Abstract
Charcot-Marie-Tooth disease type 2 (CMT2) is a clinically and genetically heterogeneous inherited neuropathy. Although new causative and disease-associated genes have been identified for CMT2 in recent years, molecular diagnoses are still lacking for a majority of patients. We here studied a cohort of 35 CMT2 patients of Chinese descent, using whole exome sequencing to investigate gene mutations and then explored relationships among genotypes, clinical features, and mitochondrial DNA levels in blood as assessed by droplet digital PCR. We identified pathogenic variants in 57% of CMT2 patients. The most common genetic causes in the cohort were MFN2 mutations. Two patients with typical CMT phenotype and neuromyotonia were detected to harbor compound heterozygous variations in the HINT1 gene. In conclusion, our work supports that the molecular diagnostic rate of CMT2 patients can be increased via whole exome sequencing, and our data suggest that assessment of possible HINT1 mutations should be undertaken for CMT2 patients with neuromyotonia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Charcot-Marie-Tooth (CMT) is a clinically and genetically heterogeneous group of disorders that is the most frequent form of inherited neuropathy [1]. Median motor nerve conduction velocities (MMNCVs) are used to classify CMT into either CMT1 (MNCV < 25 m/s), CMT2 (MNCV > 45 m/s), or ICMT (25 m/s < MNCV < 45 m/s) [2]. The contribution of CMT2 to all CMT cases ranges from 12 to 36% [3, 4]. The clinical features are characterized by distal muscle weakness and atrophy, mild or no sensory loss, depressed tendon reflexes, and deformity (e.g., pes cavus or clawed hands) Some cases could also present atypical symptoms such as hearing loss, pyramidal features, and optic atrophy [5].
Approximately 100 causative genes of CMT have been reported to date [3, 6], among which over 50 loci have been related to CMT2 (https://neuromuscular.wustl.edu/). The most common subtype of CMT2 is CMT2A2A (phenotype MIM number: 609260), associated with MFN2 gene mutations [7]. However, it has been estimated that genetic diagnosis of CMT2 was still unclear in about 75% of clinically diagnosed CMT2 individuals [8]. Moreover, owing to the genetic diversity, clinical manifestations and genotype–phenotype correlation of CMT2 are also heterogeneous and complex.
The CMT2 pathogenic genes such as MFN2 (MIM# 608507) and GDAP1 (MIM# 606598) encode outer mitochondrial membrane proteins and were associated with mitochondrial fusion and fission [7, 9]. It has been reported that MFN2 mutations might cause compensatory mitochondrial DNA proliferation, and patients with MFN2 mutations have been reported to harbor lower levels of mitochondrial DNA (mt-DNA) [10, 11]. Moreover, because of the dependence of axonal transport on a high metabolic rate, many CMT2-causative genes, including the axonal architecture regulating genes such as HSPB1 (MIM# 602195), HSPB8 (MIM# 608014), RAB7 (MIM# 602298), and axonal transport-related cytoplasmic dynein genes KIFIB (MIM# 605995), DYNC1H1 (MIM# 600112), and NEFH (MIM# 162230), may also be indirectly associated with mitochondrial function or dynamics [12,13,14,15,16,17,18].
Herein, 35 patients clinically diagnosed with CMT2 were enrolled and received a molecular diagnosis based on whole exome sequencing. We explored the impact of genotype on clinical heterogeneity and severity of CMT2, and assessed associations between disease severity and levels of mt-DNA.
Material and methods
Subjects
Thirty-five patients were enrolled in this study between 2004 and 2018. Neurological examinations were performed by two neurologists at least at the Department of Neurology of the First Affiliated Hospital of Fujian Medical University. The ratio between patients with familial history and sporadic was 1:3.4 (8:27). Muscle strength was graded bilaterally from 0 to 5 according to the Medical Research Council scale (Medical Research Council, 1976). Electrophysiological measurement was carried out using standard techniques. CMT2 was diagnosed when the median motor nerve conduction velocity was > 45 m/s and accompanied with clinical features. Compound muscle action potential (CMAP) amplitudes were also taken into account [19] Often, patients had decreased CMAP. The Charcot-Marie-Tooth Neuropathy Score (CMTNS) was used to evaluate disease severity [20]. Written informed consent was obtained from all the patients included in this study. This study was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University.
Whole exome sequencing
Total genomic DNA was extracted from the leucocyte fraction of venous blood samples using standard techniques and genetic analyses were performed with the whole exome sequencing-based assay using the Illumina Hiseq2500 platform (Illumina, USA). Clean reads were mapped to the human reference genome (UCSC hg19 http://genome.ucsc.edu/) with BWA (version 0.7.10, http://bio-bwa.sourceforge.net). Duplicate sequence reads were removed by Picard (version 1.85; http://picard.sourceforge.net), and GATK (version 3.1, https://software.broadinstitute.org/gatk/) was used to detect variants. Variants were annotated by ANNOVAR software (version 2015 Dec14, http://www.openbioinformatics.org/annovar/), which includes functional implications and allele frequency in several databases such as dbSNP138, 1000 Genomes (The 1000 Genomes Project Consortium; http://browser.1000genomes.org), and ExAC (Exome Aggregation Consortium; http://exac.broadinstitute.org/). Mutations were predicted by SIFT (http://sift.jcvi.org/), PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/), and MutationTaster (http://www.mutationtaster.org/). Variants were interpreted according to the American College of Medical Genetics and Genomics (ACMG) recommended standards. Sanger sequencing was performed to validate the putative pathogenic variants, allowing segregation analyses where possible.
Droplet digital PCR
Total genomic DNA was extracted from whole blood samples of 35 CMT2 patients and 42 age-paired healthy controls in which MFN2 and GDAP1 genes are expressed. The median age of patients and normal controls were 25 and 28 years old, respectively. The average cellular mitochondrial DNA content was quantified using the QX200 Droplet Digital PCR (ddPCR™) system (BioRad®, Hercules, CA, USA), with mitochondrial-encoded NADH dehydrogenase 1 (MT-ND1) as the mitochondrial template and RPP30 as the nuclear-encoded housekeeping template [21, 22]. For mt-DNA quantification, the primers and probe targeting the MT-ND1 gene were as follows: forward, CTAGCCGTTTACTCAATC; reverse, GGTGACTTCATATGAGATTG; and Taqman probe, AGCATCAAACTCAAACTACGCCC attached to 5′FAM and 3′TAM fluorophores. The primers and probe targeting ribonuclease P/MRP 30 kDa subunit (RPP30) were synthesized as follows: forward, GTGGTAGTGCATAGACTTTA; reverse, GTAGGAGGACATTTGAG; and the probe sequence was AGGCAGACTGACACTAGAGTTCAC with fluorescence labeling of 5′HEX and 3′TAM. Droplet digital PCR was performed according to manufacturer’s instructions. Briefly, after PCR on a thermal cycler, droplets from each sample were analyzed individually on the QX200 droplet reader, where PCR-positive droplets were read as mitochondrial DNA (MT-ND1 gene) or chromosomal housekeeping genes (RPP30 gene) by issuing specific fluorescence signals (FAM for the ND1 gene and HEX for the RPP30 gene). Then, PCR-positive and PCR-negative droplets were counted to provide absolute quantification of target DNA according to Poisson’s algorithm and mt-DNA copy numbers of each cell were quantified as the ratios of MT-ND1/RPP30*2 [21, 23].
Statistical analysis
Comparisons of levels of mt-DNA between different groups were performed using two-tailed paired Student’s t tests, unpaired t tests, or one-way ANOVA. Linear regression and the Pearson correlation analysis were performed between CMTNS and levels of mt-DNA. Statistical analyses were performed using GraphPad Prism 7 (USA, GraphPad Software). p < 0.05 was considered as statistically significant.
Results
Clinical features
Based on clinical features and electrophysiological measurements, 35 patients were enrolled in this study. The ratio of males (23/35; 34.3%) to females (12/35; 65.7%) was 1.9:1. The median age of onset was 13 years (ranging from 1 to 62). Most patients (71.4%; 25/35) developed initial symptoms in their childhood or adolescence (Fig. 1a).
Among the 35 patients, 91.4% (32/35) cases developed muscle weakness in lower limbs, of which 10 (28.6%) patients also had weakness in the upper limbs. Other signs included distal muscle atrophy (60%), hyporeflexia (71.4%), foot deformity (37.1%), and sensory disturbance (20%). In addition to these common symptoms, atypical manifestations also appeared in several cases: two patients revealed difficulties in flexing their fists after a strong voluntary hand contraction, dating back from childhood, which is a clinical presentation of neuromyotonia; three patients experienced different degrees of hearing loss.
CMTNS was applied to evaluate severity of disease. Linear regression and the Pearson correlation analysis were used to analyze the correlation between CMTNS and disease duration. The result indicated a positive correlation (r = 0.3846; p = 0.0297, Fig. 1b), meaning that participants with a longer course tended to have a higher CMTNS score. Among patients whose course of disease was more than 20 years, the median score of CMTNS was 12.
Genetic findings
We detected pathogenic variants in 20 cases (57%) (Fig. 2a). Three of these patients had genetic causes previously reported for CMT2, including HSPB1 (MIM# 602195) (NM_001540: c.539C > T (p.Thr180Ile)), GARS (MIM# 600287) (NM_002047: c.767A > G (p.His256Arg)), and GDAP1 (MIM# 606598) (NM_018972: c.1415A > G (p.His472Arg)). YARS (MIM# 603623) (NM_003680: c.1079C > A (p.Pro360Gln)) which was classified as variants with uncertain significant pathogenicity according to ACMG was also identified in one patient (Table 1). The most common genetic causes in this group were in MFN2; these accounted for 42.9% of all patients in our cohort (15/35). In total, 86.6% (13/15) of the MFN2 variants were located in the GTPase domain (exon 4 to exon 8). The most frequent variant, c.280C > T (p.Arg94Trp) (NM_014874), was detected in exon 4 (Fig. 2b). Four patients shared this variant and three of them were from the same family. Among the patients with variants identified in MFN2 gene, seven cases have familial history. The next most common causative gene after MFN2 was HINT1; two patients were identified with compound heterozygous mutations in the HINT1 gene (MIM# 600112) (NM_005340.6: p.Gly93Asp&Val97Met; p.Gly93Asp&Cys38Arg). In addition to the 20 aforementioned molecularly diagnosed CMT2 cases, fifteen cases have not yet been associated with disease-causative genes.
The association between the levels of mt-DNA and CMT1 severity
We quantified levels of mt-DNA from all 35 axonal CMT patients and age-paired healthy controls and compared them, but no significant difference was observed (Fig. 3a). To explore the possible influence of MFN2 and GDAP1 gene mutations on the levels of mt-DNA, data from patients with pathogenic variants in MFN2 and GDAP1 were compared with normal controls. However, there was no significant difference. Similarly, there was no significant difference in mt-DNA levels between patients with other causative genes and normal controls (Fig. 3b). To further explore the association between the levels of mt-DNA and disease severity, the correlation between CMTNS of CMT2 patients and levels of mt-DNA was evaluated. The results showed trend of negative correlation between CMTNS scores and levels of mt-DNA (r = − 0.1611; p = 0.3705) (Fig. 3c).
Mitochondrial DNA copy number analysis in different groups. a Comparison between normal controls and patients with CMT2. b Comparison of normal controls and the groups with identified variants in MFN2 and GDAP1 genes and other disease-causing genes. c The correlation between CMTNS of CMT2 patients and levels of mt-DNA
Discussion
In this study, whole exome sequencing identified known casual mutations in the MFN2, HINT1, HSPB1, GDAP1, and GARS genes in a cohort of 35 CMT2 patients with a total mutation detection rate of 57% (20/35). According to previous research, the mutation detection rate in our study was similar to the CMT cohorts reported in Spain (62.6%) and higher than other CMT groups in Japan (22.9%), Korea (13.3%), and the UK (25.2%) (Table 2) [24,25,26,27,28,29,30]. In our data, mutations in the MFN2 gene were the most common genetic cause; this frequency was higher than that reported in other cohorts, where the frequency was reported to be in the range of 17–23%. The higher mutation rate for MFN2 may be due to our strict control of the enrollment of CMT2 patients, which was based on their clinical features and electrophysiology (MMNCV > 45 m/s instead of > 38 m/s), which excluded intermediate CMT according to Berciano et al. who provided a proposed algorithm of the electrophysiological approach when investigating a patient with presumptive intermediate CMT [19]. About half of the patients with MFN2 gene mutation have familial history. For the reason that most of the patients were from the same province, there may be some particular founder effect that cause the higher mutation. But our sample size was limited, it lacks sufficient clue to evaluate the probability. However, GDAP1 was the most frequent causative gene in Spain and similarly for MPZ in Korea and GJB1 in Germany and Italy [31,32,33,34]. We identified GDAP1-related CMT2 with a mutation frequency of 2.9%, and no variants were identified in the MPZ or GJB1 genes. This may be related to our exclusion of cases with MNCV lower than 45 m/s, whereas other cohorts included patients with MNCV > 38 m/s.
Our findings highlight that that the distribution of CMT2-associated genes can be highly heterogeneous in different populations. In general, five genes, MFN2, GDAP1, MPZ, GJB1, and HSPB1, were the leading reasons for CMT2 in most cohorts and GDAP1 mutations may be more commonly distributed in European populations in Italy (14.5%) and Spain (25.8%) than in China [26, 35]. Moreover, mutations in the HINT1 gene were identified as the second most prevalent genetic cause for CMT2 in our study, yet mutations in this gene were rarely reported among other CMT2 cohorts.
We found that most CMT2 cases present with the typical CMT phenotype, and noted the presence of several mutation-specific phenotypic clues that may be useful for clinicians in directing future diagnosis [2, 5]. Specifically, the four CMT2A2A patients with MFN2 mutations experienced severe weakness of the distal muscles, extending to the upper limbs. But in previous studies, in addition to the typical CMT phenotype, CMT2A2A presents with atypical manifestations such as pyramidal features [5], optic atrophy [36], or retinitis pigmentosa; these manifestations were not present in our CMT2A2A patients.
In addition, two patients with compound heterozygous mutations in the HINT1 gene both experienced neuromyotonia at early ages. To date, only 81 CMT2 patients (including our study) with HINT1 mutations have been reported globally, and the frequency of these mutations was higher in our cohort than in European cohorts [37,38,39,40,41,42,43,44,45] (Supplementary Table 1). About 71.6% of the patients with HINT1 mutations exhibited neuromyotonia, a striking clinical and electrophysiological hallmark that can help to distinguish this disease and guide diagnostic screening [46]. Thus, it is recommended that for patients with difficulties flexing their fists or muscle stiffness, testing for HINT1 mutations should be an early diagnostic choice. Notably, we did not identify causal mutations for three of the CMT2 patients with hearing loss, and it is conceivable that they may share a common but as-yet-undiscovered pathogenic mechanism.
CMT2 is known to frequently feature abnormal mitochondria, including continuous changes in the position, size, shape, and levels of mt-DNA within cells [10, 11, 47, 48]. Our results indicated that in CMT2 patients with increased disease severity based on CMTNS, the levels of mt-DNA may decrease. Many CMT2-causative genes are directly or indirectly physiologically involved in mitochondrial function or dynamics. For example, the MFN2 and GDAP1 proteins are localized to the mitochondrial outer membrane. Results from Sitarz et al. (2012) indicated that CMT2A2A patients (n = 58) with MFN2 mutations exhibited compensatory mitochondrial DNA proliferation in blood [10]. Here, we used ddPCR to quantify levels of mt-DNA; however, we found no significant differences between normal controls and (i) the CMT2 patients generally or (ii) CMT2 patients with MFN2 and GDAP1 mutations. This may relate to the relatively small size of our CMT2 patient cohort. However, it could be possible that lower mt-DNA in blood could correlate with severity not only in MFN2 and GDAP1 cases but also with other CMT2 patients in a larger cohort. Thus, any influence of CMT2-causative genes and mt-DNA levels will require further exploration.
In conclusion, the molecular diagnostic rate of CMT2 patients increased with the use of whole exome sequencing in our data, and we suggest that HINT1 mutations should be assessed for CMT2 patients with neuromyotonia. Moreover, we found that levels of mt-DNA may be associated with CMT2 severity, which requires further exploration.
References
Braathen GJ, Sand JC, Lobato A, Hoyer H, Russell MB (2010) MFN2 point mutations occur in 3.4% of Charcot-Marie-Tooth families. An investigation of 232 Norwegian CMT families. BMC Med Genet 11:48. https://doi.org/10.1186/1471-2350-11-48
Stojkovic T (2016) Hereditary neuropathies: an update. Rev Neurol (Paris) 172(12):775–778. https://doi.org/10.1016/j.neurol.2016.06.007
Bacquet J, Stojkovic T, Boyer A, Martini N, Audic F, Chabrol B, Salort-Campana E, Delmont E, Desvignes JP, Verschueren A, Attarian S, Chaussenot A, Delague V, Levy N, Bonello-Palot N (2018) Molecular diagnosis of inherited peripheral neuropathies by targeted next-generation sequencing: molecular spectrum delineation. BMJ Open 8(10):e021632. https://doi.org/10.1136/bmjopen-2018-021632
Barreto LC, Oliveira FS, Nunes PS et al (2016) Epidemiologic study of Charcot-Marie-Tooth disease: a systematic review. Neuroepidemiology. 46(3):157–165. https://doi.org/10.1159/000443706
Gemignani F, Marbini A (2001) Charcot-Marie-Tooth disease (CMT): distinctive phenotypic and genotypic features in CMT type 2. J Neurol Sci 184(1):1–9
Yoshimura A, Yuan JH, Hashiguchi A, Ando M, Higuchi Y, Nakamura T, Okamoto Y, Nakagawa M, Takashima H (2019) Genetic profile and onset features of 1005 patients with Charcot-Marie-Tooth disease in Japan. J Neurol Neurosurg Psychiatry 90(2):195–202. https://doi.org/10.1136/jnnp-2018-318839
Chandhok G, Lazarou M, Neumann B (2018) Structure, function, and regulation of mitofusin-2 in health and disease. Biol Rev Camb Philos Soc 93(2):933–949. https://doi.org/10.1111/brv.12378
Rossor AM, Polke JM, Houlden H, Reilly MM (2013) Clinical implications of genetic advances in Charcot-Marie-Tooth disease. Nat Rev Neurol 9(10):562–571. https://doi.org/10.1038/nrneurol.2013.179
Pezzini I, Geroldi A, Capponi S, Gulli R, Schenone A, Grandis M, Doria-Lamba L, la Piana C, Cremonte M, Pisciotta C, Nolano M, Manganelli F, Santoro L, Mandich P, Bellone E (2016) GDAP1 mutations in Italian axonal Charcot-Marie-Tooth patients: phenotypic features and clinical course. Neuromuscul Disord 26(1):26–32. https://doi.org/10.1016/j.nmd.2015.09.008
Sitarz KS, Yu-Wai-Man P, Pyle A, et al. (2012) MFN2 mutations cause compensatory mitochondrial DNA proliferation. Brain. 135(Pt 8):e219, 211–213; author reply e220, 211–213. https://doi.org/10.1093/brain/aws049
Rouzier C, Bannwarth S, Chaussenot A, Chevrollier A, Verschueren A, Bonello-Palot N, Fragaki K, Cano A, Pouget J, Pellissier JF, Procaccio V, Chabrol B, Paquis-Flucklinger V (2012) The MFN2 gene is responsible for mitochondrial DNA instability and optic atrophy ‘plus’ phenotype. Brain. 135(Pt 1):23–34. https://doi.org/10.1093/brain/awr323
Kalmar B, Innes A, Wanisch K, Kolaszynska AK, Pandraud A, Kelly G, Abramov AY, Reilly MM, Schiavo G, Greensmith L (2017) Mitochondrial deficits and abnormal mitochondrial retrograde axonal transport play a role in the pathogenesis of mutant Hsp27-induced Charcot Marie Tooth disease. Hum Mol Genet 26(17):3313–3326. https://doi.org/10.1093/hmg/ddx216
Gentil BJ, Cooper L (2012) Molecular basis of axonal dysfunction and traffic impairments in CMT. Brain Res Bull 88(5):444–453. https://doi.org/10.1016/j.brainresbull.2012.05.003
Campbell PD, Shen K, Sapio MR, Glenn TD, Talbot WS, Marlow FL (2014) Unique function of kinesin Kif5A in localization of mitochondria in axons. J Neurosci 34(44):14717–14732. https://doi.org/10.1523/jneurosci.2770-14.2014
Eschbach J, Sinniger J, Bouitbir J, Fergani A, Schlagowski AI, Zoll J, Geny B, René F, Larmet Y, Marion V, Baloh RH, Harms MB, Shy ME, Messadeq N, Weydt P, Loeffler JP, Ludolph AC, Dupuis L (2013) Dynein mutations associated with hereditary motor neuropathies impair mitochondrial morphology and function with age. Neurobiol Dis 58:220–230. https://doi.org/10.1016/j.nbd.2013.05.015
Bomont P, Cavalier L, Blondeau F, Hamida CB, Belal S, Tazir M, Demir E, Topaloglu H, Korinthenberg R, Tüysüz B, Landrieu P, Hentati F, Koenig M (2000) The gene encoding gigaxonin, a new member of the cytoskeletal BTB/kelch repeat family, is mutated in giant axonal neuropathy. Nat Genet 26(3):370–374. https://doi.org/10.1038/81701
Wong YC, Ysselstein D, Krainc D (2018) Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature. 554(7692):382–386. https://doi.org/10.1038/nature25486
Jacquier A, Delorme C, Belotti E, Juntas-Morales R, Solé G, Dubourg O, Giroux M, Maurage CA, Castellani V, Rebelo A, Abrams A, Züchner S, Stojkovic T, Schaeffer L, Latour P (2017) Cryptic amyloidogenic elements in mutant NEFH causing Charcot-Marie-Tooth 2 trigger aggresome formation and neuronal death. Acta neuropathologica communications 5(1):55. https://doi.org/10.1186/s40478-017-0457-1
Berciano J, Garcia A, Gallardo E et al (2017) Intermediate Charcot-Marie-Tooth disease: an electrophysiological reappraisal and systematic review. J Neurol 2017 264:1655–1677. https://doi.org/10.1007/s00415-017-8474-3
Fridman V, Bundy B, Reilly MM, Pareyson D, Bacon C, Burns J, Day J, Feely S, Finkel RS, Grider T, Kirk CA, Herrmann DN, Laurá M, Li J, Lloyd T, Sumner CJ, Muntoni F, Piscosquito G, Ramchandren S, Shy R, Siskind CE, Yum SW, Moroni I, Pagliano E, Zuchner S, Scherer SS, Shy ME (2015) CMT subtypes and disease burden in patients enrolled in the Inherited Neuropathies Consortium natural history study: a cross-sectional analysis. J Neurol Neurosurg Psychiatry 86(8):873–878. https://doi.org/10.1136/jnnp-2014-308826
Memon AA, Zoller B, Hedelius A et al (2017) Quantification of mitochondrial DNA copy number in suspected cancer patients by a well optimized ddPCR method. Biomolecular detection and quantification 13:32–39. https://doi.org/10.1016/j.bdq.2017.08.001
Skuratovskaia D, Zatolokin P, Vulf M, Mazunin I, Litvinova L (2019) Interrelation of chemerin and TNF-alpha with mtDNA copy number in adipose tissues and blood cells in obese patients with and without type 2 diabetes. BMC Med Genet 12(Suppl 2):40. https://doi.org/10.1186/s12920-019-0485-8
Guerrini F, Paolicchi M, Ghio F, Ciabatti E, Grassi S, Salehzadeh S, Ercolano G, Metelli MR, del Re M, Iovino L, Petrini I, Carulli G, Cecconi N, Rousseau M, Cervetti G, Galimberti S (2016) The droplet digital PCR: a new valid molecular approach for the assessment of B-RAF V600E mutation in hairy cell leukemia. Front Pharmacol 7(363). https://doi.org/10.3389/fphar.2016.00363
Di Meglio C, Bonello-Palot N, Boulay C et al (2016) Clinical and allelic heterogeneity in a pediatric cohort of 11 patients carrying MFN2 mutation. Brain and Development 38(5):498–506. https://doi.org/10.1016/j.braindev.2015.11.006
Fridman V, Bundy B, Reilly MM, Pareyson D, Bacon C, Burns J, Day J, Feely S, Finkel RS, Grider T, Kirk CA, Herrmann DN, Laurá M, Li J, Lloyd T, Sumner CJ, Muntoni F, Piscosquito G, Ramchandren S, Shy R, Siskind CE, Yum SW, Moroni I, Pagliano E, Zuchner S, Scherer SS, Shy ME, Inherited Neuropathies Consortium (2015) CMT subtypes and disease burden in patients enrolled in the Inherited Neuropathies Consortium natural history study: a cross-sectional analysis. J Neurol Neurosurg Psychiatry 86(8):873–878. https://doi.org/10.1136/jnnp-2014-308826
Manganelli F, Tozza S, Pisciotta C, Bellone E, Iodice R, Nolano M, Geroldi A, Capponi S, Mandich P, Santoro L (2014) Charcot-Marie-Tooth disease: frequency of genetic subtypes in a Southern Italy population. J Peripher Nerv Syst 19(4):292–298. https://doi.org/10.1111/jns.12092
Nam SH, Hong YB, Hyun YS, Nam da E, Kwak G, Hwang SH, Choi BO, Chung KW (2016) Identification of genetic causes of inherited peripheral neuropathies by targeted gene panel sequencing. Mol Cell 39(5):382–388. https://doi.org/10.14348/molcells.2016.2288
Murphy SM, Laura M, Fawcett K, Pandraud A, Liu YT, Davidson GL, Rossor AM, Polke JM, Castleman V, Manji H, Lunn MPT, Bull K, Ramdharry G, Davis M, Blake JC, Houlden H, Reilly MM (2012) Charcot-Marie-Tooth disease: frequency of genetic subtypes and guidelines for genetic testing. J Neurol Neurosurg Psychiatry 83(7):706–710. https://doi.org/10.1136/jnnp-2012-302451
Vaeth S, Christensen R, Duno M et al (2019) Genetic analysis of Charcot-Marie-Tooth disease in Denmark and the implementation of a next generation sequencing platform. European journal of medical genetics 62(1):1–8. https://doi.org/10.1016/j.ejmg.2018.04.003
Wang R, He J, Li JJ, Ni W, Wu ZY, Chen WJ, Wang Y (2015) Clinical and genetic spectra in a series of Chinese patients with Charcot-Marie-Tooth disease. Clin Chim Acta 451:263–270. https://doi.org/10.1016/j.cca.2015.10.007
Casasnovas C, Banchs I, Cassereau J, Gueguen N, Chevrollier A, Martinez-Matos JA, Bonneau D, Volpini V (2010) Phenotypic spectrum of MFN2 mutations in the Spanish population. J Med Genet 47(4):249–256. https://doi.org/10.1136/jmg.2009.072488
Calvo J, Funalot B, Ouvrier RA, Lazaro L, Toutain A, de Mas P, Bouche P, Gilbert-Dussardier B, Arne-Bes MC, Carrière JP, Journel H, Minot-Myhie MC, Guillou C, Ghorab K, Magy L, Sturtz F, Vallat JM, Magdelaine C (2009) Genotype-phenotype correlations in Charcot-Marie-Tooth disease type 2 caused by mitofusin 2 mutations. Arch Neurol 66(12):1511–1516. https://doi.org/10.1001/archneurol.2009.284
Choi BO, Nakhro K, Park HJ et al (2015) A cohort study of MFN2 mutations and phenotypic spectrums in Charcot-Marie-Tooth disease 2A patients. Clin Genet 87(6):594–598. https://doi.org/10.1186/s12883-015-0430-1
Iapadre G, Morana G, Vari MS, Pinto F, Lanteri P, Tessa A, Santorelli FM, Striano P, Verrotti A (2018) A novel homozygous MFN2 mutation associated with severe and atypical CMT2 phenotype. Eur J Paediatr Neurol 22(3):563–567. https://doi.org/10.1016/j.ejpn.2017.12.020
Sivera R, Sevilla T, Vilchez JJ, Martinez-Rubio D, Chumillas MJ, Vazquez JF, Muelas N, Bataller L, Millan JM, Palau F, Espinos C (2013) Charcot-Marie-Tooth disease: genetic and clinical spectrum in a Spanish clinical series. Neurology. 81(18):1617–1625. https://doi.org/10.1212/WNL.0b013e3182a9f56a
Zuchner S, De Jonghe P, Jordanova A et al (2006) Axonal neuropathy with optic atrophy is caused by mutations in mitofusin 2. Ann Neurol 59(2):276–281. https://doi.org/10.1002/ana.20797
Zhao H, Race V, Matthijs G, de Jonghe P, Robberecht W, Lambrechts D, van Damme P (2014) Exome sequencing reveals HINT1 mutations as a cause of distal hereditary motor neuropathy. European journal of human genetics : EJHG 22(6):847–850. https://doi.org/10.1038/ejhg.2013.231
Horga A, Cottenie E, Tomaselli PJ, Rojas-García R, Salvado M, Villarreal-Pérez L, Gamez J, Márquez-Infante C, Houlden H, Reilly MM (2015) Absence of HINT1 mutations in a UK and Spanish cohort of patients with inherited neuropathies. J Neurol 262(8):1984–1986. https://doi.org/10.1007/s00415-015-7851-z
Jerath NU, Shy ME, Grider T, Gutmann L (2015) A case of neuromyotonia and axonal motor neuropathy: a report of a HINT1 mutation in the United States. Muscle Nerve 52(6):1110–1113. https://doi.org/10.1002/mus.24774
P1 L, Brožková DŠ, Krůtová M, Neupauerová J, Haberlová J, Mazanec R, Dvořáčková N, Goldenberg Z, Seeman P (2015) Mutations in HINT1 are one of the most frequent causes of hereditary neuropathy among Czech patients and neuromyotonia is rather an underdiagnosed symptom. Neurogenetics 16(1):43–54. https://doi.org/10.1007/s10048-014-0427-8
Rauchenzauner M, Fruhwirth M, Hecht M, Kofler M, Witsch-Baumgartner M, Fauth C (2016) A novel variant in the HINT1 gene in a girl with autosomal recessive axonal neuropathy with neuromyotonia: thorough neurological examination gives the clue. Neuropediatrics. 47(2):119–122. https://doi.org/10.1055/s-0035-1570493
Veltsista D, Chroni E (2016) A first case report of HINT1-related axonal neuropathy with neuromyotonia in a Greek family. Clin Neurol Neurosurg 148:85–87. https://doi.org/10.1016/j.clineuro.2016.07.012
Dohrn MF, Glockle N, Mulahasanovic L et al (2017) Frequent genes in rare diseases: panel-based next generation sequencing to disclose causal mutations in hereditary neuropathies. J Neurochem 143(5):507–522. https://doi.org/10.1111/jnc.14217
Meng L, Fu J, Lv H, Zhang W, Wang Z, Yuan Y (2018) Novel mutations in HINT1 gene cause autosomal recessive axonal neuropathy with neuromyotonia in two cases of sensorimotor neuropathy and one case of motor neuropathy. Neuromuscul Disord 28(8):646–651. https://doi.org/10.1016/j.nmd.2018.05.003
Wang Z, Lin J, Qiao K, Cai S, Zhang VW, Zhao C, Lu J (2018) Novel mutations in HINT1 gene cause the autosomal recessive axonal neuropathy with neuromyotonia. European journal of medical genetics 62:190–194. https://doi.org/10.1016/j.ejmg.2018.07.009
Peeters K, Chamova T, Tournev I, Jordanova A (2017) Axonal neuropathy with neuromyotonia: there is a HINT. Brain. 140(4):868–877. https://doi.org/10.1093/brain/aww301
Pareyson D, Saveri P, Sagnelli A, Piscosquito G (2015) Mitochondrial dynamics and inherited peripheral nerve diseases. Neurosci Lett 596:66–77. https://doi.org/10.1016/j.neulet.2015.04.001
Arribat Y, Broskey NT, Greggio C, Boutant M, Conde Alonso S, Kulkarni SS, Lagarrigue S, Carnero EA, Besson C, Cantó C, Amati F (2018) Distinct patterns of skeletal muscle mitochondria fusion, fission and mitophagy upon duration of exercise training. Acta Physiol (Oxford) 225:e13179. https://doi.org/10.1111/apha.13179
Acknowledgments
We would like to thank all the participants for their help and willingness to participate in this study. We thank the reviewers for the comments. This work was supported by the grant 81500980 and U1505222 from the National Natural Science Foundation of China, grant 81870929 from the National Natural Science Foundation of China, and grant 2018-CX-25 from Medical Innovation Project for Research Talents of Fujian Province.
Author information
Authors and Affiliations
Contributions
Study concept and design (Jin He and Yi Lin); acquisition of data (Jin He, Shan Lin, Liuqing Xu, Guorong Xu, Ling-Ling Guo); analysis and interpretation of data (Jin He, Shan Lin, Liuqing Xu, Ling-Ling Guo); drafting of the manuscript (Jin He, Shan Lin, Bi-Juan Lin); critical revision of the manuscript for important intellectual content (Jin He and Yi Lin); obtaining of funding (Jin He and Yi Lin); study supervision (Ning Wang and Wanjin Chen).
Corresponding authors
Ethics declarations
Written informed consent was obtained from all the patients included in this study. This study was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
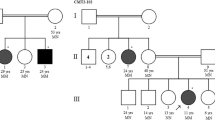
Supplementary Fig.S1
The pedigree charts of CMT2 patients. (PNG 587 kb)
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Lin, S., Xu, LQ., Xu, GR. et al. Whole exome sequencing reveals a broader variant spectrum of Charcot-Marie-Tooth disease type 2. Neurogenetics 21, 79–86 (2020). https://doi.org/10.1007/s10048-019-00591-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-019-00591-4