Abstract
Background
The purpose of this study was to investigate the effectiveness of dental intervention before and after radiation therapy (RT) for head and neck malignancy on prevention of osteoradionecrosis (ORN) of the jaws.
Methods
This is a single-arm prospective study according to intervention protocol of prophylactic dental extraction before RT and routine follow-up after RT. The primary endpoint was the occurrence of jawbone exposure during the first 2 years after RT.
Results
Sixty-seven patients were assessed. Before RT, 144 teeth among 39 patients (58%) were prophylactically extracted. The occurrence of transient jawbone exposure during the first 2 years after RT was 7%. Because those jawbone exposures healed with intervention after RT, no jawbone exposure was found at 2 years after RT.
Conclusions
Dental intervention both before and after RT seemed to be important to prevent ORN development. Further studies in larger cohorts are necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Head and neck malignancy is a heterogeneous disease arising from different anatomic sites of the upper aerodigestive tract (oral cavity, larynx, hypopharynx, oropharynx, nasopharynx, paranasal sinuses, and nasal cavity) and salivary glands. It accounts for more than 550,000 cases and 380,000 deaths annually worldwide [1]. Radiation therapy (RT) plays an indispensable role in the current management of head and neck malignancies and significantly improved the prognosis especially in patients with human papillomavirus positive oropharyngeal squamous cell carcinoma [2]. However, complications involving normal tissues that occur during (acute complications) or after (subacute and chronic complications) RT make this treatment method challenging [3]. A serious and devastating adverse effect of RT for head and neck malignancy is osteoradionecrosis (ORN) of the jaws. Although the mechanism of pathogenesis of ORN remains to be elucidated, the most frequently reported reason is radiation arteritis [4, 5]. Recent studies support the radiation-induced fibrosis theory, according to which a series of events occur in 3 phases (an initial prefibrotic phase, a subsequent phase characterized by abnormal fibroblastic activity, and late fibroatrophic phase) [6, 7]. The reported incidence of ORN has declined from approximately 20% several decades ago [8] to approximately 5% in recent large-scale studies as follows: 3.9% of 23,527 patients [9]; 4.3% of 1023 cases, all of whom received intensity-modulated RT (IMRT) [10]; 5.2% of 18,231 patients [11]; and 6.2% of 1692 patients [12]. In 2012, a systematic review by Nabil and Samman [13] showed that the estimated incidence of ORN in patients who had undergone irradiation for head and neck cancer was 2%. A short letter entitled “Osteoradionecrosis: an old toxicity in the IMRT era?” by De Felice et al. [14] noted that efforts to minimize the risk of ORN must be encouraged but that the clinical outcome in terms of the local control rate should be given priority over the risk of ORN. As mentioned above, the incidence of ORN has decreased, but this condition has not yet been eradicated.
ORN occurs spontaneously, whereas some recent studies reported that dental extraction before or after RT associated with the development of ORN [9, 15]. In recent retrospective reports involving 190 patients (among whom the incidence of ORN was 15.3%) described by Beech et al., [16, 17] dental extractions before RT were associated with a statistically significant increase in the risk of developing ORN, and a history of more than eight dental extractions before RT was associated with a statistically significant reduced quality of life (QOL). Few prospective studies have evaluated the impact of the patient’s dental condition and pre-RT dental interventions on the prevention and treatment of ORN. In a prospective trial by Ben-David et al. published in 2007 [18], 176 patients with a minimum follow-up of 6 months developed no ORN during a median follow-up of 34 months. The authors concluded that strict prophylactic dental care and the dosimetric advantages of IMRT are essential factors in reducing the risk of ORN [18]. In a 2-year prospective follow-up study by Schuurhuis et al. published in 2018 [19], ORN developed in 7% (4/56) of patients as the result of treatment of oral foci before RT.
We previously proposed appropriate prophylactic dental extraction with consideration of the irradiation field and dose [20]. We also formulated a novel protocol for dental intervention by modifying a previous protocol [21] for patients who underwent hematopoietic stem cell transplantation for hematological malignancy, and we demonstrated the validity of our dental intervention protocol for patients who underwent myelosuppressive chemotherapy for hematological malignancy [22, 23]. The primary purpose of the present single-arm prospective intervention study was to determine whether our dental intervention protocol has an impact on the prevention and treatment of ORN in patients undergoing irradiation for head and neck malignancy.
Methods
Study population
This was a single-arm prospective study. All participants provided written informed consent before involvement in this study. They underwent RT for head and neck malignancies at the Division of Radiation Oncology, Kobe University Hospital, from July 2015 to July 2016. Some patients underwent surgery at the Department of Otolaryngology-Head and Neck Surgery or Department of Oral and Maxillofacial Surgery, Kobe University Hospital. Our institutional review board approved this study (No. 1768, 2015).
Dental intervention protocol
For each patient, the Department of Oral and Maxillofacial Surgery performed the pre-RT oral examination. The radiation oncologists informed the patients of their diagnosis and planned day of RT to the attending doctors in the Department of Oral and Maxillofacial Surgery. At the first visit, the attending doctors assessed each patient’s dental condition with medical inquiries, intraoral examinations, and panoramic radiographs of all patients. The attending dental hygienists provided oral hygiene instruction and performed professional teeth cleaning before the initiation of RT. Teeth and oral mucosa cleaning by dental hygienists was continued at a frequency of once a week for patients with oral mucositis (i.e., oral cavity and oropharyngeal carcinoma patients), and as necessary for other patients. The following teeth were prophylactically extracted according to our established dental intervention protocol:
-
Teeth with marginal periodontitis and a probing depth of >8 mm, severe mobility, or severe inflammation
-
Teeth with apical periodontitis and symptoms or periapical radiolucency of > 5 mm on dental radiographs
-
Partially erupted teeth with ongoing symptoms or a history of symptoms
-
Unrestorable deep dental caries with symptoms
“Symptoms” in this study referred to spontaneous pain, percussion pain, occlusal pain, gingival redness, swelling, and pus discharge. Osseointegrated dental implants with severe mobility or symptoms were removed. Teeth near the tumor were not extracted because of the risk of bleeding.
The above-described prophylactic dental extraction was performed according to the site of the primary tumor and irradiation fields, as previously described in detail [20]. In brief, this classification stratifies RT for head and neck malignancy into the following grades according to the extent of irradiation to the jaws:
-
High risk involving both the maxilla and mandible: Cancer of the oral cavity, oropharynx, hypopharynx, or nasopharynx
-
High risk involving only the maxilla: Cancer of the nasal (paranasal) cavity
-
High risk involving only the mandible: Cancer of the submandibular gland or metastasis to level II lymph nodes
-
High risk involving the maxilla and mandible only on the diseased side: Cancer of the parotid gland
-
Prophylactic dental extraction was performed only at high-risk sites.
-
-
Low risk: Cancer of the larynx or thyroid gland or neck irradiation only
-
Prophylactic tooth extraction was not performed; only strict dental care was performed.
-
All dental extractions were performed at least 1 week before the initiation of RT under antibiotic prophylaxis.
In general, the patients were followed up at 3 and 6 months and at 1, 1.5, and 2 years after the completion of RT. Some patients (e.g., patients with severe mucositis) were followed up at shorter intervals. The dental follow-up was mostly done by the single doctor (YM). During the follow-up period after RT, teeth with symptoms such as pain, trismus, and infection were recorded and initially treated conservatively. However, when the symptoms did not resolve and extraction was the only treatment option, the teeth were extracted. During the follow-up period after RT, if the jawbone exposure was found, the interventions were started. Specifically, bone exposure was initially treated conservatively (i.e., repeated local irrigation and careful observation). However, when the symptoms caused by bone infection became worse, the surgical intervention (e.g., surgical debridement under general anesthesia) was chosen.
Data collection
The following epidemiological data were gathered: age, sex, smoking history (none, past, or current), alcohol use (none, moderate/social drinker, or heavy/daily drinker), comorbidities, histology, primary tumor site, clinical TNM classification, RT technique and dose, surgery, concurrent chemotherapy, antiresorptive agent use, pre- and post-RT dental extraction site and number of extracted teeth, and time interval between RT and dental extraction.
In the previous literature, ORN has been defined as loss of mucosal coverage and bone exposure lasting 3 to 6 months [12, 17, 20]. Although the onset of ORN is time-independent [24], the risk of developing spontaneous ORN is higher within the first 2 or 3 years after RT [15]. This prospective study assessed the incidence of jawbone exposure during the first 2 years after RT. Jawbone exposure was defined as a condition in which the jawbone was obviously detected by visual inspection or palpation by using tweezers. The duration, treatment content, and treatment outcome for jawbone exposure were recorded. The jawbone exposure at 2 years after RT in patients who could be followed up for 2 years after RT was also assessed.
Statistical analysis
Statistical analyses were performed using R software (R Development Core Team, 2011). The Mann–Whitney U test and Fisher exact test were performed. A P value less than 0.05 was considered statistically significant.
Results
In total, 88 patients were introduced to our department during the study period. One patient aged < 20 years was excluded; therefore, 87 patients participated in this study. The follow-up in 16 patients was interrupted because of transfer to another hospital and 4 patients died before the completion of RT; therefore, 67 patients were enrolled for evaluation. The detailed patient characteristics are shown in Table 1. The median age of the study population was 65.5 years (range, 24–88 years). Most patients were male (79%). Two patients had osteoporosis, one of whom was administered bisphosphonate and the other parathyroid hormone (these two patients with osteoporosis did not develop ORN). The most common histology was squamous cell carcinoma (92.5%); the others were malignant lymphoma in two patients (lower gingiva and nasal cavity) and maxillary sinus small cell carcinoma in one patient. The most common primary site was the oropharynx (25%). Of the radiation techniques, IMRT was more common than conventional RT (69% and 31%, respectively) in this study. The radiation dose for the primary lesions was <60 Gy in only three patients (4%), in whom no ORN occurred. Twenty-three patients (34%) underwent surgery. Fifty-two patients (78%) underwent concurrent chemotherapy, and cisplatin was the most common agent (46/52 patients, 88%). Cetuximab was used in 3 patients. Three patients received denosumab for bone metastasis after the completion of RT during the study period.
In total, 144 teeth in 39 patients (58%) were prophylactically extracted according to the above-mentioned dental intervention protocol before RT. The indication for prophylactic dental extraction was marginal periodontitis in 92 teeth (64%), apical periodontitis in 10 (7%), impacted wisdom teeth in 6 (4%), and dental caries in 36 (25%). After the completion of RT, 7 teeth in 4 patients underwent dental extraction involving almost spontaneous loss of teeth without surgical invasion. The incidence of jawbone exposure during the first 2 years after RT was 7% (5/67 patients). Descriptive and bivariate analyses revealed no factors associated with the development of jawbone exposure (Table 1).
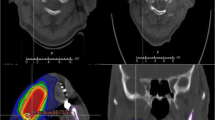
Table 2 shows the detailed information of five patients with jawbone exposure. Three of the five patients underwent prophylactic dental extraction, and only one case of jawbone exposure occurred in the socket of the prophylactic dental extraction (case 5). Therefore, the incidence of jawbone exposure due to pre-RT prophylactic dental extraction was 3% (1/39 patients). The one affected patient underwent extraction of a symptomatic mandibular impacted wisdom tooth with a dentigerous cyst. Although the socket of the dental extraction healed, a fistula with pus drainage was found after completion of RT and persisted for 15 months. Another patient (case 4) developed jawbone exposure following dental extraction after the completion of RT. The tooth extracted in case 4 was similar to a floating tooth with severe mobility caused by resorption of the surrounding jawbone. In this patient, the jawbone exposure probably existed before the spontaneous loss of the tooth; it did not occur due to surgical trauma such as that induced by dental extraction. Two cases of jawbone exposure occurred in patients who did not undergo prophylactic dental extraction. In case 1, the occurrence of jawbone exposure was associated with asymptomatic apical periodontitis. In case 2, the jawbone exposure occurred in association with a lingual anterior lesion of the mandible within the irradiation field but was unrelated to the dentition. In case 3, the wisdom tooth was not prophylactically extracted because the tooth was asymptomatic and deeply impacted. However, 11 months after the completion of RT, this patient developed severe pain in the left mandible, paralysis in the left mental region, and trismus (maximum mouth-opening amount was < 20 mm). Bone exposure on the lingual side of the left mandibular wisdom teeth was detected (Fig. 1). The symptoms became worse, and surgical debridement including bone resection and extraction of the left second and third molars was performed under general anesthesia. The bone exposure and trismus healed completely after the surgery. The other four patients with jawbone exposure achieved complete healing with conservative treatments such as sequestrectomy under local anesthesia, repeated local irrigation, or antibiotic use only when acute inflammation with pain, swelling, and trismus due to infection occurred (Table 2).
A representative case of jawbone exposure (case 3). a Positron emission tomographic image before radiation therapy (RT). b Simulation computed tomographic (CT) image. Jawbone exposure developed around the left mandibular wisdom teeth. c Note the bone destruction on the lingual side of the wisdom tooth in CT image. The wisdom tooth was not prophylactically extracted because the tooth was asymptomatic and deeply impacted. d Panoramic X-ray image before RT showing the deeply impacted left mandibular wisdom tooth. e Intraoral finding after 11 months RT showing severe trismus and the deep periodontal pocket into which the tips of tweezers were deeply inserted. f Intraoperative finding. Note the minimal mucosal defect on the lingual side of the deeply impacted wisdom tooth. g Intraoral finding 5 months postoperatively showing sufficient mouth opening and epithelialization
Fourteen patients died during the 2 first years after RT. No jawbone exposure was found at 2 years after RT in 53 patients who could be completely followed up for 2 years after RT.
Discussion
The prevention and treatment of ORN, the most problematic complication of RT for head and neck malignancy, are important tasks for dental oncologists. This novel single-arm prospective study was performed to determine the efficacy of dental intervention performed according to uniform protocol on the prevention of ORN. This study was conducted based on the hypothesis that a strict dental intervention protocol has the potential to prevent ORN. This study revealed that jawbone exposure occurred at an incidence of 7% during the first 2 years after RT. The result that jawbone exposure healed with intervention after RT indicates that the not only prophylactic tooth extraction before RT but also proper interventions for jawbone exposure after RT may be necessary for prevention of ORN development.
In the prospective trial by Ben-David et al. [18], no ORN occurred after strict prophylactic dental care and IMRT. The incidence of jawbone exposure in our study (7%) was similar to that in the 2-year prospective study by Schuurhuis et al. [19], in which ORN occurred in 7% of patients in spite of treatment of oral foci before RT. The details of the dental intervention protocol (e.g., the indications for tooth extraction) were slightly different among these three prospective studies, including ours [18, 19]. Schuurhuis et al. [19] concluded that patients with severe periodontal disease before RT are more prone to develop ORN or bone healing problems after RT. In their retrospective study, Kojima et al. [25] concluded that extraction of mandibular molars with periapical periodontitis before RT may reduce the risk of ORN because periapical periodontitis was shown to be a significant independent risk factor for ORN. Beech et al. [17] carefully discussed the indications for extraction: (1) sound teeth should not be extracted because such extractions increase the risk of ORN and may reduce patients’ QOL after RT; (2) whether to extract compromised teeth (i.e., those that would generally not be extracted in healthy people but would require some degree of restoration) is difficult to judge, but an alternative to extraction such as endodontic treatment should be considered; and (3) extraction of hopeless teeth (i.e., those that would be extracted regardless of RT planning) does not warrant an argument against performing the extraction, but no evidence regarding the appropriate timing of dental extraction exists. In their systematic review, Schuurhuis et al. [26] hypothesized that the following oral foci should be effectively treated or eliminated before the initiation of RT: deep caries that may lead to pulpal exposure, active periodontal disease with pockets of > 6 mm, nonrestorable teeth with large restorations extending the gum line or with root caries, periapical granuloma and avital teeth, impacted or partially erupted teeth not fully covered by bone, and cysts. These indications for dental intervention before RT are not substantially different from our protocol. We consider that our protocol of prophylactic dental extraction is not so invasive. Specifically, extraction is generally indicated for teeth affected by marginal periodontitis with a probing depth of > 8 mm or severe mobility and inflammation, partially erupted teeth with symptoms, and teeth containing unrestorable deep dental caries with symptoms, even in healthy subjects. How to treat teeth with apical periodontitis is difficult to judge, because apical periodontitis is generally asymptomatic and infected root canal treatment (e.g., complete removal of previous filling material and repeated root canal irrigation) is sometimes difficult and time-consuming. In fact, one case of jawbone exposure occurred among the teeth affected by asymptomatic apical periodontitis in the present study (case 1). Our study also revealed a dilemmatic result: that jawbone exposure can develop from both the socket of prophylactic tooth extraction (case 5) and the preserved tooth (case 3). Jawbone exposure seems to be inevitable in certain patients, especially those in whom the mandible is included in the irradiated field for locoregional control, regardless of whether prophylactic dental extraction is performed. In fact, jawbone exposure occurs unrelated to the dentition like case 2 in this study. Prophylactic dental extraction cannot prevent such jawbone exposure. Another key finding in our study is that although the number of patients who underwent dental extraction was small, the number of extracted teeth before RT was not significantly associated with the occurrence of jawbone exposure. In our opinion, based upon the results of this study, simple criteria for prophylactic dental extraction comparable with judgment of the need for extraction in healthy subjects are probably sufficient for patients who are scheduled for RT for head and neck malignancy.
Careful follow-up after the completion of RT may play an important role in the prevention and treatment of ORN, as do oral examination and prophylactic dental extraction. Although this study focused on the incidence of jawbone exposure during the first 2 years after RT through a specific dental intervention protocol, the onset of ORN is time-independent [24]. A systematic review by Nabil and Samman in 2011 [15] differentiated between spontaneous and trauma-induced ORN. The first 2 or 3 years after RT is a high-risk period for spontaneous ORN [15, 27, 28]. The period from 2 to 5 years after RT had the highest incidence of trauma-induced ORN (e.g., ORN secondary to dental extraction) in their review [15]. The authors found that dental extraction within 1 year after RT resulted in a 7.5% risk of ORN [15]. The risk of ORN caused by dental extraction increases to 22.6% from 2 to 5 years after RT and then decreases to 16.7% after 5 years [15]. Spontaneous ORN within 2 years after RT may be due to treatment-related trauma to the mandible (i.e., RT), and the second increase in the incidence of ORN from 2 to 5 years after RT is probably due to the increase in extractions of teeth that have broken down after a certain period of time [9, 15]. A recent large-scale national-based cohort study by Wang et al. [9] showed that the frequency of dental extractions increase during the first 2 years after RT and then gradually decreased, whereas the hazard ratio for ORN still increased after the first 2 years post-RT and peaked at 4 years after RT. As shown in case 3, jawbone exposure is sometimes so difficult to be detected unless careful observation is done by specialists. If such a cryptic jawbone exposure is left untreated, it may be found in an advanced state of ORN about 4 to 5 years after RT. Based on these findings, Wang et al. [9] encouraged prophylactic dental extraction and efforts to avoid dental extraction after RT, especially during the first 4 years after RT. In their systematic review, Schuurhuis et al. [26] recommended a follow-up period of > 2 years to detect late sequelae of RT.
This prospective study had several limitations. First, as mentioned above, the follow-up duration after RT was short (2 years). We are currently continuing this prospective study and will report the long-term results in the future. Moreover, this study is a small sample size. Further studies in larger cohorts are necessary to improve treatment outcome in patients with head and neck malignancies. Second, this study had no control. A Cochrane review published in 2013 concluded that no randomized controlled trials have been performed to assess the effect of extracting teeth before RT versus leaving teeth in the mouth during RT to the jaws [29]. However, we consider that the use of controls in such a study is ethically problematic. Third, we did not assess patients’ QOL. Ideally, dental intervention before and after RT should have the potential to both prevent ORN and improve QOL. Finally, this study established a strict protocol of dental intervention, especially prophylactic dental extraction, rather than post-RT intervention. Notably, however, careful follow-up and early intervention (as in case 3) after RT probably help to prevent the exacerbation of jawbone exposure leading to intractable ORN, because all cases of jawbone exposure in this study healed after post-RT management. An establishment of protocol focusing on appropriate post-RT dental intervention is also our future task.
Conclusions
There were jawbone exposures in 7% of the cases during 2 years after RT in spite of prophylactic tooth extraction performed in accordance with a strict protocol. Although dental intervention seemed to be important to prevent ORN, further studies in larger cohorts are necessary to improve treatment outcome in patients with head and neck malignancies.
References
Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R et al (2017) Global, regional, and National Cancer Incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol 3(4):524–528
Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, Gillison ML (2008) Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100(4):261–269
Ragin CC, Taioli E (2007) Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer 121(8):1813–1820
Chronopoulos A, Zarra T, Tröltzsch M, Mahaini S, Ehrenfeld M, Otto S (2015) Osteoradionecrosis of the mandible: a ten year single-center retrospective study. J Craniomaxillofac Surg 43(6):837–846
Chronopoulos A, Zarra T, Ehrenfeld M, Otto S (2018) Osteoradionecrosis of the jaws: definition, epidemiology, staging and clinical and radiological findings. A concise review. Int Dent J 68(1):22–30
Rivero JA, Shamji O, Kolokythas A (2017) Osteoradionecrosis: a review of pathophysiology, prevention and pharmacologic management using pentoxifylline, α-tocopherol, and clodronate. Oral Surg Oral Med Oral Pathol Oral Radiol 124(5):464–471
Sroussi HY, Epstein JB, Bensadoun RJ, Saunders DP, Lalla RV, Migliorati CA, Heaivilin N, Zumsteg ZS (2017) Common oral complications of head and neck cancer radiation therapy: mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med 6(12):2918–2931
Moon DH, Moon SH, Wang K, Weissler MC, Hackman TG, Zanation AM, Thorp BD, Patel SN, Zevallos JP, Marks LB, Chera BS (2017) Incidence of, and risk factors for, mandibular osteoradionecrosis in patients with oral cavity and oropharynx cancers. Oral Oncol 72:98–103
Wang TH, Liu CJ, Chao TF, Chen TJ, Hu YW (2017) Risk factors for and the role of dental extractions in osteoradionecrosis of the jaws: a national-based cohort study. Head Neck 39(7):1313–1321
Owosho AA, Tsai CJ, Lee RS, Freymiller H, Kadempour A, Varthis S, Sax AZ, Rosen EB, Yom SK, Randazzo J, Drill E, Riedel E, Patel S, Lee NY, Huryn JM, Estilo CL (2017) The prevalence and risk factors associated with osteoradionecrosis of the jaw in oral and oropharyngeal cancer patients treated with intensity-modulated radiation therapy (IMRT): the Memorial Sloan Kettering Cancer Center experience. Oral Oncol 64:44–51
Chang CT, Liu SP, Muo CH, Tsai CH, Huang YF (2017) Dental prophylaxis and osteoradionecrosis: a population-based study. J Dent Res 96(5):531–538
Chen JA, Wang CC, Wong YK, Wang CP, Jiang RS, Lin JC, Chen CC, Liu SA (2016) Osteoradionecrosis of mandible bone in patients with oral cancer--associated factors and treatment outcomes. Head Neck 38(5):762–768
Nabil S, Samman N (2012) Risk factors for osteoradionecrosis after head and neck radiation: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol 113(1):54–69
De Felice F, Musio D, Tombolini V (2015) Osteoradionecrosis: an old toxicity in the IMRT era? Oral Oncol 51(6):e60–e61
Nabil S, Samman N (2011) Incidence and prevention of osteoradionecrosis after dental extraction in irradiated patients: a systematic review. Int J Oral Maxillofac Surg 40(3):229–243
Beech N, Porceddu S, Batstone MD (2016) Preradiotherapy dental extractions and health-related quality of life. Oral Surg Oral Med Oral Pathol Oral Radiol 122(6):672–679
Beech NM, Porceddu S, Batstone MD (2017) Radiotherapy-associated dental extractions and osteoradionecrosis. Head Neck 39(1):128–132
Ben-David MA, Diamante M, Radawski JD, Vineberg KA, Stroup C, Murdoch-Kinch CA, Zwetchkenbaum SR, Eisbruch A (2007) Lack of osteoradionecrosis of the mandible after intensity-modulated radiotherapy for head and neck cancer: likely contributions of both dental care and improved dose distributions. Int J Radiat Oncol Biol Phys 68(2):396–402
Schuurhuis JM, Stokman MA, Witjes MJH, Reintsema H, Langendijk JA, Vissink A, Spijkervet FKL (2018) Patients with advanced periodontal disease before intensity-modulated radiation therapy are prone to develop bone healing problems: a 2-year prospective follow-up study. Support Care Cancer 26(4):1133–1142
Wanifuchi S, Akashi M, Ejima Y, Shinomiya H, Minamikawa T, Furudoi S, Otsuki N, Sasaki R, Nibu KI, Komori T (2016) Cause and occurrence timing of osteoradionecrosis of the jaw: a retrospective study focusing on prophylactic tooth extraction. Oral Maxillofac Surg 20(4):337–342
Yamagata K, Onizawa K, Yanagawa T, Hasegawa Y, Kojima H, Nagasawa T, Yoshida H (2006) A prospective study to evaluate a new dental management protocol before hematopoietic stem cell transplantation. Bone Marrow Transplant 38(3):237–242
Tsuji K, Shibuya Y, Akashi M, Furudoi S, Yakushijin K, Kawamoto S, Okamura A, Matsuoka H, Komori T (2015) Prospective study of dental intervention for hematopoietic malignancy. J Dent Res 94(2):289–296
Kishimoto M, Akashi M, Tsuji K, Kusumoto J, Furudoi S, Shibuya Y, Inui Y, Yakushijin K, Kawamoto S, Okamura A, Matsuoka H, Komori T (2017) Intensity and duration of neutropenia relates to the development of oral mucositis but not odontogenic infection during chemotherapy for hematological malignancy. PLoS One 12(7):e0182021
Reuther T, Schuster T, Mende U, Kübler A (2003) Osteoradionecrosis of the jaws as a side effect of radiotherapy of head and neck tumour patients--a report of a thirty year retrospective review. Int J Oral Maxillofac Surg 32(3):289–295
Kojima Y, Yanamoto S, Umeda M, Kawashita Y, Saito I, Hasegawa T, Komori T, Ueda N, Kirita T, Yamada SI, Kurita H, Senga Y, Shibuya Y, Iwai H (2017) Relationship between dental status and development of osteoradionecrosis of the jaw: a multicenter retrospective study. Oral Surg Oral Med Oral Pathol Oral Radiol 124(2):139–145
Schuurhuis JM, Stokman MA, Witjes MJ, Dijkstra PU, Vissink A, Spijkervet FK (2015) Evidence supporting pre-radiation elimination of oral foci of infection in head and neck cancer patients to prevent oral sequelae. A systematic review. Oral Oncol 51(3):212–220
Marx RE, Johnson RP (1987) Studies in the radiobiology of osteoradionecrosis and their clinical significance. Oral Surg Oral Med Oral Pathol 64(4):379–390
Thorn JJ, Hansen HS, Specht L, Bastholt L (2005) Osteoradionecrosis of the jaws: clinical characteristics and relation to the field of irradiation. J Oral Maxillofac Surg 58(10):1088–1093 discussion 1093–1095
Eliyas S, Al-Khayatt A, Porter RW, Briggs P (2013) Dental extractions prior to radiotherapy to the jaws for reducing post-radiotherapy dental complications. Cochrane Database Syst Rev 28(2):CD008857. https://doi.org/10.1002/14651858.CD008857.pub2
Acknowledgments
We thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical committee approval
Obtained from the Institutional Ethics Committee [No. 1768, 2015].
Informed consent
Obtained.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Muraki, Y., Akashi, M., Ejima, Y. et al. Dental intervention against osteoradionecrosis of the jaws in irradiated patients with head and neck malignancy: a single-arm prospective study. Oral Maxillofac Surg 23, 297–305 (2019). https://doi.org/10.1007/s10006-019-00783-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10006-019-00783-0