Abstract
An analysis of secreted proteins by the signal sequence trap method using a cDNA library of the rat pituitary anlage at embryonic days (E) 13.5 revealed the abundant expression of delta-like protein 1 (Dlk1) in the pituitary gland. Dlk1, an epidermal growth factor-like repeat protein in preadipocytes, functions in maintaining the preadipose state. Expression of Dlk1 mRNA in the pituitary at E13.5 and in the adult pituitary was confirmed by in situ hybridization. The expression pattern of Dlk1 during pituitary development was also studied by immunohistochemistry. Dlk1 protein first appeared in Rathke’s pouch and the infundibulum at E11.5; as development proceeded, expression became restricted to the pars distalis and pars tuberalis (PT). Dlk1 was expressed in most ACTH cells during the embryonic stages, but its expression was limited to only a few ACTH cells in the adult pituitary. It was also expressed in a small population of TSH, GTH, and PRL cells throughout development, whereas it was present in the cytoplasm of most GH cells at all developmental stages. Similarly, Dlk1 was localized in the cytoplasm of PT cells during development. These findings provide new insights into the mechanism of Dlk1 regarding its regulation of pituitary hormone-secreting cells during development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The hypothalamic pituitary axis plays a pivotal role in the integrative response of vertebrate endocrine systems to various environment stimuli. Neurohypophyseal hormones are directly secreted from the hypothalamic neurons, whereas hormones in the PD are regulated by hypothalamic neurohormones that are produced in the hypothalamic area and transported to the PD via portal vessels. The PD is vascularized by hypophysial portal vessels that arise from the capillary beds in the median eminence of the hypothalamus (Murakami et al. 1987), and this hypophyseal portal system functions as an important link by carrying hormonal information from the central nervous system to the pituitary. The pars intermedia (PI), on the other hand, is under the direct control of dopaminergic (DA) neurons arising in the periventricular nucleus (PeV) and arcuate nucleus in the hypothalamus (Goudreau et al. 1992, 1995). We previously reported that vascular endothelial growth factor-A is involved in the formation of the primary capillaries and in the vascularization of the PD (Nakakura et al. 2006), and that brain-derived neurotrophic factor plays a role directing the elongation of DA neurons from the PeV to the PI (Nakakura et al. 2007). However, it has yet to be fully clarified how molecular and cellular mechanisms are involved in the architecture of the vascular system and neuronal network in the hypothalamus pituitary axis.
In the study reported here, we extensively analyzed secreted proteins by the signal sequence trap method (Kitamura et al. 1995; Kojima and Kitamura 1999) using the cDNA library of the rat pituitary anlage on embryonic day (E) 13.5, when the formation of the portal vessels starts (Nakakura et al. 2006). We cloned a number of delta-like protein 1 (Dlk1) cDNAs that have been previously reported by Hansen et al. (1998). Dlk1, also referred to as preadipocyte factor 1 (pref-1; Smas and Sul 1993) or zona glomerulosa-specific factor (ZOG; Okamoto et al. 1997), is a type I membrane protein that has six epidermal growth factor (EGF)-like repeats in the extracellular domain. It functions either as a membrane protein (Dlk1) or a soluble protein (FA1) that is generated through the proteolytic cleavage of the Dlk1 by a disintegrin and metalloproteinase 17 (ADAM17)/tumor necrosis factor-alpha converting enzyme (TACE) (Friedrichsen et al. 2003; Jensen et al. 1994; Smas et al. 1997; Wang and Sul 2006).
Dlk1 is mainly expressed in the preadipocytes where its function is to maintain the preadipocyte state (Smas and Sul 1993; Smas et al. 1997, 1998). At early developmental stages, Dlk1 is expressed in the insulin-producing cells of the human and rat pancreas (Carlsson et al. 1997; Tornehave et al. 1993), Schwann cells of the mouse peripheral nervous system (Costaglioli et al. 2001), and hepatoblasts of the mouse liver (Tanimizu et al. 2003), but the mechanism of its function and regulation in these tissues is still unknown. The results of several studies have indicated that Dlk1 regulates the proliferation of hematopoietic stem cells (Moore et al. 1997) and thymocytes (Kaneta et al. 2000). It would therefore appear that Dlk1 is involved in the regulation of the timing of cell differentiation or proliferation.
In the pituitary, Dlk1 has been reported to be expressed in growth hormone (GH) cells and to regulate GH mRNA transcription (Ansell et al. 2007; Floridon et al. 2000; Hedlund et al. 2003; Larsen et al. 1996; Yevtodiyenko and Schmidt 2006). However, its function in the other pituitary hormone-secreting cells is unknown. We therefore investigated the spatial and temporal expression of Dlk1 in the rat pituitary glands during development by in situ hybridization (ISH) and immunohistochemistry to define the function of Dlk1 in the pituitary gland.
Materials and methods
Animals
All animal experiments were conducted in compliance with the Guide for Care and Use of Laboratory Animals of Shizuoka University. Normal adult Wistar rats were housed in a temperature-controlled room (22 ± 2°C) with automatically regulated artificial lighting (07:00–19:00 hours daily). Food and water were supplied ad libitum. Mature rats were mated at night. If spermatozoa were found in vaginal smears taken the next morning, that day was designated as day 0.5 of gestation. Pregnant rats were decapitated on days 10.5–19.5 of gestation, and adult rats (8 weeks) were decapitated and prepared for ISH and immunohistochemical examination. Rat pituitary anlages at E13.5 were used to isolate mRNA for the signal sequence trap method.
Signal sequence trap method
To identify cDNAs encoding both secreted and membrane proteins from the rat pituitary anlage at E13.5, we constructed a cDNA library from the rat pituitary anlage at E13.5, and screened it using the signal sequence trap method (Kojima and Kitamura 1999). Briefly, poly-A+-RNA was isolated from the rat pituitary anlages at E13.5 using the FastTrack 2.0 kit (Invitrogen, Carlsbdad, CA, USA) according to the manufacturer’s instructions. The poly-A+-RNA was amplified using the BD SMARTTM mRNA Amplification Kit (BD Biosciences, San Jose, CA, USA). Complementary DNA (cDNA) was synthesized from sense RNA with random hexamers as primers using a SuperScriptTM Double-Stranded cDNA Synthesis kit (Invitrogen) and then inserted into the BstXI sites of the pMXs-SST vector (Kojima and Kitamura 1999) using BstXI adapters (Invitrogen). The ligated DNA was then introduced into ElectroMAXTM DH10BTM cells (Invitrogen) and the plasmid DNA was prepared using a QIAGEN Plasmid Maxi Kit (QIAGEN, Hilden, Germany). High-titer retroviruses representing the SST-REX library were produced using PlatE cells (Kojima and Kitamura 1999) and infected into Ba/F3 cells as previously described (Kitamura et al. 1995). One day after the infection, we started selecting factor-independent Ba/F3 cells in the absence of IL-3 using 96-well plates. Integrated cDNA was isolated from factor-independent Ba/F3 clones by genomic PCR using PrimeStar polymerase (Takara, Shiga, Japan). The PCR fragments were labeled by Dye Terminator Cycle Sequencing with a CEQ2000 Quick Star Kit (Beckman Coulter, Fullerton, CA, USA) and analyzed on an automatic sequencer model (CEQ2000; Beckman Coulter).
In situ hybridization
Digoxigenin (DIG)-labeled antisense and sense cRNA probes were prepared from the coding region of rat Dlk1 cDNA (accession no. D84336) by in vitro transcription, as described previously (Saito et al. 2002). The following primers were commercially synthesized (Operon, Tokyo, Japan): primer 1, 5′-AGCATGGATTCTGTGAGGCT-3′ (233–252 bp); primer 2, 5′-ATTCGTACTGGCTTTCTCGA-3′ (452–472 bp). For fixation, 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB) (pH 7.4) was perfused through the umbilical vein of the fetal rats. Following perfusion, the rats were decapitated and the heads fixed by immersion in the same fixative solution for 2 days at 4°C. Adult rats were perfused through the heart and their pituitary glands were subsequently excised fixed by immersion in the same fixative for 2 days, then dehydrated through a graded ethanol series, and embedded in Paraplast. The embedded tissue was cut into 4 μm-thick sections and mounted on silane-coated slides. In situ hybridization was carried out according to a method described previously (Saito et al. 2002). Briefly, the deparaffinized sections were digested with 5 μg/ml proteinase K for 20 min at 37°C, postfixed in 4% PFA for 20 min at 4°C, and then incubated with the DIG-labeled cRNA probe at 40°C for 15 h. After hybridization, the sections were treated with 1 μg/ml RNase A solution for 30 min at 37°C and then incubated with alkaline phosphatase-conjugated sheep anti-DIG Fab antibody (1:1,000; Roche Molecular Biochemical, Meylan, France) for 15 h at room temperature. The label was detected with nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolylphosphate (NBT/BCIP; Roche).
Preparation and immunization of antigen
To prepare the bovine Dlk1 protein, we performed PCR using primers 1 (5′-TTCGGATCCAGTGCCCATGGAGCTGAA-3′) and 2 (5′-GGTGAATTCGTTGAGGCACGGCTCGCT-3′) to cover the 20–219 amino acid residues of bovine Dlk1 (accession no. AB009278). After the PCR, the fragment obtained was treated by EcoRI and BamHI and inserted into pGEX-2T (Pharmacia Biotech, Piscataway, NJ, USA). The plasmid DNA was transformed into Escherichia coli BL21, and glutathione-S-transferase (GST)-Dlk1 fused protein was expressed. The fused protein was purified by using GST–sepharose. For immunization, chickens were injected with the antigen every 2 weeks for 9 weeks by the multiple sites method.
Western blot analysis
The GST–Dlk fusion-protein (10 ng) used as an immunogen was denatured at 100°C for 3 min in denaturation buffer comprising 3% sodium dodecyl sulphate, 70 mM Tris–HCl, pH 6.8, 11.2% glycerol, 5% 2-mercaptoethanol, and 0.01% bromophenol blue, subjected to electrophoresis on a 10% polyacrylamide gel, and then transferred to an Immobilon-P membrane (Millipore, Tokyo, Japan). The proteins on the membrane were reacted sequentially with chicken anti-bovine Dlk1 (1:5,000) serum, biotinylated donkey anti-chicken IgY (Jackson Immunoresearch, West Grove, PA, USA), and streptavidin-conjugated horseradish peroxidase (DAKO Japan Co., Ltd, Kyoto, Japan). The reaction products on the membrane were then visualized using an ECL Western blot detection kit (Amersham Pharmacia Botech, Buckinghamshire, UK). To check the specificity of the immunoreaction, we performed an absorption test by preincubating anti-bovine Dlk1 with the antigen protein (10 μg/ml).
Immunofluorescence method
Fetal rats were perfused through the umbilical vein with 4% PFA in 0.1 M PB (pH 7.4), and adult rats (8 weeks) were perfused through the heart with 4% PFA in 0.1 M PB (pH 7.4). Following perfusion, the rat heads and pituitary were removed and fixed by immersion in the same fixative solution for 2 days at 4°C, dehydrated through a graded ethanol series, and embedded in Paraplast. The section were then deparaffinized and rinsed with distilled water and phosphate-buffered saline (PBS). For staining of Dlk1, the sections were then treated for antigen retrieval by heating in a retrieval solution (1 mM EDTA in Milli-Q) at 120°C for 3 min in an autoclave (Sanyo, Osaka, Japan), washed three times with PBS, blocked with 1% BSA–PBS for 1 h, and immunolabeled by the immunofluorescence method as described previously (Tanaka et al. 1997). The sections were incubated overnight with chicken anti-bovine Dlk1 (1:2,000) and guinea pig anti-amidated joining peptide (JP) (ST-3; 1:2,000; Tanaka and Kurosumi 1992), goat anti-human thyroid stimulating hormone β (TSHβ; 1:2,000; Biogenesis, New Fields, UK), rabbit anti-rat TSH (1:2,000; supplied by Prof. K. Wakabayashi), rabbit anti-rat prolactin (PRL; 1:1,000; Kato et al. 2004), guinea pig anti-human GH (1:2,000; Kato et al. 2004), mouse monoclonal antibody against ovine luteinizing hormone β (LHβ; 1:1,000; Uehara et al. 2001), or mouse monoclonal antibody against S-100 protein (Immuno Biological Laboratories, Fujioka, Japan), followed by a 2-h incubation with indocarbocyanine (Cy3)-labeled affinity-purified donkey anti-chicken IgY (1:400; Jackson Immunoresearch), fluorescein isothiocyanate (FITC)-labeled donkey anti-guinea pig IgG (1:400; Jackson), FITC-labeled donkey anti-mouse IgG (1:400; Jackson), Alexa 488-donkey anti-rabbit IgG (1:200; Molecular Probes, Eugene, OR, USA) or Alexa 488-donkey anti-goat IgG (1:200; Molecular Probes). For nuclear counterstaining, 4′, 6-diamidino-2-phenylindole (DAPI) was included with the secondary antibody solution. The sections were washed with PBS, mounted in Permafluor (Immunon, Pittsburgh, PA, USA), and examined with an Olympus BX61 microscope equipped with a BX-epifluorescence attachment (Olympus Optical, Tokyo, Japan). The specificity of the immunostaining was checked using an absorption test by preincubating the anti-Dlk1 antiserum with the antigen protein (4 μg/ml).
Results
Identification of secreted protein expressed in the rat E13.5 pituitary by the signal sequence trap method
To identify signal molecules involved in the formation of the pituitary portal vessels in the hypothalamus pituitary axis, we extensively analyzed secreted proteins expressed in the rat pituitary anlage at E13.5 by the signal sequence trap method. The results of this experiments revealed that Dlk1 was abundantly present in the cDNA library of the pituitary at E13.5 (Table 1). Clones encoding collagen type II, collagen α1 type XVII, prolyl 4-hydroxylase, follistatin-like 1, and 120-kDa lysosomal membrane glycoprotein were also identified.
Expression of Dlk1 mRNA in the pituitary at E13.5 and adult stage
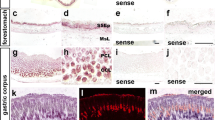
The Dlk1-expressing cells in the developing and adult rat pituitary were identified by ISH (Fig. 1). Dlk1 mRNA was expressed in the upper part of the Rathke’s pouch and in the infundibulum at E13.5 (Fig. 1a). Similarly, following the hybridization of adult pituitary sections with the antisense probe for Dlk1, we observed a large number of positive cells in the PD, but no positive cells were found in the PI and PN (Fig. 1c). In contrast, Dlk1 mRNA was intensely detected in the cells of the PT (Fig. 1e). No mRNA signal was detected in any parts of the pituitary glands on E13.5 or in those of adult rats when the sense probe was employed in the ISH (Fig. 1b, d, f).
In situ hybridization images of Dlk1 in the rat pituitary. At embryonic day (E)13.5, expression of Dlk1 mRNA is observed in the upper part of the primodial pars distalis (PD), pars intermedia (PI), and pars nervosa (PN) a. No detectable signal of Dlk1 mRNA is present in any regions with the sense probe at E13.5 b. An intense signal of Dlk1 mRNA is detected in the PD, but not in the PI and PN of the adult pituitary c. With the sense probe, no signal is visible in any region of the adult PD d. In the pars tuberalis (PT), a similar positive signal is seen (e), but no detectable signal is observed in the PT of the adult pituitary using sense probes (f). III: The third ventricle. Bar in a–f, 50 μm
Characterization of anti-Dlk1 antibody
Western blot analysis using the fused-proteins revealed a positive band at 56.8 kDa, which was the fusion-protein comprising the Dlk1 protein (20–219 amino acid residues) linked to the trombin cutting site (21.75 kDa) and GST (35 kDa) (Fig. 2, lane 1). When the antibody was preabsorbed with the immunogen, no positive band was observed in any region (Fig. 2, lane 2).
Immunolocalization of Dlk1 protein during development
When pituitary sections at E13.5 were immunolabeled for Dlk1, labeling was observed in the upper part of the PD and PI anlages and in the infundibulum (Fig. 3a, b). The immunopositive labeling was abolished when the antiserum was preabsorbed with the antigen (Fig. 3c). Intense labeling was observed throughout the cytoplasm of the cells constituting the primordial PD and PT (Fig. 3d). As shown in Fig. 3, the pattern of immunolabeling for Dlk1 changed drastically during development. Dlk1 protein was not detected in Rathke’s pouch and infundibulum at E10.5 (Fig. 4a). The first expression of Dlk1 protein was observed on E11.5 in the region, that links the Rathke’s pouch with the infundibulum (Fig. 4b) and on E12.5 in the same region (Fig. 4c). From E13.5 to E14.5, Dlk1 protein was found in the upper part of PD, PI, and PN (Figs. 3a, 4d). On E15.5, Dlk1 protein was observed in the anlage of pars tuberalis (PT) in addition to the upper parts of the PD, PI, and PN (Fig. 4e). On E19.5, the number of Dlk1-positive cells had increased in the PD and PT, while it had decreased in the PI and PN (Fig. 4f).
Immunofluorescence localization of Dlk1 in the rat pituitary at E13.5. Imunofluorescence images of Dlk1 (a, c, d) and the corresponding Nomarski image (b) are shown. The labels (red) are clearly visible in the upper part of the primordial pars distalis (PD), pars intermedia (PI) and pars nervosa (PN). No labeling is detected in any cells of the pituitary when the anti-Dlk1 was preabsorbed with the corresponding antigen (c). Enlarged view of Dlk1-positive cells (red) in the upper part of the primordial pituitary anlage (d). III: The third ventricle nuclei are counterstained with DAPI (blue). Bars in a–c, 50 μm, Bar in d, 10 μm
Immunolocalization of Dlk1 in the rat pituitary gland during development. No positive label is seen in Rathke’s pouch (R) and infundibulum (I) at embryonic days (E) 10.5 (a). The labels for Dlk1 (red) are observed in Rathke’s pouch and the inner and outer lines along the third ventricle side (III) of an infundibulum at E11.5 (b). Similar localizations (red) are visible at E12.5 (c). At E14.5, the labels (red) are detected in the upper part of the primordial pars distalis (PD), pars intermedia (PI) and pars nervosa (PN) at E14.5 (d). The labels for Dlk1 (red) are observed in all parts of pituitary at E15.5 (e). At E19.5, the labels (red) are in the PD and PT, but no labels are visible in the PI and PN (f). Nuclei are counterstained with DAPI (blue). Bars in a–e, 50 μm, Bar in f, 100 μm
In a subsequent experiment, Dlk1-expressing cells in the developing and the adult pituitary were identified by double-labeling for Dlk1 protein and pituitary hormones. On E15.5, adenocorticotropic hormone (ACTH) cells, which were identified using anti-amidated JP, first appeared in the ventral–dorsal region of the PD, and Dlk1 protein was expressed in the cytoplasm of most ACTH cells (Fig. 5a–c). At the E18.5 the number of ACTH cells had increased in the PD, but the number of Dlk1-positive ACTH cells had decreased slightly (Fig. 5d–f). In the adult PD, the number of Dlk1-positive ACTH cells decreased still further, and Dlk1 protein was observable only as a dot-like signal only in several ACTH cells (Fig. 5g–i). During the development of the PD, TSH cells, GTH cells (identified by anti-LHβ), and PRL cells first appeared on E17.5, E18.5, and E20.5, respectively (Watanabe and Daikoku 1979). In this study, we focused on the cell types that were the first to appear during the development of the PD. At the time when the TSH cells, GTH cells, and PRL cells first appeared, labels for Dlk1 were found in the Golgi region of these cells (Fig. 6a–c, g–i, m–o). These cells continued to display similar immunolabeling behavior until the adult stage (Fig. 6d–f, j–l, p–r). In most GH cells in the PD, which first appeared at E19.5, Dlk1 protein was expressed throughout the cytoplasm at both the embryonic and adult stages (Fig. 7a–f).
Immunolocalization of Dlk1 in ACTH cells of the pars distalis (PD) during development. Double-immunofluorescence images for Dlk1 (red) and ACTH (green) at E15.5 (a–c), E18.5 (d–f), and the adult PD (g–i) are shown. The labels for ACTH are first visible in the Dlk1-positive cells at E15.5. At E18.5 and in the adult pituitary, the labels for Dlk1 are observed in the cytoplasm at E15.5 and in the Golgi region at the adult stage. The arrows indicate the same cells. Bar in a–f, 50 μm, Bar in g–i, 50 μm
Immunolocalization of Dlk1 in TSH cells, GTH cells, and PRL cells during development. Double-immunofluorescence images for Dlk1 (red), TSH (green), LH (green), and PRL (green) are shown. Labels for Dlk1 are visible in a small number of each type of hormone-secreting cell: TSH cells at E17.5 (a–c) and the adult (d–f), GTH cells at E18.5 (g–i) and the adulthood (j–l), and PRL cells at E20.5 (m–o) and the adult (p–r). The labels for Dlk1 are seen as a spot-like fashion in the cytoplasm of all the hormone-secreting cells. The arrows show the same cells. Bar in a–c, g–i, and m–o, 50 μm, Bar in d–f, j–l, and p–r, 10 μm
Immunolocalization of Dlk1 in GH cells during development. Double-immunofluorescence images for Dlk1 (red) and GH (green) in the pars distalis at E 19.5 (a–c) and the adult (d–f) are shown. Labels for Dlk1 are visible throughout the cytoplasm of the GH cells. The arrows indicate the same cells. Bar in a–c, 50 μm, and bar in d–f, 10 μm
In the PT, the expression of Dlk1 protein first appeared in the cytoplasm of cells constituting of the PT on E15.5 (Fig. 8a) and Dlk1 protein continued to be expressed in most of the cells of the PT at later embryonic stages (Fig. 8b–c). Dlk1 protein was predominately observed in TSH cells on E19.5 (Fig. 9a–c). In the adult PT, Dlk1 protein was localized in the cytoplasm of several PT cells (Fig. 9d). These Dlk1-expressing cells were identified as TSH cells and immunonegative cells with a little cytoplasm (Fig. 9d–f). However, folliculo-stellate cells, which were identified with anti-S-100 protein, were not labeled with anti-Dlk1 in the adult PT (data not shown).
Immunolocalization of Dlk1 in TSH cells of the pars tuberalis (PT) at different developmental stages. Double-immunofluorescence images for Dlk1 (red) and TSH (green) are shown. At E19.5, the labels for Dlk1 are observed in the cytoplasm of the immunopositive TSH cells in the PT (a–c; arrows). The labels for Dlk1 are visible in the immunopositive TSH cells (arrows) and non-immunopositive cells (arrowheads) of the adult PT (d–f). Bar in a–f, 10 μm
Discussion
It is well known that many different secreted proteins and transcription factors are involved in the differentiation of pituitary hormone-secreting cells (Zhu et al. 2007) and the architecture of the vascular system and neuronal network of pituitary glands (Nakakura et al. 2006, 2007). Our analysis of secreted protein expressed in the rat pituitary anlage on E13.5, when the portal vessels first appear in the Atwell’s recess (Nakakura et al. 2006), by the signal sequence trap method resulted in the identification of 152 clones containing genes encoding Dlk1 from among 550-positive clones. Dlk1 is a type I membrane protein that has six EGF-like repeats in its extracellular domain, is presumed to function in the regulation of the development of pituitary hormone-secreting cells. Knowledge of the spatial and temporal expression of Dlk1 in the pituitary gland is important in term of defining the function of Dlk1.
We first revealed that Dlk1 mRNA and protein were expressed in the rat pituitary anlage at E13.5 and in the PD and PT at adult pituitary. These positive signals were not detected when the sense probe was used in ISH, or when the antiserum was preabsorbed with the immunogen. We detected a positive band corresponding to the GST Dlk-fusion protein when the fusion protein was used in the Western blot analysis. However, we were not able to detect a specific band of Dlk1 or FA1 protein in the adult rat pituitary extracts by Western blot analysis. The detection of Dlk1 fusion-protein by Western blot analysis has been reported in a number of publications (Kaneta et al. 2000; Halder et al. 1998; Mei et al. 2002), but failure to detect its difficulty of extracting Dlk1 protein from rat tissues. The antibody used in this study is considered to be specific to the Dlk protein.
Our immunohistochemical results demonstrated that the expression pattern of Dlk1 varied depending upon the developmental stage. The adult pituitary gland is divided into four parts: the PD, PI, PN, and PT, with each part forming a particular compartment for regulating the secretion of the pituitary hormones. We found that the expression pattern of Dlk1 changed during the development of the pituitary gland: it was initially localized to from the upper parts of Rathke’s pouch during the early developmental stages, but then was restricted to the PD and the PT as the formation of the pituitary gland proceeded. Similar findings have been reported on the development of the liver or pancreas: Dlk1 mRNA levels were found to be higher in hepatoblasts or hepatocytes during the early developmental stages (E12.5–E16.5) and to decrease gradually from E18.5 to the adult stage (Tanimizu et al. 2003). Tanimizu et al. (2004) reported that Dlk1 was expressed in oval cells, which are adult hepatic progenitors, in the rat 2-acetylaminofluorene/partial hepatectomy model and that Dlk1 levels continued to be high 18 days after the operation, disappearing at 28 days post-surgery. Carlsson et al. (1997) found that most of the pancreatic anlage was Dlk 1 positive at an early embryonic stage (E13), becoming predominantly restricted to the insulin-producing cells during development. Therefore, our finding on the development of pituitary suggests that Dlk1 plays a role in the early formation and cell differentiation of pituitary glands.
In our study, Dlk1 was expressed in all the anterior pituitary hormone-producing cells during all developmental stage of the pituitary gland. In the other hormone-producing cells with the exception of GH cells, Dlk1 expression was observed predominately during the early stage of differentiation of each cell type. The Notch signaling pathway has been indicated in the formation and differentiation of anterior pituitary hormone-producing cells. Additional targets of this pathway include a member of the Hairy enhancer of split (Hes) family (Zhu et al. 2007). Cell differentiation of ACTH cells and gene expression of proopiomelanocortin (POMC) in the anterior pituitary are stimulated by Tpit/Tbx19 and NeuroD1 (Lamolet et al. 2001, 2004; Pulichino et al. 2003). However, because the expression of Tpit/Tbx19, NeuroD1, and POMC is found at the earlier developmental stages in the pituitary gland in the Hes1-knockout mice, and not at later stages, Hes1 is considered to be an inhibitor of Tpit/Tbx19 and NeuroD1 expression (Zhu et al. 2006). The transfection of the Dlk1 construct into the Balb/c cells induces a decrease of Hes1 expression, while Hes1 expression increases when antisense Dlk1-constructs are transfected into the Dlk1-positive 3T3-L1 cells (Baladron et al. 2005). Consequently, based on the observation that the expression of Hes1 is inhibited by Dlk1, the differentiation of ACTH cells in the anterior pituitary is believed to be stimulated by inducing Tpit/Tbx19 and NeuroD1 expressions. The differentiation of the Pit1-lineage, especially TSH cells and GH cells is inhibited in transgenic mice overexpressing Notch intracellular domain (NICD) under the control of the Pit1 promoter (Zhu et al. 2006). However, the differentiation of TSH and GTH cells is also inhibited in transgenic mice overexpressing the intracellular domain fragment of Notch2 under the control of the pituitary α-glycoprotein promoter and enhancer (Raetzman et al. 2006, 2007). Using the yeast two-hybrid system, Baladron et al. (2005) observed that the extracellular domain of Dlk1 interacted with the EGF-like domain of Notch1 and that Dlk1 inhibited the Notch1 activity when the activity was measured by an assay system using luciferase as a reporter gene under the control of a VSL/RGP-Jk/CBF-1-dependent promotor in the Dlk1-negative, Notch1-positive Balb/c 14 cell line. These data suggest that Dlk1 inhibits Hes1expression by interacting with Notch1. Both Notch2 and Notch3—but not Notch1—are expressed in the pituitary glands (Raetzman et al. 2004, 2006; Zhu et al. 2006). The Notch family commonly contains both the EGF-like motif and NICD (Lardelli et al. 1995): consequently, Dlk1 may interact with Notch2 in a manner similar to Notch1. Thus, there is a possibility that Dlk1 plays a role as an inhibitory factor during the developmental formation of the anterior pituitary glands based on experimental results showing that the differentiation of anterior pituitary hormone-producing cells is inhibited by activation of Notch pathway.
We found that Dlk1 was expressed throughout the cytoplasm of GH cells immediately after differentiation of GH cells to their adult state. Previous reports using immunohistochemistry and in situ hybridization have indicated that Dlk1 is expressed in GH cells (Larsen et al. 1996; Yevtodiyenko and Schmidt 2006). Studies using GH3 cells have shown that Dlk1 specifically inhibits the transcription of the GH gene (Ansell et al. 2007). Furthermore, because the Dlk1-knockout mice displayed growth retardation and accelerated adiposity (Moon et al. 2002), it has been suggested that Dlk1 may inhibit the expression of GH from the early development onwards and play an important role in the regulation of fetal growth.
The Hes1-knockout mice induced a hypoplasia in the PD, while the expression of α-MSH and prohormone convertase 2 (PC2) was not found in the PI, although the number of Pit1-positive cells increased and many GH-positive cells were observed (Raetzman et al. 2007). This decrease of Dlk1 levels may be necessary for inducing Hes1, resulting in the formation of the PI. Therefore, our observation provides further insight into the involvement of Dlk1 in the development of the PI.
The results of our study suggest that Dlk1 was expressed in the PT during development from the fetal stage to the adult, and that Dlk1 protein was always expressed in the cytoplasm during development. In the PT, Dlk1 protein was found in both TSH-negative cells and TSH cells. During the development of the PT, Notch and Hes1 were not expressed in the PT (Zhu et al. 2006; Kita et al. 2007; Raetzman et al. 2004, 2006, 2007). Consequently, the function of Dlk1 remains unclear, because Dlk1 is not involved in the regulation of the Notch pathway and transcriptional regulation of hormone genes in the PT. Taken together, the differential expression levels of Dlk1 may be involved in the timing of the hormone expression or the determination of hormone-secreting cell types in the pituitary gland.
References
Ansell PJ, Zhou Y, Schjeide BM, Kerner A, Zhao J, Zhang X, Klibanski A (2007) Regulation of growth hormone expression by Delta-like protein 1 (Dlk1). Mol Cell Endocrinol 271:55–63
Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284:770–776
Baladron V, Ruiz-Hidalgo MJ, Nueda ML, Díaz-Guerra MJ, García-Ramírez JJ, Bonvini E, Gubina E, Laborda J (2005) dlk acts as a negative regulator of Notch1 activation through interactions withspecific EGF-like repeats. Exp Cell Res 303:343–359
Carlsson C, Tornehave D, Lindberg K, Galante P, Billestrup N, Michelsen B, Larsson LI, Nielsen JH (1997) Growth hormone and prolactin stimulate the expression of rat preadipocyte factor—1/delta-like protein in pancreatic islets: molecular cloning and expression pattern during development and growth of the endocrine pancreas. Endocrinology 138:3940–3948
Costaglioli P, Côme C, Knoll-Gellida A, Salles J, Cassagne C, Garbay B (2001) The homeotic protein dlk is expressed during peripheral nerve development. FEBS Lett 509:413–416
Floridon C, Jensen CH, Thorsen P, Nielsen O, Sunde L, Westergaard JG, Thomsen SG, Teisner B (2000) Does fetal antigen 1 (FA1) identify cells with regenerative, endocrine and neuroendocrine potentials? A study of FA1 in embryonic, fetal, and placental tissue and in maternal circulation. Differentiation 66:49–59
Friedrichsen BN, Carlsson C, Moldrup A, Michelsen B, Jensen CH, Teisner B, Nielsen JH (2003) Expression, biosynthesis and release of preadipocyte factor-1/delta-like protein/fetal antigen-1 in pancreatic beta-cells: possible physiological implications. J Endocrinol 176:257–266
Goudreau JL, Falls WM, Lookingland KJ, Moore KE (1995) Periventricular-hypophysial dopaminergic neurons innervate the intermediate but not the neural lobe of the rat pituitary gland. Neuroendocrinology 62:147–154
Goudreau JL, Lindley SE, Lookingland KJ, Moore KE (1992) Evidence that hypothalamic periventricular dopamine neurons innervate the intermediate lobe of the rat pituitary. Neuroendocrinology 56:100–105
Hansen LH, Madsen B, Teisner B, Nielsen JH, Billestrup N (1998) Characterization of the inhibitory effect of growth hormone on primary preadipocyte differentiation. Mol Endocrinol 12:1140–1149
Halder SK, Takemori H, Hatano O, Nonaka Y, Wada A, Okamoto M (1998) Cloning of a membrane-spanning protein with epidermal growth factor-like repeat motifs from adrenal glomerulosa cells. Endocrinology 139:3316–3328
Hedlund GP, Carlsson HE, Shieck E, Nilsson I, Lundblad C, Arons S, Iversen AK, Looman C, Jensen HE, Hau J (2003) Fetal antigen 1 (FA1) in the adult rat adrenal gland, ovary and pituitary gland. In Vivo 17:1–4
Jensen CH, Krogh TN, Hojrup P, Clausen PP, Skjodt K, Larsson LI, Enghild JJ, Teisner B (1994) Protein structure of fetal antigen 1 (FA1). A novel circulating human epidermal-growth-factor-like protein expressed in neuroendocrine tumors and its relation to the gene products of dlk and pG2. Eur J Biochem 225:83–92
Kaneta M, Osawa M, Sudo K, Nakauchi H, Farr AG, Takahama Y (2000) A role for pref-1 and HES-1 in thymocyte development. J Immunol 164:256–264
Kato H, Kuwako K, Suzuki M, Tanaka S (2004) Gene expression patterns of pro-opiomelanocortin-processing enzymes PC1 and PC2 during postnatal development of rat corticotrophs. J Histochem Cytochem 52:943–957
Kita A, Imayoshi I, Hojo M, Kitagawa M, Kokubu H, Ohsawa R, Ohtsuka T, Kageyama R, Hashimoto N (2007) Hes1 and Hes5 control the progenitor pool, intermediate lobe specification, and posterior lobe formation in the pituitary development. Mol Endocrinol 21:1458–1466
Kitamura T, Onishi M, Kinoshita S, Shibuya A, Miyajima A, Nolan GP (1995) Efficient screening of retroviral cDNA expression libraries. Proc Natl Acad Sci USA 92:9146–9150
Kojima T, Kitamura T (1999) A signal sequence trap based on a constitutively active cytokine receptor. Nat Biotechnol 17:487–490
Laborda J (2000) The role of the epidermal growth factor-like protein dlk in cell differentiation. Histol Histopathol 15:119–129
Lamolet B, Pulichino AM, Lamonerie T, Gauthier Y, Brue T, Enjalbert A, Drouin J (2001) A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell 104:849–859
Lamolet B, Poulin G, Chu K, Guillemot F, Tsai MJ, Drouin J (2004) Tpit-independent function of NeuroD1(BETA2) in pituitary corticotroph differentiation. Mol Endocrinol 18:995–1003
Lardelli M, Williams R, Lendahl U (1995) Notch-related genes in animal development. Int J Dev Biol 39:769–780
Larsen JB, Jensen CH, Schroder HD, Teisner B, Bjerre P, Hagen C (1996) Fetal antigen 1 and growth hormone in pituitary somatotroph cells. Lancet 347:191
Mei B, Zhao L, Chen L, Sul HS (2002) Only the large soluble form of preadipocyte factor-1 (Pref-1), but not the small soluble and membrane forms, inhibits adipocyte differentiation: role of alternative splicing. Biochem J 364:137–144
Moon YS, Smas CM, Lee K, Villena JA, Kim KH, Yun EJ, Sul HS (2002) Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Mol Cell Biol 22:5585–5592
Moore KA, Pytowski B, Witte L, Hicklin D, Lemischka IR (1997) Hematopoietic activity of a stromal cell transmembrane protein containing epidermal growth factor-like repeat motifs. Proc Natl Acad Sci USA 94:4011–4016
Murakami T, Kikuta A, Taguchi T, Ohtsuka A, Ohtani O (1987) Blood vascular architecture of the rat cerebral hypophysis and hypothalamus. A dissection/scanning electron microscopy of vascular casts. Arch Histol Jpn 50:133–176
Nakakura T, Yoshida M, Dohra H, Suzuki M, Tanaka S (2006) Gene expression of vascular endothelial growth factor—A in the pituitary during formation of the vascular system in the hypothalamic-pituitary axis of the rat. Cell Tissue Res 324:87–95
Nakakura T, Suzuki M, Watanabe Y, Tanaka S (2007) Possible involvement of brain-derived neurotrophic factor (BDNF) in innervation of dopaminergic neurons from the periventricular nucleus to the rat pars intermedia. Zool Sci 24:1086–1093
Okamoto M, Takemori H, Halder SK, Hatano O (1997) Zona glomerulosa-specific factor: cloning and function. Steroids 62:73–76
Pulichino A, Vallette-Kasic S, Tsai J, Couture C, Gauthier Y, Drouin J (2003) Tpit determines alternate fates during pituitary cell differentiation. Genes Dev 17:738–747
Raetzman LT, Cai JX, Camper SA (2007) Hes1 is required for pituitary growth and melanotrope specification. Dev Biol 304:455–466
Raetzman LT, Ross SA, Cook S, Dunwoodie SL, Camper SA, Thomas PQ (2004) Developmental regulation of Notch signaling genes in the embryonic pituitary: Prop1 deficiency affects Notch2 expression. Dev Biol 265:329–340
Raetzman LT, Wheeler BS, Ross SA, Thomas PQ, Camper SA (2006) Persistent expression of Notch2 delays gonadotrope differentiation. Mol Endocrinol 20:2898–2908
Saito A, Kano Y, Suzuki M, Tomura H, Takeda J, Tanaka S (2002) Sequence analysis and expressional regulation of messenger RNAs encoding beta subunits of follicle-stimulating hormone and luteinizing hormone in the red-bellied newt, Cynops pyrrhogaster. Biol Reprod 66:1299–1309
Smas CM, Chen L, Sul HS (1997) Cleavage of membrane-associated pref-1 generates a soluble inhibitor of adipocyte differentiation. Mol Cell Biol 17:977–988
Smas CM, Kachinskas D, Liu CM, Xie X, Dircks LK, Sul HS (1998) Transcriptional control of the pref-1 gene in 3T3–L1 adipocyte differentiation. Sequence requirement for differentiation-dependent suppression. J Biol Chem 273:31751–31758
Smas CM, Sul HS (1993) Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell 73:725–734
Tanaka S, Kurosumi K (1992) A certain step of proteolytic processing of proopiomelanocortin occurs during the transition between two distinct stages of secretory granules maturation in rat anterior pituitary corticotrophs. Endocrinology 131:779–786
Tanaka S, Yora T, Nakayama K, Inoue K, Kurosumi K (1997) Proteolytic processing of pro-opiomelanocortin occurs in acidifying secretory granules of AtT-20 cells. J Histochem Cytochem 45:425–436
Tanimizu N, Nishikawa M, Saito H, Tsujimura T, Miyajima A (2003) Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J Cell Sci 116:1775–1786
Tanimizu N, Tsujimura T, Takahide K, Kodama T, Nakamura K, Miyajima A (2004) Expression of Dlk/Pref-1 defines a subpopulation in the oval cell compartment of rat liver. Gene Expr Patterns 5:209–218
Tornehave D, Jansen P, Teisner B, Rasmussen HB, Chemnitz J, Moscoso G (1993) Fetal antigen 1 (FA1) in the human pancreas: cell type expression, topological and quantitative variations during development. Anat Embryol (Berl) 187:335–341
Uehara M, Yaoi Y, Suzuki M, Takata K, Tanaka S (2001) Differential localization of prohormone convertase (PC1 and PC2) in two distinct types of secretory granules in rat pituitary gonadotrophs. Cell Tissue Res 304:43–49
Yevtodiyenko A, Schmidt JV (2006) Dlk1 expression marks developing endothelium and sites of branching morphogenesis in the mouse embryo and placenta. Dev Dyn 235:1115–1123
Wang Y, Sul HS (2006) Ectodomain shedding of preadipocyte factor 1 (Pref-1) by tumor necrosis factor alpha converting enzyme (TACE) and inhibition of adipocyte differentiation. Mol Cell Biol 26:5421–5435
Watanabe YG, Daikoku S (1979) An immunohistochemical study on the cytogenesis of adenohypophysial cells in fetal rats. Dev Biol 68:557–567
Zhu X, Wang J, Ju BG, Rosenfeld MG (2007) Signaling and epigenetic regulation of pituitary development. Curr Opin Cell Biol 19:605–611
Zhu X, Zhang J, Tollkuhn J, Ohsawa R, Bresnick EH, Guillemot F, Kageyama R, Rosenfeld MG (2006) Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev 20:2739–2753
Acknowledgments
We are grateful to Professor Toshio Kitamura, Institute of Medical Science, University of Tokyo for his gift of vectors and cells and advice on the signal sequence trap method. We also thank Professor Hideaki Tanaka, Kumatoto University for stimulating and helpful discussion. This work was supported in part by a grant-in-aid for science research from the Ministry of Education, Science, Sports, and Culture of Japan (to S.T.) and in a grant-in-aid from the Japan Society for the Promotion of Science (to T.N).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakakura, T., Sato, M., Suzuki, M. et al. The spatial and temporal expression of delta-like protein 1 in the rat pituitary gland during development. Histochem Cell Biol 131, 141–153 (2009). https://doi.org/10.1007/s00418-008-0494-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-008-0494-8