Abstract
S100β-positive cells exist in the marginal cell layer (MCL) of the adenohypophysis and follicle structure in the parenchyma of anterior lobe (ALFS) in pituitary. They have multiple functions as phagocytes or cells that regulate hormone secretion. Majority of S100β-positive cells in the adenohypophysis express sex determining region Y-box 2 protein (SOX2), a stem cell marker; therefore, S100β/SOX2 double positive cells are also considered as one type of stem/progenitor cells. MCL and ALFS are consisting of morphologically two types of cells, i.e., multiciliated cells and non-ciliated cells. However, the relationship between the S100β-positive cells and multiciliated cells in the pituitary is largely unknown. In the present study, we first immunohistochemically verified the feature of multiciliated cells in MCL and ALFS. We then examined the expression patterns of FOXJ1, an essential expression factor for multiciliated cell-differentiation, and SOX2 in the S100β-positive multiciliated cells by in situ hybridization and immunohistochemistry. We identified anew the S100β/SOX2/FOXJ1 triple positive multiciliated cells, and revealed that they were dispersed throughout the MCL and ALFS. These results indicate that the MCL and ALFS are consisting of morphologically and functionally distinct two types of cells, i.e., S100β/SOX2 double positive non-ciliated cells and S100β/SOX2/FOXJ1 triple positive multiciliated cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pituitary, one of the central endocrine organs regulating the universal endocrine system in the vertebrates, is composed of adenohypophysis consisting of an anterior lobe (AL) and intermediate lobe (IL), and neurohypophysis comprising the posterior lobe (PL). AL consists of five types of endocrine cells (corticotrophs, thyrotrophs, gonadotrophs, mammotrophs, and somatotrophs), S100β-positive cells, and endothelial cells of the fenestrated sinusoids. S100β is a low molecular weight calcium-binding protein and S100β-positive cells lack the secretory granules for endocrine function. S100β-positive cells in the rat and mouse pituitaries are observed in not only the AL but also the marginal cell layer (MCL), which is a single-cell layer around Rathke’s cleft [1, 2]. Majority of S100β-positive cells in the MCL and AL express sex-determining region Y-box 2 protein (SOX2), a transcription factor essential for cell-potency of stem cells, and function as one type of stem/progenitor cells in the pituitary [3, 4]. These cells play multiple functions, e.g., as phagocytes and cells regulating hormone secretion in the AL [5]; therefore, it is considered that S100β-positive cells comprise functionally heterogeneous subpopulations [6–8].
Motile cilia extend from the basal bodies of multiciliated cells and afford motility for the circulation of cerebrospinal fluid in the brain, protective mucus clearance in the airway, and ovum transport in the oviduct tubes [9]. They are also known to contain various receptors (for hormones, cytokines, etc.) and channels (for water, Ca2+, etc.); therefore, motile cilia function not only in cell motility but also as mechano- and chemo-sensors [10]. Previous reports have shown that multiciliated cells are located in the MCL and follicle structure in the parenchyma of AL (ALFS) in the pituitary [11–14]. Motile cilia in these cells are elongated toward the lumen of Rathke’s cleft or the follicle structure composed of S100β-positive cells. However, the relationship between the multiciliated cells and S100β-positive cells is largely unknown.
Forkhead box (FOX) proteins are a family of transcription factors that play important roles in the regulation of expression of genes involved in developmental events, such as cell proliferation, differentiation, glucose homeostasis, cancer, and longevity [15, 16]. FOXJ1, also known as hepatocyte nuclear factor-3/forkhead homologue-4, is expressed mainly in multiciliated cells of various epithelial tissues, such as the trachea and oviduct [17–21]. Deletion and mutation of Foxj1 results in motile cilia defects in mice, Xenopus, and zebrafish [22–26]; thus, it is thought that FOXJ1 is evolutionally conserved in vertebrates and is essential for motile cilia formation.
In the present study, we first immunohistochemically verified the existence of S100β-positive multiciliated cells in the MCL and AL. We also examined the expression patterns of FOXJ1 and SOX2 in S100β-positive multiciliated cells by in situ hybridization and immunohistochemistry.
Materials and methods
Animals
Male Wistar rats (10 weeks old) were purchased from Japan SLC, Inc. (Shizuoka, Japan). All animals were maintained in a temperature-controlled room (22 ± 2 °C) with automatically controlled lighting (light ON between 0600 and 1800 h, daily) and were supplied with food and water ad libitum. All animal experiments were conducted in compliance with the Guide for Care and Use of Laboratory Animals established by Teikyo University.
Immunohistochemistry
Immunohistochemistry was performed as previously described [27, 28]. Briefly, rats were perfused through the heart with 4 % paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The pituitary was removed and fixed by immersion in the same fixative solution for 1 day at 4 °C, dehydrated through a graded ethanol series, and embedded in paraffin. The sections (3-μm thick) were cut with a microtome (SM 2000 R, Leica Biosystems, Wetzlar, Germany) and placed on glass slides (PLATINUM PRO, Matsunami, Osaka, Japan). Sections were deparaffinized and underwent antigen retrieval by heating in an autoclave in 1 mM EDTA at 121 °C for 1 min. The sections were then incubated overnight at 25 °C with the antibodies described in Table 1 in phosphate-buffered saline (PBS) containing 1 % bovine serum albumin (BSA). After incubation with each primary antibody, the sections were washed with PBS, and then incubated with a solution of secondary antibody containing immunoglobulins specific to the animal hosts of primary antibodies and labeled with Cy3, Alexa Fluor 488, or Alexa Fluor 647 (Jackson Immunoresearch, PA, USA), and with 4′, 6-diamidino-2-phenylindole (DAPI; Dojindo, Kumamoto, Japan) for 2 h at 25 °C (Table 1). The sections were washed in PBS, mounted in PermaFluor (Thermo Fisher Scientific), and examined using a confocal laser scanning microscope system A1 (Nikon, Tokyo, Japan). The specificity of the all antibodies was checked by negative staining removed the antibody (data not shown). The far-red signals by Alexa Fluor 647 are indicated by yellow color in Figs. 1, 3, and 4, and by blue color in Fig. 5, to show four or three-components each with different colors in one image. We confirmed that these yellow signals were not the overlapping colors produced by mixing red and green colors.
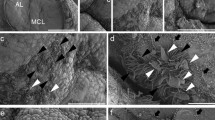
Immunohistochemical analysis of S100β-positive multiciliated cells in the rat pituitary. Low-magnification images of the rat pituitary where acetylated (Ac) α-tubulin (a red) and S100β (b green) were visualized with antibodies (a, b). Broken lines indicate the edge of Rathke’s cleft (RC). The single-cell layers facing Rathke’s cleft comprise the marginal cell layer (a–c). Acetylated α-tubulin (a) and S100β (b) signals are observed in the anterior lobe (AL), intermediate lobe (IL), and posterior lobe (PL). In the marginal cell layer, intense signals of acetylated α-tubulin are localized at the apical surface of S100β-positive cells (c arrows). Triple immunofluorescence images of acetylated α-tubulin (red), S100β (green), and γ-tubulin (yellow) in the marginal cell layer (d–g) and anterior lobe (h–k) are indicated. Motile cilia and basal bodies were labeled with anti-acetylated α-tubulin and anti-γ-tubulin antibodies, respectively. Motile cilia elongated from the basal bodies in S100β-positive cells (g, k). Arrows indicate the S100β-positive multiciliated cells. Nuclei were counterstained with DAPI (blue). Bars 100 μm (a–c), 10 μm (d–g, h–k)
In situ hybridization
In situ hybridization was carried out according to a method described previously [29, 30]. Briefly, digoxigenin (DIG)-labeled antisense and sense cRNA probes were prepared based on a partial rat Foxj1 cDNA sequence (GenBank accession no. NM_053832) inserted into pGEM-T vector (Promega, WI, USA) by in vitro transcription using T7 and SP6 RNA polymerases (Roche, Basel, Switzerland). The following primers were used for the amplification of cDNA fragment of rat Foxj1: forward, 5′-AGTATGCAGAACGCCTGCTC-3′; and reverse, 5′-TGCTTCAAAGAGGGGTTCTG-3′. Cryosections of the rat pituitary were digested in 0.5 μg/mL proteinase K solution for 5 min at 37 °C, postfixed in 4 % paraformaldehyde for 5 min, and incubated with DIG-labeled cRNA probe for 20 h at 55 °C. After hybridization, the sections were treated, 15 min each, at 55 °C in 4×, 2×, and 0.2× saline-sodium citrate buffer series, and then incubated with alkaline phosphatase-conjugated sheep anti-DIG Fab antibodies (1:1000; Roche) for 2 h at 25 °C. The label was visualized with nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolylphosphate (NBT/BCIP; Roche).
Results
In the present study, we examined the distribution of multiciliated cells in the rat pituitary employing immunohistochemistry with antibodies specific to acetylated α-tubulin, γ-tubulin, and S100β (Fig. 1). The acetylated α-tubulin and S100β signals were widely distributed in AL, IL, and PL (Fig. 1a). In MCL, intense acetylated α-tubulin signals were also detected on apical surfaces of S100β-positive cells along Rathke’s cleft (Fig. 1a–c), and acetylated α-tubulin/S100β double positive cells were observed more frequently on the IL side of MCL than on the AL side (Fig. 1a–c). Some S100β-positive cells in the MCL and ALFS (follicle structure in the parenchyma of AL) contained motile cilia that extended from the basal bodies (Fig. 1d–k). Motile cilia of S100β-positive multiciliated cells in ALFS and MCL elongated toward the central area of follicle structure composed of S100β-positive cells and Rathke’s cleft, respectively.
We investigated the expression of Foxj1 in the rat pituitary by in situ hybridization (Fig. 2a–d). We found Foxj1 signals in MCL (Fig. 2c) and parenchyma of AL (Fig. 2d). To determine the localization of FOXJ1 protein in the rat pituitary, we conducted immunohistochemical examination with anti-FOXJ1, anti-S100β, and anti-acetylated α-tubulin antibodies (Fig. 3). FOXJ1 signal was detected mainly in MCL (Fig. 3a–c). FOXJ1-expressing cells were observed abundantly on the IL side of MCL, compared with those on the AL side of MCL (Figs. 2a, 3a). We also noted that FOXJ1 signals were localized only in the nuclei of S100β-positive multiciliated cells in MCL (Fig. 3d–g) and ALFS (Fig. 3h–k).
Analysis of Foxj1 expression in the rat pituitary by in situ hybridization. Foxj1 signals (arrows) are detected in the marginal cell layer and anterior lobe (AL) in the rat pituitary (a). Foxj1 signal is not observed when the sections were treated with a DIG-labeled sense RNA probe (b). Broken lines indicate the edge of marginal cell layer facing Rathke’s cleft (RC a, b). Foxj1-expressing cells (arrows) exist in the marginal cell layer (c) and anterior lobe (d). Arrowheads point to the motile cilia. IL intermediate lobe, PL posterior lobe. Bars 100 μm (a, b), 10 μm (c, d)
Immunolocalization of FOXJ1 in the rat pituitary. Under low-magnification, FOXJ1 signals (red) are found around the marginal cell layer indicated by broken lines (a–c). Arrows indicate FOXJ1-positive cells (a–c). Triple immunofluorescence images of FOXJ1 (red), S100β (green), and acetylated (Ac) α-tubulin (yellow) in the marginal cell layer (d–g) and anterior lobe (AL h–k) are shown. FOXJ1 signals localize in the nucleus of S100β-positive multiciliated cells (g, k arrowheads). Nuclei were counterstained with DAPI (b–c, e–g, i–k blue). IL intermediate lobe, PL posterior lobe, RC Rathke’s cleft. Bars 50 μm (a–c), 10 μm (d–g, h–k)
To elucidate SOX2 expression in these cells, we used immunohistochemical approach with anti-SOX2, anti-S100β, and anti-acetylated α-tubulin antibodies (Fig. 4). SOX2 was localized in the nuclei of S100β-positive multiciliated cells in MCL (Fig. 4a, b) and ALFS (Fig. 4e–h). We also immunohistochemically examined the relationship between FOXJ1 and SOX2 in S100β-positive multiciliated cells of the pituitary (Fig. 5). We discovered that FOXJ1 and SOX2 colocalized in the nuclei of S100β-positive cells in MCL (Fig. 5a, b) and ALFS (Fig. 5e–h).
Immunolocalization of SOX2 in S100β-positive multiciliated cells of the rat pituitary. Triple immunofluorescence images of SOX2 (red), S100β (green), and acetylated (Ac) α-tubulin (yellow) in the marginal cell layer (a–d) and anterior lobe (e–h) are shown. SOX2 signals localize in the nucleus of S100β-positive cells in the marginal cell layer (a–d arrows) and anterior lobe (e–h arrows). Nuclei were counterstained with DAPI (b–d, f–h blue). IL intermediate lobe, RC Rathke’s cleft. Bar 10 μm
Colocalization of FOXJ1 and SOX2 in S100β-positive cells of the rat pituitary. Triple immunofluorescence images of FOXJ1 (red), SOX2 (blue), and S100β (green) in the marginal cell layer (a–d) and anterior lobe (e–h) are shown. Signals from the anti-FOXJ1 and anti-SOX2 antibodies colocalized in the nucleus of S100β-positive cells in the marginal cell layer (a–d arrows) and anterior lobe (e–h arrows). IL intermediate lobe, RC Rathke’s cleft. Bars 10 μm (a–d, e–h)
Discussion
Because specificity of antibodies against acetylated α-tubulin and γ-tubulin were already confirmed [27], we used these antibodies as the maker of cilia and basal body, respectively. Reliability of antibodies against S100β, FOXJ1, and SOX2 were previously reported [3, 31–33]. In the present study, we demonstrated that acetylated α-tubulin signals were localized at the apical surface of S100β-positive cells in MCL and ALFS. Because motile cilia in multiciliated cells generally consist of microtubules containing α-tubulin that is acetylated by α-tubulin-specific acetyltransferase 1 [27], it is considered that the acetylated α-tubulin/S100β double positive cells are in fact S100β-positive multiciliated cells in the pituitary. The motile cilia of multiciliated cells are involved not only in the circulation of extra cellular fluid or transport of substances, such as ovum [9], but also as cellular mechano- and chemo-sensors via a variety of receptors and channels present on these structures [10]. Water channel protein aquaporin 5 is distinctively localized on motile cilia in the MCL [34]; therefore, it is considered that the multiciliated cells in MCL transport water between Rathke’s cleft and interstitial fluid in the adenohypophysis.
Previous reports have demonstrated that FOXJ1 is localized at the nuclei of multiciliated cells of the trachea, bronchioles, brain, choroid plexus, and oviduct [17–21], and is involved in the modulation of expression of genes encoding ciliation-associated functions, such as intraflagellar transport proteins, tubulins and tubulin-modifying enzymes [26, 31, 35]. Deletion of Foxj1 in mice results in a failure of basal bodies to localize at the apical cell surface of multiciliated cells [31]. In this study, we elucidated the expression of Foxj1 gene and localization of FOXJ1 protein in S100β-positive multiciliated cells in the MCL and ALFS. Thus, it is considered that FOXJ1 is essential for motile cilia formation in S100β-positive multiciliated cells in the pituitary. Using immunohistochemistry, we also discovered that S100β/SOX2/FOXJ1 triple positive multiciliated cells were scattered throughout MCL and ALFS. Because MCL and ALFS were composed of S100β/SOX2 double positive non-ciliated cells and S100β/SOX2/FOXJ1 triple positive multiciliated cells, it is considered that S100β- and SOX2-positive cells are not uniform but heterogeneous.
Jacquet et al. [31] reported that FOXJ1 was present in the subventricular zone, a stem cell niche in the adult mouse brain, and was required for the differentiation of radial glia, one type of the neural stem cells, into ependymal cells. Moreover, FOXJ1 and SOX2 are also co-expressed in ependymal cells, and brain-specific deletion of Sox2 resulted in ciliary loss in ependymal cells in mice [31, 36]. SOX2 also promotes the expression of FOXJ1 in Clara cells, progenitor cells in the conducting airway; thus, it is thought that SOX2 regulates the differentiation of Clara cells into multiciliated cells together with FOXJ1 [37, 38]. Therefore, co-expression of FOXJ1 and SOX2 is probably important for the differentiation and maintenance of S100β-positive multiciliated cells in the pituitary. Taken together, we found that the MCL and ALFS in the pituitary were consisting of morphologically and functionally distinct two types of cells, i.e., S100β/SOX2 double positive non-ciliated cells and S100β/SOX2/FOXJ1 triple positive multiciliated cells. To identify the cellular function of these cells, further physiological investigation using knockout animals will be valid to reveal whether knockout of individual factors affect differentiation of these cells in the pituitary.
References
Shirasawa N, Kihara H, Yamaguchi S, Yoshimura F (1983) Pituitary folliculo-stellate cells immunostained with S-100 protein antiserum in postnatal, castrated and thyroidectomized rats. Cell Tissue Res 231:235–249
Soji T, Sirasawa N, Kurono C, Yashiro T, Herbert DC (1994) Immunohistochemical study of the post-natal development of the folliculo-stellate cells in the rat anterior pituitary gland. Tissue Cell 26:1–8
Yoshida S, Kato T, Yako H, Susa T, Cai LY, Osuna M, Inoue K, Kato Y (2011) Significant quantitative and qualitative transition in pituitary stem/progenitor cells occurs during the postnatal development of the rat anterior pituitary. J Neuroendocrinol 23:933–943
Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC (2008) SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci USA 105:2907–2912
Allaerts W, Vankelecom H (2005) History and perspectives of pituitary folliculo-stellate cell research. Eur J Endocrinol 153:1–12
Höfler H, Denk H, Walter GF (1984) Immunohistochemical demonstration of cytokeratins in endocrine cells of the human pituitary gland and in pituitary adenomas. Virchows Arch A Pathol Anat Histopathol 404:359–368
Allaerts W, Fluitsma DM, Hoefsmit EC, Jeucken PH, Morreau H, Bosman FT, Drexhage HA (1996) Immunohistochemical, morphological and ultrastructural resemblance between dendritic cells and folliculo-stellate cells in normal human and rat anterior pituitaries. J Neuroendocrinol 8:17–29
Yoshida S, Kato T, Kato Y (2016) Regulatory system for stem/progenitor cell niches in the adult rodent pituitary. Int J Mol Sci 17:75
Choksi SP, Lauter G, Swoboda P, Roy S (2014) Switching on cilia: transcriptional networks regulating ciliogenesis. Development 141:1427–1441
Bloodgood RA (2010) Sensory reception is an attribute of both primary cilia and motile cilia. J Cell Sci 123:505–509
Correr S, Motta PM (1981) The rat pituitary cleft: a correlated study by scanning and transmission electron microscopy. Cell Tissue Res 215:515–529
Yoshimura F, Soji T, Kiguchi Y (1977) Relationship between the follicular cells and marginal layer cells of the anterior pituitary. Endocrinol Jpn 24:301–305
Ciocca D (1980) Scanning electron microscopy of the cleft of the rat pituitary. Cell Tissue Res 206:139–143
Correr S, Motta PM (1985) A scanning electron-microscopic study of “supramarginal cells” in the pituitary cleft of the rat. Cell Tissue Res 241:275–281
Hannenhalli S, Kaestner KH (2009) The evolution of Fox genes and their role in development and disease. Nat Rev Genet 10:233–240
Lam EWF, Brosens JJ, Gomes AR, Koo C-Y (2013) Forkhead box proteins: tuning forks for transcriptional harmony. Nat Rev Cancer 13:482–495
Okada A, Ohta Y, Brody S, Watanabe H, Krust A, Chambon P, Iguchi T (2004) Role of foxj1 and estrogen receptor alpha in ciliated epithelial cell differentiation of the neonatal oviduct. J Mol Endocrinol 32:615–625
Lim L, Zhou H, Costa RH (1997) The winged helix transcription factor HFH-4 is expressed during choroid plexus epithelial development in the mouse embryo. Proc Natl Acad Sci USA 94:3094–3099
Pelletier GJ, Brody SL, Liapis H, White RA, Hackett BP (1998) A human forkhead/winged-helix transcription factor expressed in developing pulmonary and renal epithelium. Am J Physiol 274:L351–L359
Blatt EN, Yan XH, Wuerffel MK, Hamilos DL, Brody SL (1999) Forkhead transcription factor HFH-4 expression is temporally related to ciliogenesis. Am J Respir Cell Mol Biol 21:168–176
Tichelaar JW, Wert SE, Costa RH, Kimura S, Whitsett JA (1999) HNF-3/forkhead homologue-4 (HFH-4) is expressed in ciliated epithelial cells in the developing mouse lung. J Histochem Cytochem 47:823–831
Yu X, Ng CP, Habacher H, Roy S (2008) Foxj1 transcription factors are master regulators of the motile ciliogenic program. Nat Genet 40:1445–1453
Tichelaar JW, Lim L, Costa RH, Whitsett JA (1999) HNF-3/forkhead homologue-4 influences lung morphogenesis and respiratory epithelial cell differentiation in vivo. Dev Biol 213:405–417
Brody SL, Yan XH, Wuerffel MK, Song S-K, Shapiro SD (2000) Ciliogenesis and left–right axis defects in forkhead factor HFH-4—null mice. Am J Respir Cell Mol Biol 23:45–51
Chen J, Knowles HJ, Hebert JL, Hackett BP (1998) Mutation of the mouse hepatocyte nuclear factor/forkhead homologue 4 gene results in an absence of cilia and random left-right asymmetry. J Clin Invest 102:1077–1082
Stubbs JL, Oishi I, Izpisua Belmonte JC, Kintner C (2008) The forkhead protein Foxj1 specifies node-like cilia in Xenopus and zebrafish embryos. Nat Genet 40:1454–1460
Nakakura T, Suzuki T, Nemoto T, Tanaka H, Asano-Hoshino A, Arisawa K, Nishijima Y, Kiuchi Y, Hagiwara H (2016) Intracellular localization of α-tubulin acetyltransferase ATAT1 in rat ciliated cells. Med Mol Morphol 49:133–143
Nakakura T, Sato M, Suzuki M, Hatano O, Takemori H, Taniguchi Y, Minoshima Y, Tanaka S (2009) The spatial and temporal expression of delta-like protein 1 in the rat pituitary gland during development. Histochem Cell Biol 131:141–153
Fujiwara K, Maekawa F, Kikuchi M, Takigami S, Yada T, Yashiro T (2007) Expression of retinaldehyde dehydrogenase (RALDH)2 and RALDH3 but not RALDH1 in the developing anterior pituitary glands of rats. Cell Tissue Res 328:129–135
Nakakura T, Suzuki M, Watanabe Y, Tanaka S (2007) Possible involvement of brain-derived neurotrophic factor (BDNF) in the innervation of dopaminergic neurons from the rat periventricular nucleus to the pars intermedia. Zool Sci 24:1086–1093
Jacquet BV, Salinas-Mondragon R, Liang H, Therit B, Buie JD, Dykstra M, Campbell K, Ostrowski LE, Brody SL, Ghashghaei HT (2009) FoxJ1-dependent gene expression is required for differentiation of radial glia into ependymal cells and a subset of astrocytes in the postnatal brain. Development 136:4021–4031
Nakakura T, Soda A, Unno K, Suzuki M, Tanaka S (2010) Expression of IGFBP7 mRNA in corticotrophs in the anterior pituitary of adrenalectomized rats. J Histochem Cytochem 58:969–978
Horiguchi K, Higuchi M, Yoshida S, Nakakura T, Tateno K, Hasegawa R, Takigami S, Ohsako S, Kato T, Kato Y (2014) Proton receptor GPR68 expression in dendritic-cell-like S100β-positive cells of rat anterior pituitary gland: GPR68 induces interleukin-6 gene expression in extracellular acidification. Cell Tissue Res 358:515–525
Matsuzaki T, Inahata Y, Sawai N, Yang CY, Kobayashi M, Takata K, Ozawa H (2011) Immunohistochemical localization of the water channels AQP4 and AQP5 in the rat pituitary gland. Acta Histochem Cytochem 44:259–266
Didon L, Zwick RK, Chao IW, Walters MS, Wang R, Hackett NR, Crystal RG (2013) RFX3 modulation of FOXJ1 regulation of cilia genes in the human airway epithelium. Respir Res 14:70
Ferri ALM, Cavallaro M, Braida D, Di Cristofano A, Canta A, Vezzani A, Ottolenghi S, Pandolfi PP, Sala M, DeBiasi S, Nicolis SK (2004) Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development 131:3805–3819
Tompkins DH, Besnard V, Lange AW, Wert SE, Keiser AR, Smith AN, Lang R, Whitsett JA (2009) Sox2 is required for maintenance and differentiation of bronchiolar clara, ciliated, and goblet cells. PLoS One 4:e8248
Tompkins DH, Besnard V, Lange AW, Keiser AR, Wert SE, Bruno MD, Whitsett JA (2011) Sox2 activates cell proliferation and differentiation in the respiratory epithelium. Am J Respir Cell Mol Biol 45:101–110
Acknowledgments
We are grateful to Ms. Yuri Amakawa at Teikyo University for useful technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported in part by a Grant-in-Aid for Young Scientists (B) (16K18982) to T. N. from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by a research Grant from Teikyo University School of Medicine.
Conflict of interest
The authors declare no conflict of interest that might prejudice the impartiality of this research.
Rights and permissions
About this article
Cite this article
Nakakura, T., Suzuki, T., Horiguchi, K. et al. Expression and localization of forkhead box protein FOXJ1 in S100β-positive multiciliated cells of the rat pituitary. Med Mol Morphol 50, 59–67 (2017). https://doi.org/10.1007/s00795-016-0148-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-016-0148-1