PG and in multivariate analysis adjusting for confounding factors. RA preceded PG in the majority of patients with dual diagnoses by a mean latency of 9.2 years. Relative to other patients with PG, patients with RA-associated PG were significantly younger at the onset of PG. Further research is required to reproduce these findings in other study populations. Awareness of the likelihood of this severe extra-articular manifestation is of great help for physicians managing RA. Patients with RA should be advised to avoid further predisposing factors of PG.
References
Braswell SF, Kostopoulos TC, Ortega-Loayza AG (2015) Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol 73:691–698. https://doi.org/10.1016/j.jaad.2015.06.021
Ortega-Loayza AG, Nugent WH, Lucero OM, Washington SL, Nunley JR, Walsh SW (2018) Dysregulation of inflammatory gene expression in lesional and nonlesional skin of patients with pyoderma gangrenosum. Br J Dermatol 178:e35–e36. https://doi.org/10.1111/bjd.15837
Wang EA, Steel A, Luxardi G, Mitra A, Patel F, Cheng MY, Wilken R, Kao J, de Ga K, Sultani H, Merleev AA, Marusina AI, Brassard A, Fung MA, Konia T, Shimoda M, Maverakis E (2018) Classic ulcerative pyoderma gangrenosum is a T cell-mediated disease targeting follicular adnexal structures: a hypothesis based on molecular and clinicopathologic studies. Front Immunol 8. https://doi.org/10.3389/fimmu.2017.01980
Kridin K, Cohen AD, Amber KT (2018) Underlying systemic diseases in pyoderma gangrenosum: a systematic review and meta-analysis. Am J Clin Dermatol 19:479–487. https://doi.org/10.1007/s40257-018-0356-7
Calabresi E, Petrelli F, Bonifacio AF et al (2018) One year in review 2018: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol
Lora V, Cerroni L, Cota C (2018) Skin manifestations of rheumatoid arthritis. G Ital di Dermatologia e Venereol. https://doi.org/10.23736/S0392-0488.18.05872-8
Sayah A, English JC (2005) Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol 53:191–209. https://doi.org/10.1016/j.jaad.2004.07.023
Cohen AD, Dreiher J, Regev-Rosenberg S et al (2010) The quality indigators program in Clalit Health Services: the first decade. Harefuah
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. https://doi.org/10.1016/0021-9681(87)90171-8
Chang K, Yang SM, Kim SH, Han K, Park S, Shin J (2014) Smoking and rheumatoid arthritis. Int J Mol Sci 15:22279–22295. https://doi.org/10.3390/ijms151222279
Manguso F, Sanges M, Staiano T, Gargiulo S, Nastro P, Gargano D, Somma P, Mansueto G, Peluso R, Scarpa R, D'Armiento FP, Astarita C, Ayala F, Renda A, Mazzacca G, D'Arienzo A (2004) Cigarette smoking and appendectomy are risk factors for extraintestinal manifestations in ulcerative colitis. Am J Gastroenterol 99:327–334
States V, O’Brien S, Rai JP et al (2020) Pyoderma gangrenosum in inflammatory bowel disease: a systematic review and meta-analysis. Dig Dis Sci. https://doi.org/10.1007/s10620-019-05999-4
Roberts H, Rai SN, Pan J, Rao JM, Keskey RC, Kanaan Z, Short EP, Mottern E, Galandiuk S (2014) Extraintestinal manifestations of inflammatory bowel disease and the influence of smoking. Digestion. 90:122–129. https://doi.org/10.1159/000363228
Karmiris K, Avgerinos A, Tavernaraki A, Zeglinas C, Karatzas P, Koukouratos T, Oikonomou KA, Kostas A, Zampeli E, Papadopoulos V, Theodoropoulou A, Viazis N, Polymeros D, Michopoulos S, Bamias G, Kapsoritakis A, Karamanolis DG, Mantzaris GJ, Tzathas C, Koutroubakis IE (2016) Prevalence and characteristics of extra-intestinal manifestations in a large cohort of Greek patients with inflammatory bowel disease. J Crohn's Colitis 10:429–436. https://doi.org/10.1093/ecco-jcc/jjv232
Marzano AV, Trevisan V, Lazzari R, Crosti C (2011) Pyoderma gangrenosum: study of 21 patients and proposal of a “clinicotherapeutic” classification. J Dermatol Treat 22:254–260. https://doi.org/10.3109/09546631003686069
Powell FC, Schroeter AL, Su WP, Perry HO (1985) Pyoderma gangrenosum: a review of 86 patients. Q J Med 55:173–186
Adisen E, Erduran F, Gurer MA (2016) Pyoderma gangrenosum: a report of 27 patients. Int J Low Extrem Wounds 15:148–154. https://doi.org/10.1177/1534734616639172
Vidal D, Puig L, Gilaberte M, Alomar A (2004) Review of 26 cases of classical pyoderma gangrenosum: clinical and therapeutic features. J Dermatolog Treat 15:146–152. https://doi.org/10.1080/09546630410031909
Suárez-Pérez JA, Herrera-Acosta E, López-Navarro N, Vilchez-Márquez F, Prieto JD, Bosch RJ, Herrera E (2012) Pyoderma gangrenosum: a report of 15 cases and review of the literature. Actas Dermosifiliogr 103:120–126. https://doi.org/10.1016/j.ad.2011.04.010
Pereira N, Brites MM, Goncalo M et al (2013) Pyoderma gangrenosum--a review of 24 cases observed over 10 years. Int J Dermatol 52:938–945. https://doi.org/10.1111/j.1365-4632.2011.05451.x
Ye MJ, Ye JM (2014) Pyoderma gangrenosum: a review of clinical features and outcomes of 23 cases requiring inpatient management. Dermatol Res Pract 2014:1–7. https://doi.org/10.1155/2014/461467
Saracino A, Kelly R, Liew D, Chong A (2011) Pyoderma gangrenosum requiring inpatient management: a report of 26 cases with follow up. Australas J Dermatol 52:218–221. https://doi.org/10.1111/j.1440-0960.2011.00750.x
Ashchyan HJ, Butler DC, Nelson CA, Noe MH, Tsiaras WG, Lockwood SJ, James WD, Micheletti RG, Rosenbach M, Mostaghimi A (2018) The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatology 154:409–413. https://doi.org/10.1001/jamadermatol.2017.5978
Alatab S, Sepanlou SG, Ikuta K, Vahedi H, Bisignano C, Safiri S, Sadeghi A, Nixon MR, Abdoli A, Abolhassani H, Alipour V, Almadi MAH, Almasi-Hashiani A, Anushiravani A, Arabloo J, Atique S, Awasthi A, Badawi A, Baig AAA, Bhala N, Bijani A, Biondi A, Borzì AM, Burke KE, Carvalho F, Daryani A, Dubey M, Eftekhari A, Fernandes E, Fernandes JC, Fischer F, Haj-Mirzaian A, Haj-Mirzaian A, Hasanzadeh A, Hashemian M, Hay SI, Hoang CL, Househ M, Ilesanmi OS, Jafari Balalami N, James SL, Kengne AP, Malekzadeh MM, Merat S, Meretoja TJ, Mestrovic T, Mirrakhimov EM, Mirzaei H, Mohammad KA, Mokdad AH, Monasta L, Negoi I, Nguyen TH, Nguyen CT, Pourshams A, Poustchi H, Rabiee M, Rabiee N, Ramezanzadeh K, Rawaf DL, Rawaf S, Rezaei N, Robinson SR, Ronfani L, Saxena S, Sepehrimanesh M, Shaikh MA, Sharafi Z, Sharif M, Siabani S, Sima AR, Singh JA, Soheili A, Sotoudehmanesh R, Suleria HAR, Tesfay BE, Tran B, Tsoi D, Vacante M, Wondmieneh AB, Zarghi A, Zhang ZJ, Dirac M, Malekzadeh R, Naghavi M (2020) The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol 5:17–30. https://doi.org/10.1016/S2468-1253(19)30333-4
Wolf R, Ruocco V (1998) Nicotine for pyoderma gangrenosum. Arch Dermatol 134:1071–1072. https://doi.org/10.1001/archderm.134.9.1071
Patel GK, Rhodes JR, Evans B, Holt PJA (2004) Successful treatment of pyoderma gangrenosum with topical 0.5% nicotine cream. J Dermatol Treat. https://doi.org/10.1080/09546630310019364
Greenland S, Thomas DC (1982) On the need for the rare disease assumption in case-control studies. Am J Epidemiol 116:547–553. https://doi.org/10.1093/oxfordjournals.aje.a113439
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The ethical committee of Ben-Gurion University approved the current study in accordance with the declaration of Helsinki.
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kridin, K., Damiani, G. & Cohen, A.D. Rheumatoid arthritis and pyoderma gangrenosum: a population-based case-control study. Clin Rheumatol 40, 521–528 (2021). https://doi.org/10.1007/s10067-020-05253-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-020-05253-7