Abstract
Sea level rise and the subsequent intrusion of saline seawater can result in an increase in soil salinity, and potentially cause coastal salinity-intolerant vegetation (for example, hardwood hammocks or pines) to be replaced by salinity-tolerant vegetation (for example, mangroves or salt marshes). Although the vegetation shifts can be easily monitored by satellite imagery, it is hard to predict a particular area or even a particular tree that is vulnerable to such a shift. To find an appropriate indicator for the potential vegetation shift, we incorporated stable isotope 18O abundance as a tracer in various hydrologic components (for example, vadose zone, water table) in a previously published model describing ecosystem shifts between hammock and mangrove communities in southern Florida. Our simulations showed that (1) there was a linear relationship between salinity and the δ18O value in the water table, whereas this relationship was curvilinear in the vadose zone; (2) hammock trees with higher probability of being replaced by mangroves had higher δ18O values of plant stem water, and this difference could be detected 2 years before the trees reached a tipping point, beyond which future replacement became certain; and (3) individuals that were eventually replaced by mangroves from the hammock tree population with a 50% replacement probability had higher stem water δ18O values 3 years before their replacement became certain compared to those from the same population which were not replaced. Overall, these simulation results suggest that it is promising to track the yearly δ18O values of plant stem water in hammock forests to predict impending salinity stress and mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The coastal vegetation structure of southern Florida has experienced noticeable changes over the past several decades, with encroachment of mangroves into freshwater marshes (Egler 1952; Meeder and others 1996; Ross and others 2000; Smith and others 2010), which is mainly caused by the intrusion of brackish seawater into previously fresh-water areas (Davis and others 2005; Saha and others 2011). This seawater intrusion is facilitated by the flat landscape in southern Florida with shallow elevation and porous soil substrate (Hoffmeister 1974), sea level rise (SLR) (Desantis and others 2007; Krauss and others 2011; Guha and Panday 2012), and by hydrologic management along the coastal area (Fourqurean and Robblee 1999). For example, canal systems in the Everglades diverted freshwater flow from freshwater areas (Sklar and others 2002; Davis and others 2005), which then experienced intrusion by more saline ocean water. Coupled with the seawater intrusion of the water table, there is an increase in the salinity of the pore water of the vadose zone, the zone that is located between the soil surface and the top of the water table (Stephens 1995), and which supplies water for the vegetation (for example, hardwood hammocks, mangroves) in the coastal areas (Figure 1). The salinity of the vadose zone has a great influence on plant community type, as plants vary in their salinity tolerance (Ross and others 1992).
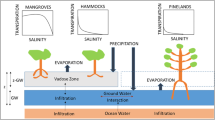
Schematic diagram of hydrological components and factors on water flow in vadose zone. H WT is thickness of the water table, H V is thickness of the vadose zone, H cell is height of a spatial cell, and H tide is height of tidal flooding. The blue arrow denotes tidal flooding. The vertical black arrows denote evaporation, precipitation, transpiration, and infiltration between the vadose zone and water table, and between water table and ocean water, respectively. The horizontal black arrow denotes excess water flow from water table in the landward neighboring cell.
The intrusion of brackish seawater inland is accompanied by a spatial shift inland of the boundary or ecotone between salinity-tolerant (halophytic) vegetation, such as mangroves, and salinity-intolerant freshwater vegetation, such as hardwood hammock or freshwater marsh (Ross and others 2000). Hardwood hammock communities occupy slightly elevated and rarely tidally inundated sites along coastal Florida (Ross and others 1992; Gunderson 1994) and comprise a suite of tropical tree species intolerant of high salinity (Ish-Shalom and others 1992). Mangroves are able to utilize water having a range of salinities, from fresh water to brackish water with salinities above 30 ppt (Chapman 1976; Sternberg and Swart 1987; Ball 1988). Mangroves cannot compete with hammock species in freshwater areas and are excluded from such areas (Silander and Antonovics 1982; Kenkel and others 1991; Greiner La Peyre and others 2001). But the increasing seawater intrusion can change the balance in favor of the mangroves, causing the boundary or ecotone between the two vegetation types to shift inland (Ewe and others 2007; Doyle and others 2010; Saha and others 2011). This boundary shift can result from a salinity-induced mortality of the hammock, which is then replaced by mangroves, or by a decline in the growth rate of hammock vegetation, allowing it to be outcompeted by the invading mangroves. Conversely, vegetation shift from mangroves to hammocks is, in principle, possible if soil water becomes less saline, and can occur in our model. However, such shifts have not been observed empirically (Lugo 1980) and are unlikely to occur in the current global scenario of SLR, so we limit our study to early-stage detection of changes from hammocks to mangroves.
Although the two plant communities, mangroves and hardwood hammocks, occupy overlapping geographical ranges (Odum and others 1982; Odum and McIvor 1990; Sklar and van der Valk 2002), they are rarely mixed, and there are sharp boundaries between them (Snyder and others 1990). Not only is there a sharp ecotone (or boundary) between the two, but the gradient in vadose zone’s salinity is also extremely sharp at the ecotone (Saha and others 2014). This results from the self-reinforcing positive feedback between the vegetation and the vadose zone below them. Both mangroves and hammock species obtain their water from the vadose zone (Sternberg and Swart 1987). In coastal areas, this vadose zone is underlain by highly saline groundwater (Fitterman and others 1999). Plant transpiration, by depleting water in the vadose zone during the dry season, can cause infiltration of the vadose zone by more saline groundwater through capillary action. Hardwood hammock trees tend to decrease their transpiration when vadose zone salinities begin to increase, thus limiting the salinization of the vadose zone (Volkmar and others 1998). However, mangroves can continue to transpire at relatively high salinities, resulting in high soil salinities (Passioura and others 1992). Thus, each vegetation type, through a self-reinforcing positive feedback, tends to promote local salinity conditions that favor itself in competition. However, under the influence of SLR, the positive feedback in mangrove forests can either help the ecosystem become resilient to future changes or strengthen the landward migration of the vegetation boundary (Jiang and others 2015). The positive feedback based on salinity can also be found in other ecosystems, for example, Armas and others (2010) proposed that salty groundwater lift by salt-tolerant species (Pistacia lentiscus) in arid coastal sand dune systems can positively affect its own growth and decrease growth of competing salt-intolerant species.
It is possible that the sharp separation of the two vegetation types caused by the apparent positive feedback relationship can be formally represented as alternative stable states, in the sense of Scheffer and others (1997), Brovkin and others (1998) and Sternberg (2001), along certain regions of a gradual gradient in groundwater salinity (Jiang and others 2012). There is no empirical proof of this. At present, the evidence for the existence of the alternative stable states are from modeling studies indicating that a mixture of plants from the two communities (mangrove and hammock) may represent an unstable state and will move toward complete dominance of one type eventually (Sternberg and others 2007; Teh and others 2008; Jiang and others 2012). This vegetation dynamics pattern is also supported by modeling studies based on other ecosystem types. For example, Morris (2006) demonstrated that two marsh species with different optimal elevations for growth can move toward stable states dominated by one species or another.
Despite short-term stability of the coastal ecosystem in the Everglades, the vegetation will shift over longer time scales as highly saline groundwater intrudes farther inland due to SLR. However, there is no guarantee that this shift will have a linear and predictable response to SLR. Other factors, including precipitation and freshwater flows, also affect the vegetation dynamics, and along with the positive feedback mechanisms described above, could cause the shift process sometimes to be delayed or sometimes accelerated. More importantly, the vegetation dynamics are also vulnerable to more rapid changes from disturbances of sufficiently large size (Ross and others 2009). For example, if a large enough pulse of salinity from a storm surge is pushed inland into the region of freshwater vegetation, and high salinity remains in the soil for a long period, it may overwhelm the favorable conditions for freshwater vegetation by killing or slowing the growth of the freshwater vegetation. If the storm surge also carries mangrove seedlings into the affected freshwater region, areas of freshwater vegetation could be set on the path to irreversible replacement by halophytic vegetation and permanent salinization of the vadose zone.
There is some empirical evidence for rapid vegetation shifts following large disturbances. For example, Baldwin and Mendelssohn (1998) used salt water inundation coupled with clipping of aboveground vegetation on two adjoining halophytic and freshwater plant communities to simulate the effects of a hurricane with a storm surge. They showed that the vegetation might shift from one type to the other, depending on the levels of flooding and salinity at the time of disturbance. Hurricanes Katrina and Rita (2005), which affected the coastal areas of Louisiana, created large storm surges. Subsequent changes in the vegetation to a more saline classification have been identified in both freshwater and brackish communities due to the salinity overwash (Steyer and others 2010), suggesting a possible regime shift. Because of the importance of freshwater habitat in affected areas of the Everglades, methods of finding indicators of rapid changes in the ecosystem will be important (Dakos and others 2012). For example, Doering and others (2002) used plant growth as an indicator of salinity to determine the amount of needed freshwater to reduce the salinity. They used shoot density of a salt-tolerant species, Vallisneria americana, to estimate a minimum freshwater flow, and leaf density of Halodule wrightii to estimate a maximum flow, because the plant growth can indicate salinity change. Presenting a new predictive methodology is the objective of this paper.
Ecosystems may show little indication of a tipping point until change from one ecosystem type to another starts to occur (Scheffer and others 2009), so it is hard to find an appropriate indicator that is practical for use in monitoring an ecosystem transition (Dakos and others 2012). In coastal ecosystems, water salinity of the vadose zone may be a potential indicator for the tipping point between hammock and mangrove trees. However, salinity distribution in the vadose zone is uneven. For example, a study in coastal southwestern Florida found that salinity varies from location to location within as little as one meter (Ewe and Sternberg 2005). In addition to the uneven salinity distribution, the extensive and complex root system of trees in tropical forests (Sternberg and others 1998; Kathiresan and Bingham 2001; Sternberg and others 2002) makes it harder to precisely determine which part of the vadose zone the roots absorb water from. A second possible approach, monitoring the salinity of xylem water, is also problematic, because plants may exclude salt during water uptake (Scholander and others 1962; Scholander 1968). A further potential indicator of salinity is predawn water potential, which can reflect the salinity of the water available to a plant (osmotic potential). But water potential is also affected by soil moisture (matric potential). Therefore, predawn water potential, depending on both the salinity and moisture of soil, may not precisely indicate the change in the salinity of the water available for plant uptake.
A more practical and precise indicator of a potential shift at the level of individual trees may be the oxygen isotope composition (δ18O value) of plant stem water. Current methods allow for easy extraction and analysis of plant stem water for hydrogen and oxygen isotopic composition (Vendramini and Sternberg 2007). The δ18O value of plant stem water is an appropriate indicator candidate for two reasons: (1) the δ18O value of water, at least in southern Florida, is indicative of its salinity, with freshwater showing less oxygen-18 enrichment than saline seawater (Sternberg and Swart 1987; Sternberg and others 1991). Thus, there are significant differences in δ18O between various water sources, such as precipitation and sea water. (2) Although plants discriminate against salt, they do not discriminate against 18O during water uptake (Dawson and others 2002); for example, Lin and Sternberg (1994) found that there were no significant differences in δ18O values between stem water of red mangrove (Rhizophora mangle) and its corresponding vadose zone. Several studies have used δ18O values of stem water to quantitatively determine the utilization of plant water source, for example, Sternberg and Swart (1987), Dawson and Ehleringer (1991), Ewe and others (2007). The other isotopic indicator of plant water uptake, the hydrogen isotope composition value of stem water (δ2H), cannot be used because some salt-tolerant plants discriminate against 2H during water uptake (Lin and Sternberg 1993; Ellsworth and Williams 2007; Wei and others 2012).
The purpose of this study was to determine if the δ18O value of plant stem water can be an appropriate predictor of future replacement of hammock species by salt-tolerant mangrove species in coastal ecosystem. We determined this by including the δ18O value of water as a tracer in a previously published spatially explicit model describing the dynamics of an initially mixed hardwood hammock and mangrove stand in southern Florida (Sternberg and others 2007). We incorporated the δ18O values of various water compartments in the model, including vadose zone, water table and plant stem water. We then followed the water salinities and δ18O values of these compartments in areas of the landscape where hammocks were replaced by mangroves in the model, and determined if the change of δ18O value of plant stem water preceded the replacement of a hammock species by a mangroves species. Being able to predict this replacement using the easily measured δ18O value of plant stem water has practical implications in the monitoring and conservation of coastal ecosystems, particularly in relation to a rising sea level and increasing storm surges.
Methods
Model: General Aspects
The competition of mangroves and hardwood hammocks was modeled on a 100×100 grid of spatial cells, with each cell being roughly the size of a mature tree. The assumptions of plant physiology and factors on water movement in the vadose zone in our model were the same as in Sternberg and others (2007) and Teh and others (2008). Mangroves tolerate a large range of salinities from freshwater to a high salinity environment (Chapman 1976; Sternberg and Swart 1987; Ball 1988). Hammocks, which are intolerant of salinity, however, are limited to freshwater areas, where they can outcompete and exclude mangroves (Silander and Antonovics 1982; Kenkel and others 1991; Greiner La Peyre and others 2001). Both vegetation types utilize water from the vadose zone. Water movement in the vadose zone is mainly determined by precipitation, evaporation, plant transpiration, and tidal flooding (Figure 1). Precipitation introduces freshwater with zero salinity and low δ18O values to the vadose zone, which drives the infiltration downward and decreases the salinity and δ18O values of the vadose zone. Tidal flooding carries saline seawater with relatively high δ18O values to the vadose zone. Both evaporation and plant transpiration can cause a water deficit in the vadose zone and drive infiltration upward, which may move water with high salinity and δ18O values from the water table up into the vadose zone.
The effects of the vegetation on the salinity of the vadose zone can be spread horizontally by lateral water uptake by a tree, because a tree’s roots extend beyond the location of its central stem into the locale of neighboring trees. It has been documented by stable isotope studies that small tropical tree species can access and presumably affect areas as far as 10 meters from the central stem (Sternberg and others 2002). Gill and Tomlinson (1977) recorded that the lengths of mangrove roots can extend over two meters, which is longer than the spatial cell length in our model. In our simulation, which is represented by 2D horizontal space with a grid of spatial cells, the transpiration by a cell containing a mangrove tree will lead to infiltration of saline water from the water table into the vadose zone. This upward water infiltration occurs not only in the immediate area of the mangrove tree, but also in neighboring areas which may not be occupied by mangroves but still have mangrove roots that extend from the focal mangrove-occupied cell. Therefore, if the above focal-neighboring interaction is sufficient to increase salinity in the neighboring area, there could potentially be a further expansion of the mangrove area. Conversely, the lateral water uptake of vegetation can enlarge hammock areas by suppressing salinity in neighboring areas. Therefore, in the model, the lateral water uptake affects soil salinity beyond the area occupied by the individual tree, and there is a tendency to eliminate lone individuals of one vegetation type surrounded by vegetation of the opposite type, which appears to be the case in nature as well. The overall effect is to sharpen the ecotone between the two vegetation types. Soil pore horizontal diffusion of salinity was ignored in our simulation, because this diffusion in soil is very small (<0.0003 m2/day) (Hollins and others 2000) compared to horizontal salinity transfer by roots.
Model: Mathematical Details
As in Sternberg and others (2007), the hydrological components of our model include vadose zone’s pore water, water table, and ocean water, and our study also added plant stem water to these compartments. These hydrological components and factors responsible for water flow in the vadose zone are shown in Figure 1. We used the same initial water salinity for the first three of these compartments as in Sternberg and others (2007) (See Appendix). In addition, we tagged the water from the above compartments with realistic isotopic signatures and followed the flow of 18O as well as salinity.
We used the usual expression to describe isotopic abundance:
where R represents the 18O/16O ratio of a sample and the standard, respectively. The standard is standard mean ocean water (SMOW). We labeled ocean water (O O) and tidal influx water (O tide) with a δ18O value of +4‰ (Sternberg and Swart 1987). Sea water in the bays and inlets of Florida is isotopically enriched because these systems are semi-closed and undergo extensive evaporation (Lloyd 1964). Sea water isotopic composition did not change through time in our model. The vadose zone pore water had an initial δ18O value of −3‰, but changed as a function of precipitation, infiltration and tidal input. The δ18O value of precipitation (O P ) in southern Florida was calculated daily by the monthly average δ18O of precipitation (\( \bar{O}_{\text{P}} \)) and the monthly standard deviation (\( \sigma_{{O_{\text{P}} }} \)) multiplied by a normally distributed random number ranging from −1 to +1 (RN) (Price and others 2008) (equation 2). The information about precipitation and tidal flooding is shown in Figure 2, and their calculations are shown in the Appendix. The equation for O P is
For every month, daily averages and standard deviations of precipitation (mm) (NOAA, National Weather Services Forecast Office, Florida) and its δ18O (0/00) (Price and others 2008), and height of tidal flooding in Florida (NOAA, Tide & Current Historic data base, Key West Station) were derived from 162, 3, and 5 years of empirical data, respectively.
The initial δ18O value of water table decreased uniformly from +4‰ on the seaward side to −3‰ in the landward side. The δ18O values of the water table can change depending on percolation from vadose zone, the water table of neighboring cells, or infiltration from the underlying ocean water.
We used the same basic flux equations dictating vertical movement of water and changes of salinity as those in Sternberg and others (2007) (see Appendix). We also added equations that followed the flux of 18O between the various hydrological compartments in soil. The fluxes of 18O in and out of the vadose zone carry the signature of the δ18O values from the water table, ocean water, and precipitation, but the δ18O value of the vadose zone is not concentrated by transpiration, as, unlike salinity, it is not excluded by the roots. It is given by
As in Sternberg and others (2007), values of infiltration (IF) (\( {\text{IF}} = {\text{Evaporation}} + {\text{Plant transpiration}} - {\text{Precipitation}} - {\text{Tidal flooding}} \)) greater than 0 imply that water is infiltrating from the water table to the vadose zone (Figure 1). In the above equation, p, H V and \( \frac{{{\text{d}}O_{\text{V}} }}{{{\text{d}}t}} \) define the porosity of the vadose zone, the thickness of the vadose zone, and the change in δ18O value of the vadose zone per unit time, respectively. The subscripted O symbols represent the δ18O values of the water table (O WT), the vadose zone (O V), the precipitation (O P), and the tidal water (O tide), respectively. The water table in the model is assumed to be the upper zone of groundwater that can vary in salinity and δ18O concentrations and that can flow from high to lower elevations when there is an upstream head. The amount of precipitation and tidal height above the surface of the vadose zone are represented by P and T RH, respectively. The last term of the above equation, \({T_{\text{RH}} \times (O_{\text{tide}} - O_{\text{V}} )}\), is only applied when T RH is greater than 0, that is, the tidal height is above the surface of the vadose zone. In the case of water infiltrating from the vadose zone to the water table (IF < 0), the following equation is applied:
To determine the δ18O value of plant stem water, we first determined the total transpiration (water uptake) by each plant (T plant). Plant transpiration in a particular cell consists of contributions from the central cell and four of its adjacent cells, and is given by the following equation:
where the superscripted TT’s represent the contribution of transpiration in the central and neighboring cells by either a hammock or mangrove species, which will depend on salinity and is predicted by previously published equations (Sternberg and others 2007). The δ18O value of stem water (Oplant) is then given by the mean values of δ18O in the water transpiration streams from the vadose zones in center cell and four adjacent cell weighted by the size of the transpiration in each stream:
The water table underlying the vadose zone is also subject to fluxes that might alter its salinity (see Appendix) and δ18O. When water percolates from the vadose zone to the water table and the water table height increases beyond that of the initial conditions, the excess water (α) flows horizontally to the next water table cell seaward to it. By mass balance principles, the changing rate of the δ18O value in the water table of a particular cell should be
On the other hand, when IF is greater than 0, that is, water flow is from the water table to the vadose zone, water from the underlying ocean water infiltrate upward to make up for the water table deficit. By mass balance, the δ18O dynamics of the water table in a particular cell is then predicted by
Model: Simulation
The purpose of simulations was to investigate whether given hammock occupied cells that switched to mangrove due to salinity stress could be predicted beforehand from δ18O levels in the plant stem water. If there is a particular vadose zone’s salinity level at which change from hammock to mangrove becomes inevitable, this is referred to as the tipping point. Simulations were based on a previously published model (Sternberg and others 2007; Teh and others 2008; Jiang and others 2012), and performed on a 2D grid of 100 × 100 cell landscape, which was built by an algorithm (Sternberg and others 2007) that produces natural-looking topographies based on a transect by Ross and others (1992), and the landscape elevation increased by 10 mm per cell length along the dimension from the 1st to 100th row. The initial height of the water table was assumed to increase uniformly from 50 mm to 400 mm along the same dimension. The thickness of the vadose zone is the difference between cell elevation and the height of the water table (Figure 1). The above differential equations were discretized and applied incrementally day by day to the initial conditions.
Simulation Process
Initially, each spatial cell was assigned randomly as either mangrove or hammock, and the whole landscape had 50% frequency of hammocks and mangroves. On a daily time step, the simulation computed the flux of water into and out of each cell, and the salinity and δ18O value of each hydrological compartment. After the 1st and 2nd years, the running average salinity of the vadose zone in each cell was used as an indicator of whether the type of tree in that cell would ultimately remain or be replaced by the other type of vegetation. If the running average salinity was at least 5 ppt in a cell, it was seen that a mangrove tree, if present, was either maintained in the cell or a mangrove replaced the hammock tree. On the other hand, if the running average salinity of a cell was less than 5ppt, it was seen that a hammock tree, if present, was maintained, or a hammock tree replaced a mangrove tree. (By replace we mean ‘will immediately replace,’ but the actual replacement of one tree type by another tree type may take decades to occur.) The simulation was continued, and the running average was calculated again at the end of each succeeding year. The vegetation distribution was updated according to the new running average of vadose zone’s salinity. At the end of the 10th year, the final distribution of vegetation was recorded. The simulation stopped in the 10th year, because there were few changes in the plant distribution after 10 years. The modeling thus artificially speeds up the rate at which the cells on the landscape sort into final vegetation types, but this still properly connects the tipping of each particular tree with the previous value of stem water δ18O.
Simulation Data Sampling
Two types of simulations were performed: (1) The purpose of the first set, in which 100 simulations were performed, was to identify specific cells on the grid that were initially populated by hammocks and that had specific probabilities (0, 25, 50, 75, and 100%) of being replaced through time by a mangrove species, when the 100 independent simulations were performed with different random number simulators (recall there is stochasticity in precipitation and tidal flux). The replacement probability was found to be related to distance of the cell from the ocean; that is, if a cell with a hammock tree was closer to ocean, it tended to be replaced by a mangrove tree with higher probability. (2) The second set was a single data-sampling simulation in which we recorded the salinities of the vadose zone water available to the plant and the δ18O of hammock species stem water. In this simulation, we monitored the vadose zone’s salinity and δ18O of stem water for hammock cells showing different probabilities of being eventually replaced by mangroves. In addition, we monitored the vadose zone’s salinity and δ18O of stem water for hammock cells with 0% probability of replacement and a subset of the set with 50% probability of replacement that were always stable as hammock during this single 10-year simulation. These two types of simulations are described below.
-
(1)
Identifying hammock cells with different probabilities of being replaced by mangrove species
We performed 100 simulations (10 years each) starting with the same vegetation distribution landscape, but with daily stochastically determined precipitation and tidal flux. For each simulation we compared the plant species in each cell between the 1st and 10th years to identify which cells would ultimately shift from hammock to mangrove. After the 100 simulations, we identified particular cells that shifted from hammocks to mangroves with probabilities of 0, 25, 50, 75, and 100% at a 5% significance level. These cells were designated as H0, H25, H50, H75, and H100, respectively. The confidence interval of these probabilities was calculated by the following equation:
in which p is the replacement probability, \( z_{\alpha /2} \) = 1.96, and n = 100 (simulation times).
-
(2)
Data-sampling simulation
The data-sampling simulation was performed with the same initial vegetation distribution as above, where we selected subsets from every cell set of hammock cells for which specific probabilities of switching to mangroves (H0, H25, H50, H75, and H100) emerged, and we monitored the salinities and the δ18O values of the hydrological compartments of these selected subsets. Hammocks in the selected subsets, in addition to passing the tipping point threshold for shifting to mangroves, had to (1) have passed this threshold exactly in the same year during the simulation, because we want to compare yearly differences in the salinities and the δ18O values between the different cell types before and at the tipping point. The hammocks also had to (2) have been stable as hammocks before the shift and remained stable as mangroves after the shift. These newly identified cells, which we call ‘shifting subclasses’ of cells, were designated as H25→M, H50→M, H75→M, and H100→M, accordingly. In the case of H0, we selected a subset of cells that remained stable as a hammock during the 10 year simulation (designated as H0→H); that is, there is no flipping back and forth between hammock and mangrove trees before ending as hammock in the 10th year. In addition, from the set of hammock cells that had a 50% replacement probability (H50), we selected a subset that was not replaced by mangroves for this particular simulation (designated as H50→H). These cells must be stable as a hammock cell during the 10 years simulation. For statistical purposes, the data-sampling simulation above was carried out until at least five cells could be found in each cell subset (H0→H, H25→M, H50→M, H50→H, H75→M, and H100→M). For the simulation that produces sufficient cells of each type, we recorded monthly and yearly average values of salinity and δ18O of the vadose zone, water table, and plant stem water in the above cell subsets, and the vegetation distribution of landscape at the end of every year. Note that we are using ‘tipping point’ to represent the threshold running average of vadose zone’s salinity at which a single tree (or cell) will inevitably shift from hammock to mangrove.
The numbers of cells satisfying all the selection criteria, in which the replacement took place in the same year and remained stable before and after the replacement, are shown in Figure 3B. Although cells that were replaced in the 6th year had the greatest total cell number, they just had two H75→M cells. Thus, we chose cells that passed the threshold in the 7th year, because those cells had an appropriate cell number for each cell type. We examined the salinities and δ18O values of their vadose zone, water table, and plant stem water in detail for this selected group of cells.
A Horizontal view of landscape grid with 100 × 100 cells showing final distribution of mangroves (black) and hammocks (white) along a topographic gradient from 85 mm above sea level (top of A) to the highest inland elevation of about 1200 mm (bottom of A). All cells were occupied by either one mangrove or one hammock tree. B Number of cells in each shifting subclass (not shown here are H100→M, H0→H etc), with only the 7th year showing enough cells of the three types of shifting subclasses.
Data Analysis
The data-sampling simulation was analyzed as follows. First, we compared the monthly average salinities and δ18O values of the vadose zone and water table between the dry season (November through April) and wet season (May through October). For this seasonal comparison, the Mann-Whitney-Wilcoxon test was used because of the non-normal distribution of salinity and δ18O values. Second, we compared the average δ18O values of plant stem water from the H0→H, H25→M, H50→M, H75→M, and H100→M cell subset in the 5th year. The 5th year was the earliest year we could identify the significant δ18O value difference among the above five cell subsets. Third, we monitored the yearly average salinity and δ18O values of water available to the trees from the following cell subsets; H0→H, H50→H, H50→M, and H100→M, and then the yearly data were analyzed to check when the significant differences of salinity and δ18O value appear by repeated measures ANOVA.
Results
After the 10-year simulation, a clear boundary emerged between mangrove and hammock species in our model landscape (Figure 3A). We also observed that most of H25→M cells tended to pass the threshold or tipping point in the 5th and 6th years (Figure 3B). They shifted faster than most of H50→M cells, which shifted in the 7th years, and H75→M cells, which shifted in the 7th and 8th years (Figure 3B). Compared with slow shifting H50→M and H75→M cells, fast shifting H25→M cells were more landward with less salinity variation of the vadose zone. It is counterintuitive for the landward cells to shift to mangroves at a faster rate than seaward cells, but this is due to the requirement for sampling noted above that the selected cells had to remain stable in their new state after the tipping. The tidal influence caused the more seaward cells to flip back and forth more frequently, delaying the time at which we declared the cells to have shifted. The lower salinity variation in these plants made plant distribution of the landward area more stable (that is, less flipping back and forth between hammocks and mangroves) allowing for a faster stabilization. Our stabilization criterion is reasonable because the tree shift may not occur in such a short time in nature, and mangroves usually achieve maturity in 20–30 years (Lugo and others 1976).
Dynamics of Salinities and δ18O Values of Vadose Zone and Water Table
Salinity
In our simulations, a gradient emerged in the salinity of the vadose zone from around 30 ppt seaward to about 2 ppt landward, whereas the water table developed a gradient of salinity from about 20 ppt seaward to about 8 ppt landward. The salinity gradient corresponded with the distinct distribution of hammocks and mangroves (Figure 3A). The comparison between hammocks and mangroves showed that under hammock forest, the salinity in the water table was higher than that in the vadose zone, but under the mangrove forest, the salinity in the vadose zone was higher than that of the water table (Figure 4A). We also found that there was greater variance in the salinity of the mangrove soil than that of the hardwood hammock soil in figure 4A.
During the dry season, the average salinities of the vadose zone (27.8 ppt ± 5.1 below mangroves, 2.2 ppt ± 0.1 below hammocks) were close to those of the wet season (26.5 ppt ± 4.5 below mangroves, 2.2 ppt ± 0.01 below hammocks). However, the Mann-Whitney-Wilcoxon test showed that the vadose zone in the dry season had significantly higher salinity than that in the wet season (χ 2 = 3136.49, P < 0.0001 for mangroves, χ 2 = 516.97, P < 0.0001 for hammocks). The water table had significantly higher salinity in the dry season (16.9 ppt ± 2.7 under mangroves, 8.5 ppt ± 0.2 under hammocks) compared to the wet season (13.2 ppt ± 2.6 under mangroves, 8.0 ppt ± 0.1 under hammocks) (χ 2 = 20639.62, P < 0.0001 for mangroves, χ 2 = 49199.25, P < 0.0001 for hammocks). Comparing the water table with the vadose zone, we found that the water table had a greater seasonal variation of salinity (Figure 4A).
δ18O Values
In the 10th year, the δ18O values of vadose zone pore water developed a gradient from about +3.6‰ to about −2‰ with the distance from the ocean. The gradient ranged from about +1‰ to about −1‰ for the water table. The average δ18O values of the vadose zone in the dry season (+1 ‰ ± 1.5 below mangroves, −1.9 ‰ ± 0.02 below hammocks) were significantly different from those of the wet season (+1.7 ‰ ± 1.7 below mangroves, −1.8 ‰ ± 0.01 below hammocks) (χ 2 = 4569.56, P < 0.0001 for mangroves, χ 2 = 49199.25, P < 0.0001 for hammocks) (Figure 4B). In the water table, the average δ18O value was higher during the dry season (+0.8 ‰ ± 0.4 under mangroves, −0.5 ‰ ± 0.03 under hammocks) relative to the wet season (+0.1 ‰ ± 0.4 under mangroves, −0.6 ‰ ± 0.01 under hammocks) and this seasonal difference was significant in both cases (χ 2 = 31642.4, P < 0.0001 for mangroves, χ 2 = 11375.04, P < 0.0001 for hammocks). During most of the wet season, whereas the monthly δ18O values of the water table were decreasing, the values in the vadose zone were relatively stable (Figure 4B).
The Relationship Between Water Salinities and δ18O Values
The range of vadose zone salinities and δ18O values was from 1.8 to 30 ppt to −2 to +3.5‰, respectively (Figure 5A), whereas the ranges of salinities and δ18O values in the water table were from 7.5 to 18.4 ppt and from −0.8 to 1‰, respectively (Figure 5B). The simulation results showed that salinities of the vadose zone and water table are highly correlated with δ18O values of the water, with the trend that water with higher salinity has a higher δ18O value. However, the pattern of this positive correlation differed between the water table and the vadose zone. In the water table, there was a clear linear relationship between δ18O value and salinity (Figure 5B). The relationship between salinity and δ18O values tends to be curvilinear in the vadose zone (Figure 5A). This relationship is composed of two parts: the first part (below δ18O = 0) is linear, whereas in the second part (above δ18O = 0), the slope asymptotes toward a maximum salinity of about 30 ppt. In the H0→H, H50→H, H50→M, and H100→M cells, from the linear relationship, it is very clear that hammock trees with higher replacement probabilities absorbed water with higher salinities and therefore higher δ18O values (Figure 5C).
The relationship between δ18O values versus salinities of water in the vadose zone (A) and water table (B) for all the cells in the landscape in the 10th year. In A, the straight line represents ideal linear relationship between δ18O and salinity expected if salinity was only determined by the mixing of freshwater and ocean water. The vertical arrow means the increase of salinity without change of δ18O value in vadose zone caused by salt exclusion by mangrove roots. C This is the relationship between δ18O values of the plant stem water and salinities of the water available for uptake in the 10th year from cell types with the different shifting probabilities (H0→H, H100→M, H50→H, and H50→M). The H0→H cells showed less variation in the bottomleft of C.
Prediction of Hammock Replacement by Mangroves
There were significant differences in the average δ18O values of stem water from hammock trees with different replacement probabilities (H0→H, H25→M, H50→M, and H100→M) 2 years before the tipping point for the replacement occurred (Figure 6A). The H0→H cells, that is, no probability of shifting to mangroves, had the lowest δ18O values, whereas the H100→M cells, that is, a certainty of shifting, had the highest values. There were significant differences in the average δ18O values of stem water in these five types of cells in the 5th year (F = 1355, P < 0.0001).
A The average δ18O values and their standard deviations of plant stem water for hammocks in the H0→H, H25→M, H50→M, H75→M, and H100→M cells. Isotope ratios of stem water were recorded 2 years before replacement occurred (the 7th year). Shifting subclasses having the same letter are not significantly different at the 5% level of confidence from Tukey’s Post Hoc Test. B The yearly average and standard deviation of vadose zone salinities in the identified cell and its four neighboring cells during the data-sampling simulation. C The yearly average and standard deviation of δ18O values of plant stem water in the identified cells during the data-sampling simulation. Error bars represent the standard deviation. The dash line indicates the year when hammocks were replaced by mangroves.
We also tracked yearly average salinities of the vadose zone used by individual trees (Figure 6B) and δ18O values of their stem water (Figure 6C) in H0→H, H50→H, H50→M, and H100→M cells. Stem water of trees in H0→H and H100→M cells generally had the lowest and highest values, respectively. Trees in H50→H and H50→M cells are from the same hammock population having a 50% replacement probability. The clear salinity difference between the two types of cells in H50→M and H50→H appeared in the 4th year (F = 4.9325, P = 0.0290), 3 years before the replacement happened (7th year) (Figure 6B). Moreover, the δ18O values of plant stem water had similar trends as the salinity of the water available to the plants. Trees in H0→H and H100→M cells generally had uptake of water with the lowest and highest δ18O values, respectively. There was a significant difference of around 0.1‰ in the δ18O value of stem water between H50→M and H50→H cells in the 4th year (F = 6.7213, P = 0.0535). In the 7th year, when the tipping point occurred, the difference increased to about 0.2‰.
Discussion
The comparisons of salinities and δ18O dynamics between the two vegetation types and the two seasons demonstrate the effects of plant transpiration, precipitation, and tidal flooding on the coastal ecosystem. Differences in plant transpiration between the halophytic and freshwater vegetation influence the salinities of the vadose zone and water table, and this influence is indicated by the contrast of vertical salinity heterogeneity in the soil between the mangrove forest (where vadose zone > water table) and the hardwood hammock forest (where vadose zone < water table) (Figure 4A). Our results are consistent with field observations of southern Florida where the upper soil (vadose zone) of mangrove forest has higher salinity than that in the water table (Ewe and Sternberg 2005). This salt accumulation in the vadose zone under mangrove forests is caused by salt exclusion in mangrove roots during water uptake (Passioura and others 1992; Tomlinson 1994). The high salinity resulting from upward flow of saline groundwater by plant transpiration is also recorded in a salt marsh ecosystem in Georgia (Wilson and others 2011). On the other hand, in hammocks of southern Florida, field study of Ish-Shalom and others (1992) found that salinities of soil samples (from 0 to 10 cm and 20 to 30 cm depths) are lower than those of groundwater in both the wet and dry seasons. However, there is a limitation in our model assumption on plant transpiration that the water uptake rate is only dependent on salinity for one specific vegetation type. This rate may actually be variable through differences in energy balance, photosynthetic metabolism, and water use efficiency (Moffett and others 2012), which may be caused by changes of biotic and abiotic factors (for example, temperature, nutrient, light), affecting soil salinity.
The effect of precipitation can be detected by the significant seasonal differences of salinities in the water table under each of the vegetation types (Figure 4A). The seasonal difference is corroborated by the field study of Ewe and Sternberg (2005) in southern Florida, indicating that salinity at 50 cm belowground was lower during the end of the wet season than that during the dry season. This seasonal difference in salinities was mainly caused by the dilution effect of wet season precipitation. Our results showed that the maximum salinity of the water table occurred in April, near the end of the dry season, and the minimum salinity was in October, near the end of the wet season. The timings of the two maximum and minimum salinities match salinity measurements of a coastal hardwood hammock water table in the Everglades (Saha and others 2014). With respect to the δ18O values of the vadose zone, under the hammocks, the similarities in δ18O dynamics between the vadose zone water (Figure 4B) and that of rain water (Figure 2A) indicate that the vadose zone is recharged by rain water and not by groundwater during the wet season. A field study in southern Florida also found that δ18O values of the vadose zone water in hammocks follow temporal and seasonal patterns of rainwater δ18O (Saha and others 2009). Rainfall can change soil salinity by water input to groundwater and fresh surface water input. Therefore, in Moreton Bay of Australia, Eslami-Andargoli and others (2009) found that precipitation is the driving factor on the landward mangrove expansion by comparing the rainfall pattern and mangrove expansion from 1972 to 2004, and Snedaker (1995) also argued that changes in regional rainfall may have great influence on mangrove distribution in the short term.
We also noticed that there was greater variance in salinity and δ18O values of the mangrove soil in the model simulations (Figure 4), which might be caused by a sudden increases in the salinity and δ18O values in the area near the ocean which is under the influence of the tidal flooding. This tidal flooding effect on soil salinity was also noticed by Jiang and others (2014), who found that salinity variance of soil pore water at the mangrove site was higher than that at the freshwater marsh site. The flooding effect on δ18O is supported by Greaver and Sternberg (2006), who found that variance of stem water δ18O was negatively related to distance from the ocean. In the simulation study of a salt marsh creek ecosystem, Xin and others (2009) showed that there was a significant water table fluctuation in the near-creek zone. From the increased salinity variance, it can be inferred that there is a significant tidal influence on the coastal ecosystem. In some specific ecosystems, tidal flooding is the only detected factor affecting vegetation dynamics. For example, at Elkhorn Slough in California, Wasson and others (2013) found that only increased tidal flooding had a strong relationship with upward migration of salt marsh–upland boundaries compared with other factors, including mean sea level and precipitation. At Baja California, Mexico, López-Medellín and others (2011) found that inland mangrove colonization requires extraordinary tidal flooding, which is a main factor in establishment of the viviparous propagules of mangroves (Rabinowitz 1978). The flooding frequency and strength can be increased by SLR (Ezer and Atkinson 2014; Kriebel and others 2015); therefore, the influence of tidal flooding may be enhanced in the future. The enhanced influence will tend to raise vadose zone’s salinity and favor the growth of halophytic vegetation, pushing the vegetation boundary farther landward. Thus, there is a need to forecast this vegetation shift in coastal ecosystems, and take management steps where possible.
The positive linear relationship between salinity and δ18O in the water table (Figure 5B) observed here is corroborated in many field studies of southern Florida; for example, Sternberg and others (1991), Lin and Sternberg (1994), Ewe and others (2007), which may result from mixing of freshwater with low δ18O values and saline seawater with high δ18O values. In a tidal creek system of Australia, Wei and others (2012) also found a similar trend, in which the water table on the creek side had higher salinities and δ18O values than the inland area. If the relationship between δ18O values and salinity of the vadose zone pore water is affected only by the mixing of freshwater and seawater, one would also expect a linear relationship in the vadose zone (straight line in Figure 5A). However, the relationship between salinity and δ18O values is curvilinear (Figure 5A) for the vadose zone, and this simulation result is consistent with the field studies by Swart and others (1989) and Sternberg and others (1991). Sternberg and others (1991) observed a similar curvilinear relationship between the salinities and δ18O values for water samples at the surface of the water table in southern Florida. The linear part of the δ18O versus salinity curve at the low salinity section (salinity < 20 ppt) might be caused purely by the mixing of freshwater and saline seawater, but the asymptotic part in the high salinity region (salinity > 20 ppt) indicates that mangrove water uptake by excluding salts might increase the salinity of the vadose zone, without changing the δ18O value of vadose zone pore water. Despite the curvilinearity, the positive correlation between the salinity and δ18O values of the vadose zone suggests that we may be able to use the δ18O value of stem water to indicate the salinity of the vadose zone water available for plant uptake.
Our results indicate that the differences of vadose zone’s salinity among the four cell types (H0→H, H50→H, H50→M, and H100→M) diverge gradually during the simulation (Figure 6B). Therefore, although the vegetation tipping point can be abrupt, it is really caused by the culmination of incremental salinity shifts. However, because of the previously stated reasons, salinity measurements of the vadose zone or of the plant xylem water in the field will not necessarily determine the probability that a hammock species will be replaced by a mangrove species. The oxygen isotope ratio of the stem water, however, might be a good candidate as an indicator of the impending vegetation shift. The δ18O value ranking of the five cell types from Tukey’s post hoc test (Figure 6A) also suggested that we can use δ18O values of stem water to determine the probability of a hammock tree being replaced by a mangrove tree, with the exception for distinguishing the 25–50% probabilities, which were not significantly different.
Currently, measurement of the isotopic composition of stem water has a precision of approximately ±0.1‰ (Vendramini and Sternberg 2007), which is the difference observed for the δ18O values of stem water from H50→M and H50→H trees 3 years before hammocks in the group H50→M were replaced by mangroves (Figure 6C). Thus, we may be able to analyze an appropriate number of plant stem water samples from a given hammock tree to detect the significant difference in δ18O values by repeated measures ANOVA. For example, an 80% certainty of detecting the significant 0.1‰ difference in δ18O values of plant stem water at the 5% level of significance requires 18 replicates of stem water samples (Sokal and Rohlf 1995).
This forecasting method based on δ18O values may be applied to other ecosystems in addition to the coastal ecosystem in southern Florida, because the similar salinity and δ18O relation and the plant water use pattern can also be found in other areas. For example, Wei and others (2012) recorded a positive salinity and δ18O relation and slight differences of δ18O between the vadose zone and stem water of A. marina (mangrove species) in a sub-tropical estuary in Australia. However, more research is required to make our forecasting practical. First, to capture the topographic, geologic, and vegetative complexity of a natural site, it is important to know how to apply a general model study to specific field conditions (Moffett and others 2012). For example, our current model used a simplified structure of root and salinity distribution in the vadose zone. In a specific field, the root structure may vary by different plant species, particularly for hardwood hammock forest which may comprise different neotropical evergreen broadleaf trees (Saha and others 2009) with different root structures, and the salinity distribution of a site may be more complex than our current assumption. The root structure determines the location of the plant water resource, and this location will be very important to our prediction in the site with a complex salinity distribution in the vadose zone. Second, there is a need for long-term field measurements both on salinities and δ18O of the water components (vadose zone, water table, and plant stem water), and on vegetation dynamics during the same time period, as it is an appropriate way to test, elaborate, and calibrate our model. Third, we need to know how much time is required to decrease vadose zone’s salinity to values which prevent replacement of hammock by mangroves under current water management practices.
Conclusions
Our predictions will be useful for improving the efficiency of current ecological restoration in southern Florida. For example, the Comprehensive Everglades Restoration Plan proposes to return freshwater flow to the Everglades to historic conditions (Perry 2004), and the implementation of this plan will affect water salinity of the coastal ecosystems in southern Florida (Herbert and others 2011). Our study shows that δ18O values of plant stem water may be a promising predictor of replacement of hammocks by mangroves 3 years before it occurs. 3 years may be sufficient time to introduce appropriate amounts of freshwater flow to cease or slow the hammock replacement by mangroves.
References
Armas C, Padilla FM, Pugnaire FI, Jackson RB. 2010. Hydraulic lift and tolerance to salinity of semiarid species: consequences for species interactions. Oecologia 162:11–21.
Baldwin AH, Mendelssohn IA. 1998. Effects of salinity and water level on coastal marshes: an experimental test of disturbance as a catalyst for vegetation change. Aquat Bot 61:255–68.
Ball MC. 1988. Ecophysiology of mangroves. Trees 2:129–42.
Brovkin V, Claussen M, Petoukhov V, Ganopolski A. 1998. On the stability of the atmosphere-vegetation system in the Sahara/Sahel region. J Geophys Res: Atmos 103:31613–24.
Chapman VJ. 1976. Mangrove vegetation. Vaduz: J. Cramer.
Dakos V, Carpenter SR, Brock WA, Ellison AM, Guttal V, Ives AR, Kefi S, Livina V, Seekell DA, Van Nes EH. 2012. Methods for detecting early warnings of critical transitions in time series illustrated using simulated ecological data. PLoS One 7:e41010.
Davis SM, Childers DL, Lorenz JJ, Wanless HR, Hopkins TE. 2005. A conceptual model of ecological interactions in the mangrove estuaries of the Florida Everglades. Wetlands 25:832–42.
Dawson TE, Ehleringer JR. 1991. Streamside trees that do not use stream water. Nature 350:335–7.
Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP. 2002. Stable isotopes in plant ecology. Annu Rev Ecol Syst 33:507–59.
Desantis LR, Bhotika S, Williams K, Putz FE. 2007. Sea-level rise and drought interactions accelerate forest decline on the Gulf Coast of Florida, USA. Glob Change Biol 13:2349–60.
Doering PH, Chamberlain RH, Haunert DE. 2002. Using submerged aquatic vegetation to establish minimum and maximum freshwater inflows to the Caloosahatchee Estuary, Florida. Estuaries 25:1343–54.
Doyle TW, Krauss KW, Conner WH, From AS. 2010. Predicting the retreat and migration of tidal forests along the northern Gulf of Mexico under sea-level rise. For Ecol Manag 259:770–7.
Egler FE. 1952. Southeast saline Everglades vegetation, Florida and its management. Vegetatio 3:213–65.
Ellsworth P, Williams D. 2007. Hydrogen isotope fractionation during water uptake by woody xerophytes. Plant Soil 291:93–107.
Eslami-Andargoli L, Dale P, Sipe N, Chaseling J. 2009. Mangrove expansion and rainfall patterns in Moreton Bay, Southeast Queensland, Australia. Estuary Coast Shelf Sci 85:292–8.
Ewe SML, Sternberg LdSL. 2005. Water uptake patterns of an invasive exotic plant in coastal saline habitats. J Coast Res 23:255–64.
Ewe SML, Sternberg LdSL, Childers DL. 2007. Seasonal plant water uptake patterns in the saline southeast Everglades ecotone. Oecologia 152:607–16.
Ezer T, Atkinson LP. 2014. Accelerated flooding along the U.S. East Coast: on the impact of sea-level rise, tides, storms, the Gulf Stream, and the North Atlantic Oscillations. Earth’s Future 2:362–82.
Fitterman DV, Deszcz-Pan M, Stoddard CE. 1999. Results of time-domain electromagnetic soundings in Everglades National Park, Florida. US Department of the Interior. pp 99–426.
Fourqurean JW, Robblee MB. 1999. Florida Bay: a history of recent ecological changes. Estuaries 22:345–57.
Gill AM, Tomlinson PB. 1977. Studies on the growth of red mangrove (Rhizophora mangle L.) 4. The adult root system. Biotropica 9:145–55.
Greaver TL, Sternberg LLdS. 2006. Linking marine resources to ecotonal shifts of water uptake by terrestrial dune vegetation. Ecology 87:2389–96.
Greiner La Peyre M, Grace J, Hahn E, Mendelssohn I. 2001. The importance of competition in regulating plant species abundance along a salinity gradient. Ecology 82:62–9.
Guha H, Panday S. 2012. Impact of sea level rise on groundwater salinity in a coastal community of south Florida. J Am Water Resour Assoc 48:510–29.
Gunderson LH. 1994. Vegetation of the Everglades: determinants of community composition. In: Davis SM, Ogden JC, Eds. Everglades: the ecosystem and its restoration. Boca Raton: CRC Press. p 323–40.
Herbert DA, Perry WB, Cosby BJ, Fourqurean JW. 2011. Projected reorganization of Florida Bay seagrass communities in response to the increased freshwater inflow of Everglades restoration. Estuar Coasts 34:973–92.
Hoffmeister JE. 1974. Land from the sea: the geologic story of South Florida. Coral Gables: University of Miami Press.
Hollins S, Ridd P, Read W. 2000. Measurement of the diffusion coefficient for salt in salt flat and mangrove soils. Wetlands Ecol Manag 8:257–62.
Ish-Shalom N, Sternberg LdSL, Ross M, O’Brien J, Flynn L. 1992. Water utilization of tropical hardwood hammocks of the lower Florida Keys. Oecologia 92:108–12.
Jiang J, DeAngelis DL, Anderson GH, Smith TJIII. 2014. Analysis and simulation of propagule dispersal and salinity intrusion from storm surge on the movement of a marsh–mangrove ecotone in South Florida. Estuar Coasts 37:24–35.
Jiang J, DeAngelis DL, Smith TJIII, Teh SY, Koh HL. 2012. Spatial pattern formation of coastal vegetation in response to external gradients and positive feedbacks affecting soil porewater salinity: a model study. Landsc Ecol 27:109–19.
Jiang J, DeAngelis DL, Teh S-Y, Krauss KW, Wang H, Li H, Smith TJ, Koh H-L. 2015. Defining the next generation modeling of coastal ecotone dynamics in response to global change. Ecol Model. doi:10.1016/j.ecolmodel.2015.04.013.
Kathiresan K, Bingham BL. 2001. Biology of mangroves and mangrove ecosystems. Adv Mar Biol 40:81–251.
Kenkel N, McIlraith A, Burchill C, Jones G. 1991. Competition and the response of three plant species to a salinity gradient. Can J Bot 69:2497–502.
Krauss KW, From AS, Doyle TW, Doyle TJ, Barry MJ. 2011. Sea-level rise and landscape change influence mangrove encroachment onto marsh in the Ten Thousand Islands region of Florida, USA. J Coast Conserv 15:629–38.
Kriebel DL, Geiman JD, Henderson GR. 2015. Future flood frequency under sea-level rise scenarios. J Coast Res. doi:10.2112/JCOASTRES-D-13-00190.1.
López-Medellín X, Ezcurra E, González-Abraham C, Hak J, Santiago LS, Sickman JO. 2011. Oceanographic anomalies and sea-level rise drive mangroves inland in the Pacific coast of Mexico. J Veg Sci 22:143–51.
Lin G, Sternberg LdSL. 1993. Hydrogen isotopic fractionation by plant roots during water uptake in coastal wetland plants. In: Ehleringer J, Hall A, Farquhar G, Eds. Stable isotopes and plant carbon-water relations. San Diego: Academic Press. p 397–410.
Lin G, Sternberg LdSL. 1994. Utilization of surface water by red mangrove (Rhizophora mangle L.): an isotopic study. Bull Mar Sci 54:94–102.
Lloyd RM. 1964. Variations in the oxygen and carbon isotope ratios of Florida Bay mollusks and their environmental significance. J Geol 72:84–111.
Lugo AE. 1980. Mangrove ecosystems: successional or steady state? Biotropica 12:65–72.
Lugo AE, Sell M, Snedaker SC. 1976. Mangrove ecosystem analysis. Syst Anal Simul Ecol 4:113–45.
Meeder JF, Ross MS, Telesnicki G, Ruiz PL, Sah JP. 1996. Vegetation analysis in the C-111/Taylor Slough basin. Final Report for Contract C-4244. Miami, FL, USA: Southeast Environmental Research Program, Florida International University.
Moffett KB, Gorelick SM, McLaren RG, Sudicky EA. 2012. Salt marsh ecohydrological zonation due to heterogeneous vegetation–groundwater–surface water interactions. Water Resour Res 48:1–22.
Morris JT. 2006. Competition among marsh macrophytes by means of geomorphological displacement in the intertidal zone. Estuar Coast Shelf Sci 69:395–402.
Odum WE, McIvor CC. 1990. Mangroves. In: Myers RL, Ewel JJ, Eds. Ecosystems of Florida. Orlando: University of Central Florida Press. p 517–48.
Odum WE, McIvor CC, Smith TJIII. 1982. The ecology of the mangroves of south Florida: a community profile. Washington DC: Fish and Wildlife Service.
Passioura J, Ball M, Knight J. 1992. Mangroves may salinize the soil and in so doing limit their transpiration rate. Funct Ecol 6:476–81.
Perry W. 2004. Elements of South Florida’s comprehensive everglades restoration plan. Ecotoxicology 13:185–93.
Price RM, Swart PK, Willoughby HE. 2008. Seasonal and spatial variation in the stable isotopic composition (δ18O and δD) of precipitation in south Florida. J Hydrol 358:193–205.
Rabinowitz D. 1978. Early growth of mangrove seedlings in Panama, and an hypothesis concerning the relationship of dispersal and zonation. J Biogeogr 5:113–33.
Ross MS, Meeder JF, Sah JP, Ruiz PL, Telesnicki GJ. 2000. The southeast saline Everglades revisited: 50 years of coastal vegetation change. J Veg Sci 11:101–12.
Ross MS, O’Brien JJ, Flynn LJ. 1992. Ecological site classification of Florida Keys terrestrial habitats. Biotropica 24:488–502.
Ross MS, O’Brien JJ, Ford RG, Zhang K, Morkill A. 2009. Disturbance and the rising tide: the challenge of biodiversity management on low-island ecosystems. Front Ecol Environ 7:471–8.
Saha AK, Saha S, Sadle J, Jiang J, Ross MS, Price RM, Sternberg LdSL, Wendelberger KS. 2011. Sea level rise and South Florida coastal forests. Clim Change 107:81–108.
Saha AK, Sternberg LdSL, Miralles-Wilhelm F. 2009. Linking water sources with foliar nutrient status in upland plant communities in the Everglades National Park, USA. Ecohydrology 2:42–54.
Saha S, Sadle J, Heiden C, Sternberg LdSL. 2014. Salinity, groundwater, and water uptake depth of plants in coastal uplands of Everglades National Park (Florida, USA). Ecohydrology 8:128–36.
Scheffer M, Bascompte J, Brock WA, Brovkin V, Carpenter SR, Dakos V, Held H, Van Nes EH, Rietkerk M, Sugihara G. 2009. Early-warning signals for critical transitions. Nature 461:53–9.
Scheffer M, Rinaldi S, Gragnani A, Mur LR, van Nes EH. 1997. On the dominance of filamentous cyanobacteria in shallow, turbid lakes. Ecology 78:272–82.
Scholander PF. 1968. How mangroves desalinate seawater. Physiol Plant 21:251–61.
Scholander PF, Hammel HT, Hemmingsen E, Garey W. 1962. Salt balance in mangroves. Plant Physiol 37:722.
Silander J, Antonovics J. 1982. Analysis of interspecific interactions in a coastal plant community—a perturbation approach. Nature 298:557–60.
Sklar FH, McVoy C, VanZee R, Gawlik DE, Tarboton K, Rudnick D, Miao S, Armentano T. 2002. The effects of altered hydrology on the ecology of the Everglades. In: Porter JW, Porter KG, Eds. The Everglades, Florida Bay and coral reefs of the Florida keys: an ecosystem source book. Boca Raton: CRC Press. p 39–82.
Sklar FH, van der Valk A. 2002. Tree islands of the Everglades. Boston: Springer.
Smith TJIII, Tiling-Range G, Jones J, Nelson P, Foster A, Balentine K. 2010. The use of historical charts and photographs in ecosystem restoration: examples from the Everglades Historical Air Photo Project. In: Cowley DC, Standring RA, Abicht MJ, Eds. Landscapes through the lens: aerial photographs and the historic environment. Occasional Publication of the Aerial Archaeology Research Group. Oxford, UK: Oxbow Books. p 179–91.
Snedaker SC. 1995. Mangroves and climate change in the Florida and Caribbean region: scenarios and hypotheses. In: Wong Y-S, Tam NY Eds. Asia-Pacific symposium on mangrove ecosystems. Dordrecht: Springer. pp 43–49.
Snyder JR, Herndon A, Robertson WB. 1990. South Florida rockland. In: Myers RL, Ewel JJ, Eds. Ecosystems of Florida. Orlando: University of Central Florida Press. p 230–79.
Sokal R, Rohlf F. 1995. Biometry: the principles and practice of statistics in biological research. New York: W. H. Freeman and Company.
Stephens DB. 1995. Vadose zone hydrology. Boca Raton: CRC Press. pp 1–2.
Sternberg LdSL. 2001. Savanna-forest hysteresis in the tropics. Glob Ecol Biogeogr 10:369–78.
Sternberg LdSL, Green L, Moreira MZ, Nepstad D, Martinelli LA, Victória R. 1998. Root distribution in an Amazonian seasonal forest as derived from δ13C profiles. Plant Soil 205:45–50.
Sternberg LdSL, Ish-Shalom-Gordon N, Ross M, O’Brien J. 1991. Water relations of coastal plant communities near the ocean/freshwater boundary. Oecologia 88:305–10.
Sternberg LdSL, Moreira MZ, Nepstad DC. 2002. Uptake of water by lateral roots of small trees in an Amazonian tropical forest. Plant Soil 238:151–8.
Sternberg LdSL, Swart PK. 1987. Utilization of freshwater and ocean water by coastal plants of southern Florida. Ecology 68:1898–905.
Sternberg LdSL, Teh SY, Ewe SML, Miralles-Wilhelm FR, DeAngelis DL. 2007. Competition between hardwood hammocks and mangroves. Ecosystems 10:648–60.
Steyer GD, Cretini KF, Piazza S, Sharp LA, Snedden GA, Sapkota S. 2010. Hurricane influences on vegetation community change in coastal Louisiana. Reston: U.S. Geological Survey.
Swart PK, Sternberg LdSL, Steinen R, Harrison SA. 1989. Controls on the oxygen and hydrogen isotopic composition of the waters of Florida Bay, USA. Chem Geol 79:113–23.
Teh SY, DeAngelis DL, Sternberg LdSL, Miralles-Wilhelm FR, Smith TJ, Koh H-L. 2008. A simulation model for projecting changes in salinity concentrations and species dominance in the coastal margin habitats of the Everglades. Ecol Model 213:245–56.
Tomlinson PB. 1994. The botany of mangroves. Cambridge: Cambridge University Press.
Vendramini PF, Sternberg LdSL. 2007. A faster plant stem-water extraction method. Rapid Commun Mass Spectrom 21:164–8.
Volkmar K, Hu Y, Steppuhn H. 1998. Physiological responses of plants to salinity: a review. Can J Plant Sci 78:19–27.
Wasson K, Woolfolk A, Fresquez C. 2013. Ecotones as indicators of changing environmental conditions: rapid migration of salt marsh-upland boundaries. Estuar Coasts 36:654–64.
Wei L, Lockington DA, Poh S-C, Gasparon M, Lovelock CE. 2012. Water use patterns of estuarine vegetation in a tidal creek system. Oecologia 172:485–94.
Wilson AM, Moore WS, Joye SB, Anderson JL, Schutte CA. 2011. Storm-driven groundwater flow in a salt marsh. Water Resour Res 47:W02535.
Xin P, Jin G, Li L, Barry DA. 2009. Effects of crab burrows on pore water flows in salt marshes. Adv Water Resour 32:439–49.
Acknowledgments
We thank the Department of Biology at the University of Miami for providing the equipment and funding to Lu Zhai for this study. Dr. Carol Horvitz, Dr. Robert Stephen Cantrell, Dr. Lili Wei, Dr. Su Yean Teh, and Dr. Guanghui Lin provided useful comments on an early draft of the manuscript. We also thank the three journal reviewers as well as USGS reviewer Ken Krauss for helpful comments on the manuscript. DLD was supported by the USGS′s Greater Everglades Priority Ecosystem Science Program. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Conceived of or designed study: Lu Zhai, Leo Sternberg. Performed research: Lu Zhai. Analyzed data: Lu Zhai. Contributed new methods or models: Lu Zhai, Jiang Jiang, Don DeAngelis, Leo Sternberg. Wrote the paper: Lu Zhai.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhai, L., Jiang, J., DeAngelis, D. et al. Prediction of Plant Vulnerability to Salinity Increase in a Coastal Ecosystem by Stable Isotope Composition (δ18O) of Plant Stem Water: A Model Study. Ecosystems 19, 32–49 (2016). https://doi.org/10.1007/s10021-015-9916-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-015-9916-3