Abstract
In the anterior pituitary, S100β protein (S100β) has been assumed to be a marker of folliculo-stellate cells, which are one of the non-hormone-producing cells existing in the parenchyma of the adult anterior lobe and are composed of subpopulations with various functions. However, recent accumulating studies on S100β-positive cells, including non-folliculo-stellate cells lining the marginal cell layer (MCL), have shown the novel aspect that most S100β-positive cells in the MCL and parenchyma of the adult anterior lobe are positive for sex determining region Y-box 2 (SOX2), a marker of pituitary stem/progenitor cells. From the viewpoint of SOX2-positive cells, the majority of these cells in the MCL and in the parenchyma are positive for S100β, suggesting that S100β plays a role in the large population of stem/progenitor cells in the anterior lobe of the adult pituitary. Reportedly, S100β/SOX2-double positive cells are able to differentiate into hormone-producing cells and various types of non-hormone-producing cells. Intriguingly, it has been demonstrated that extra-pituitary lineage cells invade the pituitary gland during prenatal pituitary organogenesis. Among them, two S100β-positive populations have been identified: one is SOX2-positive population which invades at the late embryonic period through the pituitary stalk and another is a SOX2-negative population that invades at the middle embryonic period through Atwell’s recess. These two populations are likely the substantive origin of S100β-positive cells in the postnatal anterior pituitary, while S100β-positive cells emerging from oral ectoderm-derived cells remain unclear.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
S100β, a small acidic protein composed of 92 amino acids, is an EF-hand Ca2+, Cu2+, and Zn2+ binding protein (Donato et al. 2009). It was discovered from brain extracts in 1965, and its name derives from the fact that it is soluble in saturated ammonium sulfate solution (Moore 1965). Using S100β-promoter/enhanced green fluorescent protein (EGFP)-transgenic (TG) mice, S100β expression has been reported in immature and mature neurons and glial cells in the central and peripheral nervous system in addition to tissues of non-neural systems such as melanocytes, chondrocytes, and pituitary folliculo-stellate cells (Vives et al. 2003). Although S100β acts as an intracellular regulator and an extracellular signal (Donato et al. 2009), its precise function is poorly understood.
The pituitary gland is an endocrine tissue that plays essential roles in plural physiological processes via synthesis and secretion of multiple hormones. It comprises the two following anatomically distinct entities: the neurohypophysis (posterior pituitary) derived from the neural ectoderm and the adenohypophysis (anterior pituitary) derived from the oral ectoderm (developed from the anterior cranial placode) (Couly and Le Douarin 1985; Kouki et al. 2001). The posterior pituitary consists of the posterior lobe and the pituitary stalk. It is connected to the median eminence of the hypothalamus through the pituitary stalk (Rizzoti and Lovell-Badge 2017). The posterior lobe, the major part of the posterior pituitary, consists of axon terminals of oxytocin and vasopressin neurons extending from the hypothalamus, a large population of pituicytes (a special type of astrocytes), a small population of microglia, and unknown cells (Wei et al. 2009).
The anterior pituitary consists of the intermediate and anterior lobes, and the pars tuberalis. The anterior lobe comprises five types of hormone-producing cells including growth hormone (GH), prolactin (PRL), luteinizing hormone (LH)/follicle-stimulating hormone (FSH), thyroid-stimulating hormone (TSH), and adrenocorticotropic hormone (ACTH), as well as non-hormone-producing cells such as stem/progenitor cells and folliculo-stellate cells (Sheng et al. 1997; Tremblay et al. 1998; Zhu et al. 2006). The anterior lobe shows low steady-state turnover, but image analyses with apoptotic and mitotic cells show that, in young and adult male rat anterior lobes, cells either die or divide as frequently as once every 60–70 days (1.4–1.7% cell renewal daily) (Nolan et al. 1998, 1999). Nevertheless, a continuous supply of hormone-producing cells in the anterior pituitary is essential. It is reported that at least ACTH-producing cells show a substantial cell turnover by self-duplication (Langlais et al. 2013), and long term lineage tracing by two groups (Andoniadou et al. 2013; Rizzoti et al. 2013) indicated that the sex determining region Y-box 2 (SOX2)-positive stem/progenitor cells do not show significant participation in the renewal of hormone-producing cells. The mechanism of replenishment of hormone-producing cells from terminally differentiated those cells is an interesting issue.
In 1980, by immunohistochemical analyses of the anterior lobe of the adult rat pituitary gland using an anti-S100β antibody, Nakajima et al. first revealed that the folliculo-stellate cells in the parenchyma are positive for S100β (Nakajima et al. 1980). Furthermore, Cocchia and Miani (1980) and Yoshimura’s group (Shirasawa et al. 1983) reported the presence of other S100β-positive cell types, including marginal cells of the intermediate and anterior lobes lining the residual lumen of the anterior pituitary, and pituicytes in the posterior lobe. Since then, accumulating evidence has suggested that S100β-positive cells may have various functions. Until now, the identification of S100β-positive cells in the mouse anterior pituitary has been difficult and another approach to seek an alternative marker for mouse S100β-positive cells has been reported (Fujiwara et al. 2020). Nevertheless, recent analyses using S100β/GFP-TG mice (Andoniadou et al. 2013; Vives et al. 2003) and immunohistochemical analysis using an anti-S100β antibody from each different manufacturer (Fauquier et al. 2008; Horiguchi et al. 2021; O’Hara et al. 2020) reveal the presence of S100β-positive cells in the mouse anterior gland. Especially, Horiguchi et al. (2021) clearly show the S100β-positive cells both in the MCL and in the parenchyma. Detail comparison of the S100β expression profile between mouse and rat may be required further studies. In this review, we address the origin of S100β-positive cells in addition to the relationship with SOX2-positive pituitary stem/progenitor cells in the adult anterior pituitary.
Pituitary organogenesis
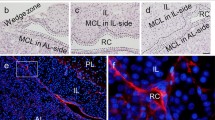
The architecture of stem/progenitor cells develops during pituitary organogenesis (Vankelecom 2012). Construction of the pituitary primordium begins with invagination of the oral ectoderm (Fig. 1a), which is composed of only SOX2-positive cells, toward the ventral diencephalon, the prospective posterior pituitary originating from the neural ectoderm (see the review Rizzoti (2015), and references therein). Subsequently, the primordium of the prospective anterior pituitary, known as Rathke’s pouch, is formed and detaches from the oral ectoderm (Fig. 1b) (Kioussi et al. 1999). Further expansion proceeds along with development through proliferation of the cell cord growing out from the marginal cell layer (MCL) facing the anterior wall of Rathke’s pouch to the parenchyma (Yoshimura et al. 1977a), followed by construction of the functional pituitary gland (Fig. 1c, d). These processes are advanced by extrinsic signals from the diencephalon and an intrinsic one within Rathke’s pouch, in addition to spatial–temporal expression of plural transcription factors (Kelberman et al. 2009; Vankelecom and Gremeaux 2010; Zhu et al. 2007).
Development of the rodent pituitary gland. a Invagination of the oral ectoderm (OE) toward the diencephalon (Di: presumptive posterior pituitary). b Development of the pituitary primordium, Rathke’s pouch (RP). All cells are positive for sex determining region Y-box 2 (SOX2, blue). c, d During development, SOX2-positive cells localize in the marginal cell layer (MCL, thick blue line) and in the parenchyma (PA, light blue) of the anterior lobe (AL) and the intermediate lobe (IL, light blue). PL posterior lobe, AR Atwell’s recess. The number at the top of each figure shows the embryonic age of rats and mice (in order of rat/mouse)
Before the discovery of SOX2-positive cells
In the 1970s, to investigate stem/progenitor cells for hormone-producing cells, many studies actively investigated non-hormone-producing cells (termed as chromophobes or agranular cells at that time) which localized in the MCL (marginal cells with polygonal shape) and in the parenchyma (follicular cells, stellate cells, and folliculo-stellate cells with stellate shape) of the anterior lobe. Vila-Porcile collectively calls the follicular and stellate cells in the parenchyma as folliculo-stellate cells, because of their similar morphology and intracellular ultrastructure, “une même famille cellulaire” (the same cell family) (Vila-Porcile 1972).
Based on ultrastructural similarities with non-hormone-producing cells, such as the presence of microfilaments, immaturity, scanty rough endoplasmic reticulum, and abundance of free ribosomes and polysomes, Yoshimura et al. suggested a clear relation between marginal layer cells and follicular cells, and predicted that they are involved in a “cell renewal system” (Yoshimura et al. 1977a, 1977b). This hypothesis is frequently discussed, but direct evidence has not been demonstrated (Vankelecom 2007a, 2007b, 2012).
Later, Nakajima et al. investigated the adult rat anterior pituitary by immunohistochemistry using anti-S100β antibody and first observed positive signals in two cell types, follicle-forming cells and stellate-shaped cells, and collectively integrated them into folliculo-stellate cells (Nakajima et al. 1980). Since then, S100β has been used exclusively as a marker for folliculo-stellate cells. However, Cocchia and Miani (1980) and Yoshimura’s group (Shirasawa et al. 1983) have reported that different shapes of S100β-positive cells are also present in the MCL (polygonal shape) of the intermediate and anterior lobes, and in the posterior lobe (pituitcytes; irregular and branched shape), thereby indicating the presence of various types of S100β-positive cells. Additionally, it has been revealed that folliculo-stellate cells are heterogeneous with different immunophenotypes, morphologies, and functions (Allaerts and Vankelecom 2005; Devnath and Inoue 2008). However, understanding for the folliculo-stellate cells is likely to be confusing.
Discovery of SOX2-positive cells as pituitary stem/progenitor cells
Since SOX2 is required for expansion of progenitors and differentiation of pituitary endocrine lineages (Goldsmith et al. 2016; Jayakody et al. 2012), various studies on stem cells using SOX2-positive cells have been conducted. Lepore et al. have reported that pituitary colony-forming cells in dispersed pituitary cells possess the ability to proliferate and differentiate into hormone-producing cells (Lepore et al. 2006, 2005). Chen et al. have reported that the cell fraction showing Hoechst 33342 dye efflux capacity suggests a universal property of stem cell populations expressing several marker genes of stem cells other than Sox2 at high levels (Chen et al. 2006, 2005). Using the sphere-forming assay that assesses stem cell properties in vitro (Reynolds and Weiss 1992), Fauquier et al. have reported that the sphere prepared from dispersed pituitary cells (pituisphere) is composed of SOX2-positive cells and they differentiate into all hormone-producing cells and S100β-positive cells by cultivation under differentiation-inducing conditions (Fauquier et al. 2008).
Currently, stem cells are divided into two groups namely embryonic stem cells and adult stem cells. Embryonic stem cells are pluripotent and are assumed to play a role in basic development, while adult stem cells are either multipotent or unipotent and contribute to maintenance and repair of the resident tissue (Bragdon and Bahney 2018). In the pituitary gland, there are several reports describing adult stem cells that are qualitatively different from the embryonic stem cells by virtue for the maintenance of postnatal pituitary function (Andoniadou et al. 2013; Gleiberman et al. 2008; Rizzoti et al. 2013). Especially, SOX2-positive cells in the adult anterior lobe are involved in the pituitary homeostasis responsible for physiological demand (Andoniadou et al. 2013; Rizzoti et al. 2013). Future elucidation of how such stem/progenitor cells emerge during the embryonic pituitary development is expected. Recently, depletion of SOX2-positive cells in the adult pituitary gland using diphtheria toxin shows that 80% obliteration of these cells does not affect adult hormone-producing homeostasis and remodeling (Roose et al. 2017). It is interesting in the existence of cell lineage with SOX2-positive signal in the cytoplasm (cSOX2-positive cells) escaped from Sox2 promoter-driven ablation.
Establishment of S100β-transgenic rats provides novel insights into pituitary stem/progenitor cells
In 2007, Itakura et al. generated S100β/GFP-TG rats, which express the gene encoding the GFP protein under the control of the rat S100β promoter (Itakura et al. 2007). TG rats show specific expression of Gfp in the S100β-positive cells in the pituitary gland and other tissues including cerebellum, chondrocytes, and adipocytes. Consequently, they enable live observation of S100β-positive cells and tissues, cell separation by a cell sorter, and multiple immunostaining with higher sensitivity. Accumulating studies using the S100β/GFP-TG rats have disclosed a novel aspect of S100β-positive cells; most of them are stem/progenitor cells of the postnatal anterior pituitary as discussed below.
Most S100β-positive cells are positive for SOX2
Until now, it has been shown that S100β is present in SOX2-positive cells of the rat and mouse anterior pituitaries (Andoniadou et al. 2013; Fauquier et al. 2008; Yoshida et al. 2011). Yoshida et al. (2013) performed immunohistochemistry for SOX2, prophet of PIT1 (PROP1, a pituitary-specific transcription factor (Sornson et al. 1996)), and S100β (substituted by GFP) using the pituitary gland of adult S100β/GFP-TG male rats and showed intriguing localization profile of the pituitary stem/progenitor cells (Yoshida et al. 2011). SOX2-, PROP1-, and S100β-positive cells show heterogeneous composition and dissimilar populations in the MCL and parenchyma of the anterior lobe, thereby indicating that they are heterogeneous (Fig. 2). The study focused on cells positive for SOX2 and/or S100β revealing that approximately 85% of the S100β-positive cells are positive for SOX2 (types 3 and 4 in Fig. 2a, b) and suggesting that they have stem/progenitor cell properties, while only approximately 15% of the S100β-single positive cells (type 5 in Fig. 2a, b) are located in both the MCL and parenchyma of the anterior lobe (Fig. 2c). From the viewpoint of SOX2-positive cells, of those positive for S100β (types 3 and 4) are 78% in the rat anterior lobe (64% and 82% in the MCL and parenchyma, respectively) (Fig. 2d). Therefore, S100β-positive cells could be divided into two groups, namely SOX2-positive (stem/progenitor cells) and SOX2-negative. S100β/SOX2-double positive cells are absent from the anterior lobe during the embryonic period (Horiguchi et al. 2016), and they eventually appear at least on postnatal day 3 (P3) (Ueharu et al. 2018). Afterward, they are abundantly located in the MCL and the parenchyma (Yoshida et al. 2011). In the mouse adult anterior lobe, the S100β/SOX2-double positive cells are certainly present and the ratio in SOX2-positive cells is substantially low at 58% (Andoniadou et al. 2013) in comparison that of the rat, remaining to be clarified the difference in future.
Immunohistochemistry and cell of the anterior lobe of male S100β/green fluorescent protein-transgenic (GFP-TG) rats at postnatal day 60. Results of immunostaining using antibodies against SOX2 (blue), prophet of PIT1 (PROP1, red), and S100β (green, GFP expression under the S100β promoter) are shown (a, b). In the stained images of the MCL (a) and parenchyma (b), five types of positive cells (types 1–5) are observed. The proportion of each cell type positive for S100β-GFP (c) and SOX2 (d) in the MCL and in the parenchyma are shown (Yoshida et al. 2011). Bars = 20 µm
Microenvironments that nurture stem/progenitor cells have been studied in many tissues after the stem cell niche theory was proposed in Drosophila melanogaster (Lin 2002). In the pituitary, two kinds of niches are postulated, the MCL niche and parenchymal niche, scattering in the parenchyma of the postnatal anterior lobe (Gremeaux et al. 2012; Vankelecom and Chen 2014; Yoshida et al. 2016a). The parenchymal niches are first found in the neonatal anterior lobe and increase in number during the postnatal pituitary growth wave, whereas the MCL niche persists throughout embryonic and postnatal period (Chen et al. 2013).
Construction of the parenchymal stem/progenitor cell niche by epithelial-mesenchymal transition in the postnatal anterior lobe
To elucidate the construction of the parenchymal niche, Chen et al. have proposed the migration of stem/progenitor cells from the MCL to the parenchyma by epithelial-mesenchymal transition (EMT) (Chen et al. 2013). EMT is known to play important role in cell migration and differentiation in embryonic development of many tissues as well as oncogenesis (Davis et al. 2014; Simoes-Costa and Bronner 2015; Yoshida et al. 2016a).
In addition to EMT markers (E-cadherin for epithelial cells and vimentin for mesenchymal cells), Chen et al. investigated EMT using the coxsackievirus and adenovirus receptor (CAR), a tight-junction molecule, and showed that EMT is preceded by the alteration of apical membrane localization of CAR to the basolateral membrane, followed by the migration to the parenchyma and formation of clusters (Chen et al. 2013). The juxtacrine signaling molecule, ephrin B2, is also involved in EMT by alteration of membrane localization (Yoshida et al. 2015, 2017).
In the parenchyma, the stem/progenitor cells form dense clusters with a pseudo-follicular structure (termed as parenchymal stem/progenitor cell clusters; PS clusters), in which CAR (Chen et al. 2013), ephrin B2, and its cis-acting receptor EphB3 (Yoshida et al. 2017) encircle the follicular lumen. Notably, in the neonatal pituitary, EphB2, which is another ephrin-B2 interacting receptor and is produced by ACTH-producing cells, shows trans-interaction between ephrin-B2 localized in the basolateral membrane of stem/progenitor cells beneath the MCL (Yoshida et al. 2017). Although this trans-interaction between ephrin-B2 and EphB2 may be involved in the acceleration of stem/progenitor cell migration from the MCL niche, this hypothesis remains to be elucidated.
Investigation of the mechanism for onset of EMT is important. Perez-Millan et al. reported that PROP1 triggers EMT-like transition in mice pituitary by comprehensive genomic profiling using Prop1-deficient Ames dwarf mice (Prop1df/df) (Perez Millan et al. 2016). Future elucidation is expected to clarify the molecules which trigger EMT in the PROP1-negative stem/progenitor cells lining the MCL (Yoshida et al. 2011) and a role of PROP1 in the parenchymal stem/progenitor niches.
In retrospect, the observations regarding EMT are in accordance with the relationship between non-hormone-producing cells in the MCL and parenchyma that is pointed out by Yoshimura et al. They reported that there are fine ultrastructural similarities between marginal layer cells and follicular cells (Yoshimura et al. 1977a) and that the cell cords grow out from marginal layer cells and follicular cell clusters around pseudo-follicles (Yoshimura et al. 1977b).
Diverse plasticity of S100β-positive stem/progenitor cells
Using the adult anterior pituitary of the S100β/GFP-TG rat, it has been demonstrated that S100β-positive cells have plasticity to differentiate into skeletal muscle cells (Osuna et al. 2012), and into hormone-producing cells (Higuchi et al. 2014). Yoshida et al. (2013) succeeded in isolating PS clusters by intense enzymatic dispersion of the adult anterior lobe with subsequent repeated pipetting, and confirmed that all cells in the PS clusters are positive for SOX2 (Yoshida et al. 2016b). They are classified into the following three types: type A, composed of all S100β-positive cells; type B, a mix of S100β-positive and S100β-negative cells; and type C, all S100β-negative cells. Cultivation of type A and type C clusters in 3D cultivation medium shows that some type A cells, but not type C, differentiate into hormone-producing cells (Yoshida et al. 2016b). Cultivation of type A clusters in 2D cultivation medium, intriguingly, shows differentiation into cells positive for various molecular markers, including myogenin (skeletal muscle cell), alpha-smooth muscle actin (αSMA, smooth muscle cell), neuron-glial antigen 2 (NG2, pericyte), and SOX17 (endoderm lineage cell) (Yoshida et al. 2018). Thus, S100β/SOX2-double positive cells have plasticity to differentiate into various cell types regardless of the germ layer. These differentiation profiles suggested that the cells in type A clusters have a capacity to differentiate into various cell types depending on cultivation conditions, and each cluster is composed of previously destined cells. Whether S100β-positive cells emerge from type C clusters, which are composed of SOX2-single positive cells and exhibit high stemness, requires further study.
Recently, it has been reported that most S100β/SOX2-double positive cells preferentially express Cd9 and Cd81, which participate in proliferation, and are able to differentiate into endothelial cells (Horiguchi et al. 2018) and hormone-producing cells (Horiguchi et al. 2021).
Invasion of extra-pituitary lineage cells during pituitary organogenesis
It has long been suggested that cells of different origins invade the pituitary gland during early development (Couly and Le Douarin 1985, 1987). Notably, as described above, S100β is expressed in several cells of tissues in the nervous and non-neural systems (Vives et al. 2003). It is now known that many of the S100β-positive cells in various tissues are neural crest-derived cells (Aquino et al. 2006; Zhang et al. 2014). In fact, using gene tracing analysis and cell markers, several types of invasion of extra-pituitary lineage cells during early pituitary organogenesis have recently been demonstrated.
P0-positive neural crest-derived cells invade the primordium of the pituitary
Neural crest cells are a transient and multipotent cell population that migrate along with EMT and reside in various organs, including endocrine system, by generating vast diverse array of cell types during vertebrate development (see reviews Perera and Kerosuo (2021), Pierret et al. (2006), Vega-Lopez et al. (2018)). Migratory neural crest-derived cells are classified into two groups, namely early migratory neural crest cells, which are positive for the P0 protein, and late migratory cells, which are negative for P0 (Nitzan et al. 2013).
Davis et al. suggested an ectopic expression of P0-Cre in Rathke’s pouch (Davis et al. 2016). However, time-dependent survey of P0 lineage cell by gene tracing analysis using P0-Cre/EGFP-TG mice (Ueharu et al. 2017) showed that P0 lineage neural crest-derived cells migrate into developing mouse pituitary in a stepwise manner at mouse embryonic day 9.5 (mE9.5 corresponds to rat E11.5) (Fig. 3a), when the pituitary primordium of Rathke’s pouch (Fig. 3b) begins to be formed, and at mE13.5 (rat E15.5), when vasculogenesis proceeds from Atwell’s recess (Fig. 3c). Thereafter, invasion of P0 lineage cells is not observed. P0 lineage neural crest-derived cells of the first wave differentiate into all hormone-producing cell lineages and reside in the postnatal anterior lobe as pituitary stem/progenitor cells accounting for 5% of the SOX2-positive cells, while P0 lineage cells of the second wave exclusively differentiate into pericytes (Davis et al. 2016; Ueharu et al. 2017). However, S100β-positive P0 lineage cell is rarely detected in the postnatal pituitary gland of the P0-Cre/EGFP-TG mice (Yoshida et al. 2013personal communication). Thus, the origin of S100β/SOX2-double positive cells requires elucidation.
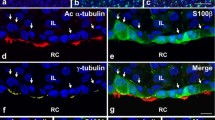
Possible invasion pathways and localization of S100β-positive cells. P0 lineage neural crest cells (NCs) invade the invaginating oral ectodermal region at mouse embryonic day 9.5 (E9.5 corresponding to rat E11.5, a, NCP0) and reside in Rathke’s pouch at rat E13.5 (b, NC/P0SOX2), but rarely produce S100β-positive cells. Then, S100β-positive/SOX2-negative cells that are also positive for p75 (NC/p75S100β) or for paired related homeobox 1 (PRRX1), desmine, vimentin, isolectin B4, or nestin (othersS100β) invaded through Atwell’s recess (AR) at rat E15.5 (c). At rat E19.5 (d), SOX10/p75/SOX2-triple positive cells (NCSOX10) of the neural crest lineage invade through the pituitary stalk and become positive for S100β (NC/SOX10S100β), which is followed by migration to the intermediate lobe and then to the anterior lobe accompanied by an eventual expression of PROP1 (NC/SOX10S100βPROP1)
Mesenchyme and neural crest-derived cells invade through Atwell’s recess
S100β-positive cells have long been thought to first appear in the postnatal anterior pituitary (Shirasawa et al. 1983). The development of S100β/GFP-TG rats and highly sensitive immuno-fluorescence antibody technique using confocal laser scanning microscopy made it possible to detect the first appearance of GFP-positive cells in the posterior lobe and Atwell’s recess at E15.5 (Horiguchi et al. 2016) (Fig. 3c), which are also positive for anti-S100β antibody (Horiguchi et al. personal communication). A small number of S100β-positive pituicytes are located in the posterior lobe and then increase in number during development. In Atwell’s recess, an intraglandular fossa that receives several blood vessels in the entry of the anterior lobe (Daikoku et al. 1981), S100β-positive cells are detected and some of them are positive for isolectin B4, a marker of vascular endothelial cells. Thereafter, a small number of S100β-positive cells are detected inside the developing anterior lobe. They are negative for SOX2, but some are positive for paired related homeobox 1 (PRRX1, a mesenchyme cell marker), isolectin B4, desmin (an immature and mature pericyte marker), nestin (a neural and mesenchymal stem/progenitor cell marker), vimentin (a mesenchymal progenitor cell marker), or p75 (a neural crest-derived cell marker) (Horiguchi et al. 2016), in addition to PRRX2 (a mesenchymal cell marker) and α-SMA (smooth muscle cell/pericyte marker) (Higuchi et al. 2015). Additionally, some of the S100β-positive cells show proliferation activity (Horiguchi et al. 2016), thereby indicating that S100β-positive cells possess diverse populations. The results suggest that these cell populations provide postnatal S100β-positive/SOX2-negative cells. At the late embryonic period, after the closing of Atwell’s recess, although there is a small number of S100β-positive cells that are negative for SOX2 remaining in the anterior lobe, a number of S100β/SOX2-double positive cells appear in the intermediate lobe mainly at the boundary region of the posterior lobe, but are absent in the anterior lobe (Horiguchi et al. 2016). Thus, two types of extra-pituitary lineage S100β-positive cells appear during pituitary organogenesis and are likely the origin of the S100β-positive cells in the adult anterior pituitary.
SOX10-positive neural crest-derived cells invade through the pituitary stalk at the late embryonic stage
Time-dependent analysis by immunohistochemistry using S100β/GFP-TG rats shows that cells triple-positive for SOX10 (a marker of neural crest-derived cells and glial cells (Yang et al. 2020)), p75 (a marker of neural crest-derived cell), and SOX2, but negative for S100β, first appear in the pituitary stalk and the rostral region of the posterior lobe at E21.5 (Fig. 3d) (Ueharu et al. 2018). Afterward, they appear in the middle and caudal region, indicating that they migrate in the posterior lobe in rostral to caudal direction. Investigation of S100β-expressing cells by using anti-GFP antibody showed that SOX10-positive cells turn positive for S100β before emerging in the intermediate lobe at P3. S100β expression may be activated by SOX10 as described (Fujiwara et al. 2014).
In the anterior pituitary at P3, S100β-positive cells are mainly located in the parenchyma and MCL of the intermediate lobe, and a small number are in the parenchyma of the anterior lobe (Ueharu et al. 2018). Those in the intermediate lobe are positive for SOX2, and some of them are positive for another neural crest cell marker p75 in addition to SOX10. Spindle-shaped S100β/SOX10-double positive cells with elongated nuclei are detected in the gap region between the lobule structure and the MCL of the intermediate lobe. Images of migrating cytoplasmic F-actin visualized by fluorescently labeled phalloidin show extension of F-actin toward the MCL of the intermediate lobe and the boundary of the anterior lobe. Afterward, S100β-positive cells invade the MCL of the anterior lobe, and the expression of Prop1 is further confirmed, thereby indicating that part of S100β-positive cells is the origin of the S100β/SOX2/PROP1-triple positive cells in the postnatal anterior lobe.
During the postnatal growth wave, the number of S100β-positive cells in the anterior lobe increases, but immuno-reactive signals are lower than those in the intermediate lobe. Then, at P60, 85% are positive for SOX2 and 82% of the SOX2-positive cells in the parenchyma show positive for S100β as described above (Yoshida et al. 2011).
These results suggested that at least two types of neural crest-derived cells invade the pituitary during embryonic development and only late migratory neural crest-derived cells are involved in the appearance of S100β/SOX2-double positive cells in the adult anterior lobe.
Unique properties of S100β-positive pituitary lineage cell lines
Given that most S100β-positive cells are stem/progenitor cells, the investigation of pituitary S100β-positive cell lines is valuable. Tpit/E, Tpit/F1, and TtT/GF cell lines have been established from the mouse pituitary gland (Chen et al. 2000; Matsumoto et al. 1993). Thus far, researchers frequently use Tpit/F1 and TtT/GF as model cell lines for folliculo-stellate cells, because of their characteristic morphology and S100β expression, while Tpit/E is not well characterized. However, recent studies have demonstrated their characteristics as follows.
Microarray analysis of three cell lines shows the expression of Sox2 together with S100β (Yoshida et al. 2014). While expression levels of S100β are in the order, Tpit/E = Tpit/F1 < TtT/GF (0.01:0.01:6.25, relative to that of TATA-box binding protein), expression of Sox2 is in the reverse order, i.e., Tpit/E > Tpit/F1 > TtT/GF (1.29:0.45:0.17), suggesting that they are in different stages of plasticity. In addition to the ability of differentiation into skeletal muscle cells (Mogi et al. 2004), a potential of Tpit/F1 to differentiate into hormone-producing cells has been recently demonstrated (Higuchi et al. 2017), thus revealing the high plasticity of Tpit/F1. TtT/GF, which expresses S100β at high levels, shows an ability to transform into cells similar to the pericytes under the action of transforming growth factor-β (Tsukada et al. 2019, 2017). Unfortunately, although Tpit/E expresses Sox2 at high levels and may have a high stemness, the conditions required to induce differentiation have not been established. Thus, taken together, these cell lines would be useful tools for the study of S100β-expressing pituitary stem/progenitor cells, although the origin of each cell line is obscure.
Conclusion: origin of S100β-positive cell lineages
In the anterior lobe of the adult pituitary, S100β-positive cells are located in the MCL and parenchyma. Current studies described above suggest that some of these S100β-positive cells originate from extra-pituitary cell lineages and are classified into two groups, namely SOX2-positive (major) and SOX2-negative (minor). The follicular-type and stellate-type S100β-positive cells described in Table 1 may correspond to SOX2-positive cells and SOX2-negative cells, respectively. Although the emergence of S100β-positive cells from the oral ectoderm has not been demonstrated and needs further validation, recent studies using S100β/GFP-TG rats provide a new perspective on the origin of S100β-positive cells. Two possible pathways of S100β-positive cell invasion are shown in Fig. 3c, d.
Stepwise invasion by two groups of S100β-positive cells may occur. The first group invades the developing pituitary through Atwell’s recess (Fig. 3c). They are negative for SOX2 but positive for various mesenchymal markers and p75, a neural crest-derived cell marker. At the late embryonic stage, the second group of SOX10/SOX2-double positive cells invades the posterior pituitary through the pituitary stalk, followed by transition to express S100β. These cells move to the MCL of the intermediate lobe to reach the anterior lobe, the final destination (Fig. 3d). A hypothetical correlation between stem/progenitor cells and S100β-positive cells of the embryonic and adult anterior lobe is indicated in Fig. 4. Considering that S100β/SOX2-double positive cells share major population of the stem/progenitor cells in the postnatal anterior lobe, they may be indispensable population of adult stem cells postulated by gene tracing analysis using nestin-Cre-TG mice (Gleiberman et al. 2008). Whether some of the S100β-positive/SOX2-negative cells have progenitor cell properties to supply non-hormone-producing cells remains unclear. Nevertheless, it is evident that extra-pituitary lineage S100β-positive cells indeed play important roles in the maintenance of the adult pituitary gland.
References
Allaerts W, Vankelecom H (2005) History and perspectives of pituitary folliculo-stellate cell research. Eur J Endocrinol 153:1–12
Andoniadou CL, Matsushima D, Mousavy Gharavy SN, Signore M, Mackintosh AI, Schaeffer M, Gaston-Massuet C, Mollard P, Jacques TS, Le Tissier P, Dattani MT, Pevny LH, Martinez-Barbera JP (2013) Sox2(+) stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell 13:433–445
Aquino JB, Hjerling-Leffler J, Koltzenburg M, Edlund T, Villar MJ, Ernfors P (2006) In vitro and in vivo differentiation of boundary cap neural crest stem cells into mature Schwann cells. Exp Neurol 198:438–449
Bragdon BC, Bahney CS (2018) Origin of reparative stem cells in fracture healing. Curr Osteoporos Rep 16:490–503
Chen J, Crabbe A, Van Duppen V, Vankelecom H (2006) The notch signaling system is present in the postnatal pituitary: marked expression and regulatory activity in the newly discovered side population. Mol Endocrinol 20:3293–3307
Chen J, Hersmus N, Van Duppen V, Caesens P, Denef C, Vankelecom H (2005) The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinology 146:3985–3998
Chen L, Maruyama D, Sugiyama M, Sakai T, Mogi C, Kato M, Kurotani R, Shirasawa N, Takaki A, Renner U, Kato Y, Inoue K (2000) Cytological characterization of a pituitary folliculo-stellate-like cell line, Tpit/F1, with special reference to adenosine triphosphate-mediated neural nitric oxide synthetase expression and nitric oxide secretion. Endocrinology 141:3603–3610
Chen M, Kato T, Higuchi M, Yoshida S, Yako H, Kanno N, Kato Y (2013) Coxsackievirus and adenovirus receptor-positive cells compose the putative stem/progenitor cell niches in the marginal cell layer and parenchyma of the rat anterior pituitary. Cell Tissue Res 354:823–836
Cocchia D, Miani N (1980) Immunocytochemical localization of the brain-specific S-100 protein in the pituitary gland of adult rat. J Neurocytol 9:771–782
Couly GF, Le Douarin NM (1985) Mapping of the early neural primordium in quail-chick chimeras. I. Developmental relationships between placodes, facial ectoderm, and prosencephalon. Dev Biol 110:422–439
Couly GF, Le Douarin NM (1987) Mapping of the early neural primordium in quail-chick chimeras. II. The prosencephalic neural plate and neural folds: implications for the genesis of cephalic human congenital abnormalities. Dev Biol 120:198–214
Daikoku S, Kawano H, Abe K, Yoshinaga K (1981) Topographical appearance of adenohypophysial cells with special reference to the development of the portal system. Arch Histol Jpn 44:103–116
Davis FM, Stewart TA, Thompson EW, Monteith GR (2014) Targeting EMT in cancer: opportunities for pharmacological intervention. Trends Pharmacol Sci 35:479–488
Davis SW, Mortensen AH, Keisler JL, Zacharias AL, Gage PJ, Yamamura K, Camper SA (2016) beta-catenin is required in the neural crest and mesencephalon for pituitary gland organogenesis. BMC Dev Biol 16:16
Devnath S, Inoue K (2008) An insight to pituitary folliculo-stellate cells. J Neuroendocrinol 20:687–691
Donato R, Sorci G, Riuzzi F, Arcuri C, Bianchi R, Brozzi F, Tubaro C, Giambanco I (2009) S100B’s double life: intracellular regulator and extracellular signal. Biochim Biophys Acta 1793:1008–1022
Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC (2008) SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci USA 105:2907–2912
Fujiwara S, Hoshikawa S, Ueno T, Hirata M, Saito T, Ikeda T, Kawaguchi H, Nakamura K, Tanaka S, Ogata T (2014) SOX10 transactivates S100B to suppress Schwann cell proliferation and to promote myelination. PLoS One 9:e115400
Fujiwara K, Tsukada T, Horiguchi K, Fujiwara Y, Takemoto K, Nio-Kobayashi J, Ohno N, Inoue K (2020) Aldolase C is a novel molecular marker for folliculo-stellate cells in rodent pituitary. Cell Tissue Res 381:273–284
Gleiberman AS, Michurina T, Encinas JM, Roig JL, Krasnov P, Balordi F, Fishell G, Rosenfeld MG, Enikolopov G (2008) Genetic approaches identify adult pituitary stem cells. Proc Natl Acad Sci USA 105:6332–6337
Goldsmith S, Lovell-Badge R, Rizzoti K (2016) SOX2 is sequentially required for progenitor proliferation and lineage specification in the developing pituitary. Development (cambridge, England) 143:2376–2388
Gremeaux L, Fu Q, Chen J, Vankelecom H (2012) Activated phenotype of the pituitary stem/progenitor cell compartment during the early-postnatal maturation phase of the gland. Stem Cells Dev 21:801–813
Higuchi M, Kanno N, Yoshida S, Ueharu H, Chen M, Yako H, Shibuya S, Sekita M, Tsuda M, Mitsuishi H, Kato T, Kato Y (2014) GFP-expressing S100β-positive cells of the rat anterior pituitary differentiate into hormone-producing cells. Cell Tissue Res 357:767–779
Higuchi M, Yoshida S, Kanno N, Mitsuishi H, Ueharu H, Chen M, Nishimura N, Kato T, Kato Y (2017) Clump formation in mouse pituitary-derived non-endocrine cell line Tpit/F1 promotes differentiation into growth-hormone-producing cells. Cell Tissue Res 369:353–368
Higuchi M, Yoshida S, Ueharu H, Chen M, Kato T, Kato Y (2015) PRRX1- and PRRX2-positive mesenchymal stem/progenitor cells are involved in vasculogenesis during rat embryonic pituitary development. Cell Tissue Res 361:557–565
Horiguchi K, Fujiwara K, Yoshida S, Nakakura T, Arae K, Tsukada T, Hasegawa R, Takigami S, Ohsako S, Yashiro T, Kato T, Kato Y (2018) Isolation and characterisation of CD9-positive pituitary adult stem/progenitor cells in rats. Sci Rep 8:5533
Horiguchi K, Yako H, Yoshida S, Fujiwara K, Tsukada T, Kanno N, Ueharu H, Nishihara H, Kato T, Yashiro T, Kato Y (2016) S100β-positive cells of mesenchymal origin reside in the anterior lobe of the embryonic pituitary gland. PLoS One 11:e0163981
Horiguchi K, Yoshida S, Tsukada T, Fujiwara K, Nakakura T, Hasegawa R, Takigami S, Ohsako S (2021) Cluster of differentiation (CD) 9-positive mouse pituitary cells are adult stem/progenitor cells. Histochem Cell Biol 155:391–404
Itakura E, Odaira K, Yokoyama K, Osuna M, Hara T, Inoue K (2007) Generation of transgenic rats expressing green fluorescent protein in S-100beta-producing pituitary folliculo-stellate cells and brain astrocytes. Endocrinology 148:1518–1523
Jayakody SA, Andoniadou CL, Gaston-Massuet C, Signore M, Cariboni A, Bouloux PM, Le Tissier P, Pevny LH, Dattani MT, Martinez-Barbera JP (2012) SOX2 regulates the hypothalamic-pituitary axis at multiple levels. J Clin Invest 122:3635–3646
Kelberman D, Rizzoti K, Lovell-Badge R, Robinson IC, Dattani MT (2009) Genetic regulation of pituitary gland development in human and mouse. Endocr Rev 30:790–829
Kioussi C, Carriere C, Rosenfeld MG (1999) A model for the development of the hypothalamic-pituitary axis: transcribing the hypophysis. Mech Dev 81:23–35
Kouki T, Imai H, Aoto K, Eto K, Shioda S, Kawamura K, Kikuyama S (2001) Developmental origin of the rat adenohypophysis prior to the formation of Rathke’s pouch. Development 128:959–963
Langlais D, Couture C, Kmita M, Drouin J (2013) Adult pituitary cell maintenance: lineage-specific contribution of self-duplication. Mol Endocrinol 27:1103–1112
Lepore DA, Jokubaitis VJ, Simmons PJ, Roeszler KN, Rossi R, Bauer K, Thomas PQ (2006) A role for angiotensin-converting enzyme in the characterization, enrichment, and proliferation potential of adult murine pituitary colony-forming cells. Stem Cells 24:2382–2390
Lepore DA, Roeszler K, Wagner J, Ross SA, Bauer K, Thomas PQ (2005) Identification and enrichment of colony-forming cells from the adult murine pituitary. Exp Cell Res 308:166–176
Lin H (2002) The stem-cell niche theory: lessons from flies. Nat Rev Genet 3:931–940
Matsumoto H, Ishibashi Y, Ohtaki T, Hasegawa Y, Koyama C, Inoue K (1993) Newly established murine pituitary folliculo-stellate-like cell line (TtT/GF) secretes potent pituitary glandular cell survival factors, one of which corresponds to metalloproteinase inhibitor. Biochem Biophys Res Commun 194:909–915
Mogi C, Miyai S, Nishimura Y, Fukuro H, Yokoyama K, Takaki A, Inoue K (2004) Differentiation of skeletal muscle from pituitary folliculo-stellate cells and endocrine progenitor cells. Exp Cell Res 292:288–294
Moore BW (1965) A soluble protein characteristic of the nervous system. Biochem Biophys Res Commun 19:739–744
Nakajima T, Yamaguchi H, Takahashi K (1980) S100 protein in folliculostellate cells of the rat pituitary anterior lobe. Brain Res 191:523–531
Nitzan E, Krispin S, Pfaltzgraff ER, Klar A, Labosky PA, Kalcheim C (2013) A dynamic code of dorsal neural tube genes regulates the segregation between neurogenic and melanogenic neural crest cells. Development 140:2269–2279
Nolan LA, Kavanagh E, Lightman SL, Levy A (1998) Anterior pituitary cell population control: basal cell turnover and the effects of adrenalectomy and dexamethasone treatment. J Neuroendocrinol 10:207–215
Nolan LA, Lunness HR, Lightman SL, Levy A (1999) The effects of age and spontaneous adenoma formation on trophic activity in the rat pituitary gland: a comparison with trophic activity in the human pituitary and in human pituitary adenomas. J Neuroendocrinol 11:393–401
O’Hara L, Christian HC, Jeffery N, Le Tissier P, Smith LB (2020) Characterisation of a mural cell network in the murine pituitary gland. J Neuroendocrinol 32:e12903
Osuna M, Sonobe Y, Itakura E, Devnath S, Kato T, Kato Y, Inoue K (2012) Differentiation capacity of native pituitary folliculostellate cells and brain astrocytes. J Endocrinol 213:231–237
Perera SN, Kerosuo L (2021) On the road again: establishment and maintenance of stemness in the neural crest from embryo to adulthood. Stem Cells 39:7–25
Perez Millan MI, Brinkmeier ML, Mortensen AH, Camper SA (2016) PROP1 triggers epithelial-mesenchymal transition-like process in pituitary stem cells. Elife 5:e14470
Pierret C, Spears K, Maruniak JA, Kirk MD (2006) Neural crest as the source of adult stem cells. Stem Cells Dev 15:286–291
Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255:1707–1710
Rizzoti K (2015) Genetic regulation of murine pituitary development. J Mol Endocrinol 54:R55-73
Rizzoti K, Akiyama H, Lovell-Badge R (2013) Mobilized adult pituitary stem cells contribute to endocrine regeneration in response to physiological demand. Cell Stem Cell 13:419–432
Rizzoti K, Lovell-Badge R (2017) Pivotal role of median eminence tanycytes for hypothalamic function and neurogenesis. Mol Cell Endocrinol 445:7–13
Roose H, Cox B, Boretto M, Gysemans C, Vennekens A, Vankelecom H (2017) Major depletion of SOX2(+) stem cells in the adult pituitary is not restored which does not affect hormonal cell homeostasis and remodelling. Sci Rep 7:16940
Sheng HZ, Moriyama K, Yamashita T, Li H, Potter SS, Mahon KA, Westphal H (1997) Multistep control of pituitary organogenesis. Science 278:1809–1812
Shirasawa N, Kihara H, Yamaguchi S, Yoshimura F (1983) Pituitary folliculo-stellate cells immunostained with S-100 protein antiserum in postnatal, castrated and thyroidectomized rats. Cell Tissue Res 231:235–249
Simoes-Costa M, Bronner ME (2015) Establishing neural crest identity: a gene regulatory recipe. Development 142:242–257
Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O’Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG (1996) Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature 384:327–333
Tremblay JJ, Lanctot C, Drouin J (1998) The pan-pituitary activator of transcription, Ptx1 (pituitary homeobox 1), acts in synergy with SF-1 and Pit-1 and is an upstream regulator of the Lim-homeodomain gene Lim3/Lhx3. Mol Endocrinol 12:428–441
Tsukada T, Isowa Y, Kito K, Yoshida S, Toneri S, Horiguchi K, Fujiwara K, Yashiro T, Kato T, Kato Y (2019) Identification of TGFβ-induced proteins in non-endocrine mouse pituitary cell line TtT/GF by SILAC-assisted quantitative mass spectrometry. Cell Tissue Res 376:281–293
Tsukada T, Yoshida S, Kito K, Fujiwara K, Yako H, Horiguchi K, Isowa Y, Yashiro T, Kato T, Kato Y (2017) TGFbeta signaling reinforces pericyte properties of the non-endocrine mouse pituitary cell line TtT/GF. Cell Tissue Res 371:339–350
Ueharu H, Yoshida S, Kanno N, Horiguchi K, Nishimura N, Kato T, Kato Y (2018) SOX10-positive cells emerge in the rat pituitary gland during late embryogenesis and start to express S100beta. Cell Tissue Res 372:77–90
Ueharu H, Yoshida S, Kikkawa T, Kanno N, Higuchi M, Kato T, Osumi N, Kato Y (2017) Gene tracing analysis reveals the contribution of neural crest-derived cells in pituitary development. J Anat 230:373–380
Vankelecom H (2007a) Non-hormonal cell types in the pituitary candidating for stem cell. Semin Cell Dev Biol 18:559–570
Vankelecom H (2007b) Stem cells in the postnatal pituitary? Neuroendocrinology 85:110–130
Vankelecom H (2012) Pituitary stem cells drop their mask. Curr Stem Cell Res Ther 7:36–71
Vankelecom H, Chen J (2014) Pituitary stem cells: where do we stand? Mol Cell Endocrinol 385:2–17
Vankelecom H, Gremeaux L (2010) Stem cells in the pituitary gland: a burgeoning field. Gen Comp Endocrinol 166:478–488
Vega-Lopez GA, Cerrizuela S, Tribulo C, Aybar MJ (2018) Neurocristopathies: new insights 150 years after the neural crest discovery. Dev Biol 444(Suppl 1):S110–S143
Vila-Porcile E (1972) The network of the folliculo-stellate cells and the follicles of the adenohypophysis in the rat (pars distalis). Z Zellforsch Mikrosk Anat 129:328–369
Vives V, Alonso G, Solal AC, Joubert D, Legraverend C (2003) Visualization of S100B-positive neurons and glia in the central nervous system of EGFP transgenic mice. J Comp Neurol 457:404–419
Wei XY, Zhao CH, Liu YY, Wang YZ, Ju G (2009) Immuohistochemical markers for pituicyte. Neurosci Lett 465:27–30
Yamashita M, Qian ZR, Sano T, Horvath E, Kovacs K (2005) Immunohistochemical study on so-called follicular cells and folliculostellate cells in the human adenohypophysis. Pathol Int 55:244–247
Yang L-N, Huang W-K, Li X-L, Bai Y-Z, Zhang S-C (2020) Sox10 is a specific biomarker for neural crest stem cells in immunohistochemical staining in Wistar rats. Dis Markers 2020:8893703
Yoshida S, Higuchi M, Ueharu H, Nishimura N, Tsuda M, Nishihara H, Mitsuishi H, Kato T, Kato Y (2014) Characterization of murine pituitary-derived cell lines Tpit/F1, Tpit/E and TtT/GF. J Reprod Dev 60:295–303
Yoshida S, Kato T, Chen M, Higuchi M, Ueharu H, Nishimura N, Kato Y (2015) Localization of juxtacrine factor ephrin-B2 in pituitary stem/progenitor cell niches throughout life. Cell Tissue Res 359:755–766
Yoshida S, Kato T, Higuchi M, Yako H, Chen M, Kanno N, Ueharu H, Kato Y (2013) Rapid transition of NESTIN-expressing dividing cells from PROP1-positive to PIT1-positive advances prenatal pituitary development. J Neuroendocrinol 25:779–791
Yoshida S, Kato T, Kanno N, Nishimura N, Nishihara H, Horiguchi K, Kato Y (2017) Cell type-specific localization of Ephs pairing with ephrin-B2 in the rat postnatal pituitary gland. Cell Tissue Res 370:99–112
Yoshida S, Kato T, Kato Y (2016a) EMT Involved in migration of stem/progenitor cells for pituitary development and regeneration. J Clin Med 5:43
Yoshida S, Kato T, Yako H, Susa T, Cai LY, Osuna M, Inoue K, Kato Y (2011) Significant quantitative and qualitative transition in pituitary stem/progenitor cells occurs during the postnatal development of the rat anterior pituitary. J Neuroendocrinol 23:933–943
Yoshida S, Nishimura N, Ueharu H, Kanno N, Higuchi M, Horiguchi K, Kato T, Kato Y (2016b) Isolation of adult pituitary stem/progenitor cell clusters located in the parenchyma of the rat anterior lobe. Stem Cell Res 17:318–329
Yoshida S, Nishimura N, Yurino H, Kobayashi M, Horiguchi K, Yano K, Hashimoto S, Kato T, Kato Y (2018) Differentiation capacities of PS-clusters, adult pituitary stem/progenitor cell clusters located in the parenchymal-niche, of the rat anterior lobe. PLoS One 13:e0196029
Yoshimura F, Soji T, Kiguchi Y (1977a) Relationship between the follicular cells and marginal layer cells of the anterior pituitary. Endocr J 24:301–305
Yoshimura F, Soji T, Sato S, Yokoyama M (1977b) Development and differentiation of rat pituitary follicular cells under normal and some experimental conditions with special reference to an interpretation of renewal cell system. Endocr J 24:435–449
Zhang D, Ighaniyan S, Stathopoulos L, Rollo B, Landman K, Hutson J, Newgreen D (2014) The neural crest: a versatile organ system. Birth Defects Res C Embryo Today 102:275–298
Zhu X, Gleiberman AS, Rosenfeld MG (2007) Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev 87:933–963
Zhu X, Zhang J, Tollkuhn J, Ohsawa R, Bresnick EH, Guillemot F, Kageyama R, Rosenfeld MG (2006) Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev 20:2739–2753
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kato, Y., Yoshida, S. & Kato, T. New insights into the role and origin of pituitary S100β-positive cells. Cell Tissue Res 386, 227–237 (2021). https://doi.org/10.1007/s00441-021-03523-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-021-03523-7