Abstract
The aim of this study is to gather evidence of head-to-head double-blind randomized-controlled trials on the efficacy and safety of available treatments for attention deficit hyperactivity disorder (ADHD) in children and adolescents. A systematic review was conducted by two independent reviewers in ten electronic databases (PROSPERO register CRD42016043239). Methodological quality of included studies was evaluated according to the Jadad scale. Network meta-analyses were performed including double-blinded head-to-head trials comparing active allopathic drugs in patients (0–18 years old) diagnosed with ADHD. The results of efficacy and safety of atomoxetine (ATX), bupropion, buspirone (BSP), dexamphetamine, edivoxetine (EDX), guanfacine (GXR), lisdexamfetamine (LDX), methylphenidate (MPH), mixed amphetamine salts, modafinil, pindolol (PDL), reboxetine (RBX), selegiline, and venlafaxine were analyzed using ADDIS software v.1.16.5. Forty-eight trials were identified (n = 4169 participants), of which 12 were used for efficacy analysis and 33 for safety analysis. On the CGI-I scale, the analysis revealed that MPH was more effective than ATX and GXR. For the safety outcomes, according to drug ranks, LDX was more likely to cause sleep disorders (39%) as well as loss of appetite (65%) and behavior problems such as irritability (60%). BSP (71%) and EDX (44%) caused less appetite decrease. For behavioral effects, PDL was considered safest (50%). For any adverse events, RBX (89%) was the safest alternative. The lack of head-to-head trials properly reporting outcomes of interest limited some comparisons. Network meta-analysis offered a broader overview on the available treatments for ADHD, especially for safety issues, and contributes towards evidence gathering and clinical practice decisions. A core outcome set for ADHD should be designed to guide the conduction and report of clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attention deficit hyperactivity disorder (ADHD) is the most common psychiatric disorder that affects around 3.4% (CI 95% 2.6–4.5%) of school-age children worldwide [1]. According to the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-V), ADHD is characterized by symptoms such as inattention, hyperactivity, and impulsivity [2]. This syndrome may be caused by several changes in the neurotransmitter system. Although its pathophysiology mechanisms have not been completely elucidated [3], studies have shown the involvement of catecholamines, especially noradrenaline and dopamine [4,5,6].
Some studies have investigated the differences between the pharmacological mechanisms of the two main group of drugs for ADHD (stimulants and non-stimulants), but there are still some gaps about their therapeutic effects and possible adverse events [7,8,9]. Stimulant medication, i.e., methylphenidate (MPH) and dexamphetamine (DEX), are the drugs of choice as the first therapeutic strategies [10, 11]. Other options include lisdexamfetamine (LDX) and mixed amphetamine salts (MAS). However, 30% of patients do not respond clinically to these stimulants or are intolerant to therapies whose major complaints are adverse effects such as insomnia, decreased appetite, irritability, mood lability, headache, and gastrointestinal symptoms [11, 12]. Atomoxetine (ATX), a non-stimulant drug, is the second-line treatment for ADHD. Some other available therapeutic alternatives of non-stimulants include: bupropion (BUP), clonidine, guanfacine (GXR), theophylline, modafinil (MOD), amantadine (AMA), selegiline (SLG), and venlafaxine (VEN), some of them of off-label use [13, 14]. The most common adverse events of these drugs are: insomnia, decreased appetite, sedation, dizziness, anxiety, abdominal pain, and headache [15,16,17,18,19,20]. Given this great amount of available strategies, therapeutic decision-making can be difficult if no clear delimitation on the clinical profile of each drug exists.
Double-blind randomized-controlled trials (DBRCT) of direct comparison between active drugs, also referred to as head-to-head (HTH) trials, can provide clear evidence on health technology profiles, being useful for therapeutic decision-making and protocols improvement. However, the high financial cost and the large amount of patients needed to provide significant results may limit this type of study being carried out [21, 22].
Systematic review and meta-analysis are useful tools for strengthening primary efficacy and safety results of clinical trials, preventing additional trials being carried out on a topic [23]. Moreover, the introduction of the statistical concept of multiple treatment meta-analysis, also referred to as multiple treatment comparison (MTC), network meta-analysis (NMA), or indirect meta-analysis, allows the comparison of more interventions simultaneously even when they have not been directly compared by clinical trials [21, 24]. This offers a broader overview of all available treatment in the same model. Compared with pairwise meta-analyses, NMA also allow rank ordering the interventions on the best, second best and so on, which support clinical decisions [24].
The previous studies have already demonstrated significant evidence on the efficacy and safety of some of the drugs used for ADHD [25,26,27,28,29,30,31]. A recent systematic review with NMA comparing some active drugs (ATX, LDX, clonidine, GXR, and MPH) and placebo found no differences among the therapies for safety outcomes [32]. However, there are still other therapies currently available or recently approved for ADHD worldwide that should be better investigated. This evaluation may also guide the update and reformulation of treatment protocols.
Thus, our goal was to perform an NMA to gather in one single model further evidence from HTH DBRCT of all available pharmacological treatments for ADHD in children and adolescents.
Methods
This systematic review is part of a larger project about drugs for ADHD and was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and Cochrane Collaboration recommendations [33,34,35]. The PROSPERO registration number is CRD 420160433239.
Search strategy and selection criteria
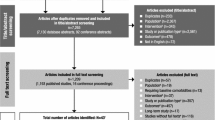
A systematic review was previously conducted by two independent reviewers in ten electronic databases (updated February 2017). Studies meeting the following criteria were included: HTH DBRCT directly comparing active allopathic drugs (any dosage or regimen) in children and adolescents (0–18 years old) diagnosed with ADHD. Crossover and parallel group studies were included. Other types of studies, records in non-Roman characters or trials using only placebo as main comparator were excluded. Initially, 24,765 registers of DBRCT were found (see Appendix 1 for complete search strategies). After exclusion of duplicate studies, titles and abstracts of 14,504 articles were read. Of these, 303 met the inclusion criteria and were fully read. Finally, 53 studies referring to 48 clinical trials were included for data extraction and analyses.
Data extraction and quality assessment
The following data were extracted from the 48 trials: (1) author name(s), publication date, patient characteristics (diagnosis, gender, age, and comorbidities); (2) type of treatment, duration, and dosage; (3) efficacy outcomes reported as psychometric scales; (4) safety outcomes assessed based on the incidence of any adverse events (overall rate reported by the authors of the studies), the most common being sleep disturbance, decreased appetite, and behavior events such as irritability, crying, anxiety, nervousness, aggression, and restlessness. Methodological quality of included trials was assessed according to the Jadad scale [36].
Statistical analysis
The included studies were analyzed using the NMA technique. We compared the efficacy and safety of drugs using ADDIS (Aggregate Data Drug Information System) software v. 1.16.5. NMA is a technique recommended by the International Society for Pharmacoeconomics and Outcome Research (ISPOR) to compare outcomes between different treatments [37]. NMA uses a Bayesian approach and allows comparisons among all treatment arms of the studies, including direct and indirect comparisons simultaneously [38].
To obtain the pooled effect sizes, a random-effect model based on the Markov chain Monte Carlo (MCMC) simulation method was built using a Bayesian approach. A consistency model was drawn for each evaluated outcome and treatments’ relative effect sizes were calculated using odds ratios (OR) for binary outcomes and mean difference (MD) for continuous outcomes. Our model adopted a final random effect rather than a fixed-effect model as it is the most appropriate and conservative analysis to account for variance among studies. The goodness of fit of the model was assessed using residual deviances (DIC). To increase the estimate precision of the relative effect sizes of comparisons and to properly account for correlations between multi-arm trials, rank probabilities involving all the interventions were built for each outcome. Results were reported with 95% credibility intervals (CrI). Rank probabilities were also built to increase the estimate precision of the relative effect sizes of comparisons, enabling conclusions to be drawn for each outcome of interest [24, 37]. These ranks account for all treatments and order them according to their probability of being the best, second best and so on. The robustness of the models was estimated using node splitting analysis, which reveals possible differences among direct and indirect comparisons of a particular node and its ramifications in the network; p values < 0.05 reveal inconsistencies in the network that should be further investigated.
Results
Electronic searches of the systematic review identified 48 HTH DBRCT (n = 4169 participants) [4, 16,17,18,19,20, 39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81]. Studies were published between 1971 and 2016, especially by the United States of America (USA) (50.0%), Iran (26%) and Canada (12.5%). The mean age of patients was 9.35 years (SD 1.46) with a majority of males 77.5%. Most patients were diagnosed with combined symptoms of ADHD (77.6%), while 22.5% were predominantly inattentive, and 2.0% were predominant hyperactive/impulsive. Overall, 23 trials (47.9%) (n = 1209) reported patient’s comorbidities, being ODD (oppositional defiant disorder) the most prevalent (23.8%), followed by conduct disorder (CD = 6.3%) and anxiety (3.0%). The mean duration of treatments was 7 weeks (SD 13.17) and the most commonly used drugs were MPH (77.0%), DEX (20.8%), MAS (16.6%), ATX (12.5%), and LDX (6.3%) (see Supplementary Material Table S1). The Jadad scale indicates that the studies presented moderate-to-good methodological quality (mean score 4, ranging from 1 to 5).
Efficacy outcomes
A total of 12 articles (n = 1552 patients) were identified as providing data on efficacy outcomes [4, 16, 18,19,20, 60, 66,67,68, 73, 81, 82]. The following drugs were reported: MPH, SLG, MAS, BUP, ATX, reboxetine (RBX), MOD, AMA, VEN, memantine (MEM), and GXR.
Considering that studies reported data on several different psychometric scales, the total number of studies that could be used in each comparison was very low. For the ADHD Rating Scale created by DuPaul in 1991, two studies were gathered [18, 60], while six trials [4, 16, 19, 20, 67, 73] informed the ADHD Rating Scale IV introduced by DuPaul in 1998. Two other studies [4, 66] reported data from the Clinical Global Impression Severity scale created by Guy in 1976, two referred to Guy’s 1976 Clinical Global Impression Improvement (CGI-I) scale [81, 82] and another two [66, 68] to the CRS proposed by Conners in 1997. NMA was conducted for each one of these outcomes. However, probably due to the lack of standardized scales and inadequate reporting of results, consistency analyses revealed few statistical differences. Only for Guy’s 1976 CGI-I scale was it possible to compare three drugs (ATX, GXR, and MPH). MPH was found to be more effective than GXR (MD 1.92 [CrI 95% 0.64–5.94]) and ATX (MD 3.15 [CrI 95% 0.75–13.71]). GXR was more effective than ATX (MD 1.65 [CrI 95% 0.65–4.17]) (see Fig. 1). Other complete analyses are shown in the supporting information (Figs. S1–S6). Node splitting analyses were not performed for these networks due to their simple geometry.
a Network of comparisons for efficacy outcome by CGI-I (Guy 1976). The nodes (drugs) are represented by circles. The grey circles represent stimulant drug and the white circles represent non-stimulant drugs. The lines connecting each drug represent direct comparisons, while indirect ones were statistically estimated. The thickness of the line represents the amount of existing comparisons and the size of the circles (nodes) indicates the sample-size number. b Consistency analysis for the outcome of efficacy. Drugs are reported in alphabetical order. The values presented correspond to the mean difference (MD) associated with its credibility interval (CrI). When the CrI does not cross the 0 null line, there is a statistically significant difference between the treatments. Comparisons are made between a first drug (e.g. ATX) and a second drug (e.g. GXR) with presentation of the estimated value (1.65 [0.65–4.17]). An MD value of less than 0 demonstrates that the first drug in the comparison is the more effective. An MD value greater than 0 indicates that the second drug in the comparison is more effective. The highlighted pictures presented statistical differences. c Rank probabilities of drugs. The values are given as the probability of each treatment occupying a position. Ranking 1 is the best therapy and the last one is the worst treatment for this outcome. ATX atomoxetine, GXR guanfacine, MPH methylphenidate
Safety outcomes
A total of 33 articles (n = 3493 patients) evaluated at least one safety outcome [4, 16,17,18,19,20, 45, 50,51,52,53, 55,56,57, 60,61,62,63,64,65,66,67,68, 72,73,74,75, 77,78,79, 81,82,83] reporting data on: (1) any adverse event, (2) sleep disturbance, (3) decreased appetite, or (4) behavior events (e.g., irritability, crying, anxiety, nervousness, aggression, and restlessness). A network of comparisons was built for each outcome of interest. For sleep disturbances, 14 drugs were included (BUP, buspirone—BSP and DEX, edivoxetine—EDX, GXR, ATX, MPH, LDX, and MOD, pindolol—PDL, RBX, SLG, VEN, and MAS), with 17 direct comparisons between them; MPH vs. ATX involved three studies, while comparisons of MPH vs. BUP, MPH vs. BSP, MPH vs. DEX, and MPH vs. SLG involved two studies each (see Fig. 2). For the outcome of decreased appetite, the analysis included 12 drugs with 14 possible direct comparisons, the most common being MPH vs. MAS (five studies). For the outcome of behavior events, EDX, DEX, BUP, GXR, ATX, MPH, LDX, PDL, VEN, MOD, and MAS were evaluated. Finally, for the outcome of any adverse event, eight drugs were analyzed (BUP, EDX, ATX, GXR, MPH, LDX, RBX, and MAS), the comparison MPH vs. ATX being the most prevalent (n = 3 studies) (see Fig. 2).
Network of comparisons for safety outcomes: sleep disturbance, decreased appetite, behavior event, and any adverse event. The nodes (drugs) are represented by circles. The grey circles represent stimulant drugs and the white circles represent non-stimulant drugs. The lines connecting each drug represent direct comparisons, while indirect ones were statistically estimated. The thickness of the line represents the amount of existing comparisons and the size of the circles (nodes) indicates the sample number. ATX atomoxetine, BUP bupropion, BSP buspirone, DEX dexamphetamine, EDX edivoxetine, GXR guanfacine, LDX Lisdexamfetamine, MPH methylphenidate, MAS mixed amphetamine salts, MOD modafinil, PDL pindolol, RBX reboxetine, SLG selegiline, VEN venlafaxine
The consistency analysis showed many statistical differences among drug profiles for the outcome of sleep disorders. ATX presented significantly more chance of causing sleep problems compared to BSP (OR 0.05 [CrI 95% 0.00–0.40]) and BUP is less safe than BSP (OR 0.04 [CrI 95% 0.00–0.57]) for this outcome. BSP was safer than DEX, EDX, GXR, LDX, MPH, MAS and PDL (see Fig. 3). In addition, DEX (OR 0.06 [CrI 95% 0.00–0.72], GXR (OR 0.08 [CrI 95% 0.00–0.99], LDX (OR 0.04 [CrI 95% 0.00–0.47]), MPH (OR 0.07 [CrI 95% 0.00–0.68]), MAS (OR 0.05 [CrI 95% 0.00–0.55]), and PDL (OR 0.05 [CrI 95% 0.00–0.76]) showed statistically unfavorable results when compared to SLG. Moreover, the drugs DEX, GXR, LDX, MPH, MAS, and PDL also presented a worst profile than VEN. The options MAS and LDX proved to cause more insomnia than MOD (OR 0.12 [CrI 95% 0.01–0.89] and OR 0.10 [CrI 95% 0.01–0.76], respectively). Finally, ATX was safe than LDX (OR 4.02 [CrI 95% 1.71–10.49]), MPH (OR 2.43 [CrI 95% 1.35–4.57]), and MAS (OR 3.31 [CrI 95% 1.39–7.44]) for this same outcome (see Fig. 3).
Consistency analysis: sleep disturbance. Drugs are reported in alphabetical order. Comparisons are made between a first drug (e.g. ATX) and a third drug (e.g. BSP), with presentation of the estimated value in the gap (0.05 [0.00–0.40]). The values presented correspond to the odds ratio (OR) associated with its credibility interval (CrI). An OR value greater than 1 demonstrates that the first drug in the comparison is the safer. An OR value of less than 1 indicates that the second drug in the comparison is safer. The highlighted pictures presented a statistically significant difference between drugs. ATX atomoxetine, BUP bupropion, BSP buspirone, DEX dexamphetamine, EDX edivoxetine, LDX Lisdexamfetamine, MPH methylphenidate, MAS mixed amphetamine salts, MOD modafinil, PDL pindolol, RBX reboxetine, SLG selegiline, VEN venlafaxine
The drug ATX caused more loss of appetite than BSP (OR 0.04 [CrI 95% 0.01–0.22]), EDX (OR 0.10 [CrI 95% 0.03–0.42], and GXR (OR 0.40 [CrI 95% 0.18–0.89]. The same occurred for DEX when compared to EDX (OR 0.10 [CrI 95% 0.02–0.59], as well for LDX vs. MPH (OR 0.45 [CrI 95% 0.21–0.93] vs. MOD (OR 0.09 [CrI 95% 0.01–0.49] and vs. VEN (OR 0.07 [CrI 95% 0.01–0.59]. On the other hand, BSP showed to be safer than LDX (OR 63.97 [CrI 95% 11.28–574.44]), MPH (OR 29.54 [CrI 95% 5.43–222.02]), MAS (OR 43.31 [8.29–359.74]), PDL (OR 17.57 [CrI 95% 2.38–191.82]), and RBX (OR 26.24 [CrI 95% 2.01–506.95]) for this outcome. Other differences were seen between EDX vs. LDX, MPH, MAS, PDL, and RBX. Both ATX and GXR were better options than LDX (see Fig. 4).
Consistency analysis decreased appetite. Drugs are reported in alphabetical order. Comparisons are made between a first drug (e.g., ATX) and a second drug (e.g., BSP), with presentation of the estimated value (0.04 [0.01–0.22]). The values presented correspond to the odds ratio (OR) associated with its credibility interval (CrI). An OR value greater than 1 demonstrates that the first drug in the comparison is the safer. An OR value of less than 1 indicates that the second drug in the comparison is safer. The highlighted pictures presented a statistically significant difference between drugs. ATX atomoxetine, BSP buspirone, DEX dexamphetamine, EDX edivoxetine, GXR guanfacine, LDX Lisdexamfetamine, MPH methylphenidate, MAS mixed amphetamine salts, MOD modafinil, PDL pindolol, RBX reboxetine, VEN venlafaxine
Concerning behavior effects, LDX, MPH, and MAS led to significantly more events when compared to PDL (OR 0.17 [CrI 95% 0.03–0.68]; OR 0.25 [CrI 95% 0.05–0.95] and OR 0.10 [CrI 95% 0.01–0.84, respectively). For the outcome of incidence of “any adverse event”, the drugs ATX, BUP, LDX, and GXR were statistically less safe than RBX (OR 0.10 [CrI 95% 0.01–0.77]; OR 0.04 [CrI 95% 0.00–0.64]; OR 0.10 [CrI 95% 0.01–0.89], and OR 0.07 [CrI 95% 0.01–0.65], respectively). No other statistical difference was obtained (see Supporting Information Figs. S7 and S8).
According to drug rankings (see Fig. 5), LDX was more likely to cause sleep disorders (39% chance of being the worst option) as well as loss of appetite (65%) and behavior problems such as irritability (60%). BSP and VEN were the best options against sleep disorders, being ranked with 62, 31% of chances, respectively. For behavioral effects, PDL was considered safest (50%). BUP was more likely to cause any adverse event (54%), while RBX (89%) followed by EDX (39%) were considered safer options for this outcome. All networks for safety outcomes were subjected to analysis by the node splitting method. All analyses revealed p values superior to 0.05, ensuring the robustness of the networks (see Supporting Information Tables S2–S5).
Rank probabilities for the security outcomes: a sleep disturbance, b decreased appetite, c behavior event, and d any adverse event. The values are given as the probability of each treatment occupying a position. Ranking 1 is the worst therapy (more likely to lead to the onset of the adverse event) and the last one is the best (safest) treatment for this outcome. ATX atomoxetine, BUP bupropion, BSP buspirone, DEX dexamphetamine, EDX edivoxetine, GXR guanfacine, LDX Lisdexamfetamine, MAS mixed amphetamine salts, MOD modafinil, MPH methylphenidate, PDL pindolol, RBX reboxetine, SLG selegiline, VEN venlafaxine
Discussion
Our study was able to gather further evidence on 14 drugs used for ADHD treatment in children and adolescents, especially for safety outcomes. Of these drugs, six are approved in the USA (ATX, MPH, DEX, LDX, GXR, and MAS) and six in many European countries (ATX, MPH, DEX, LDX, MAS, and GXR) [84, 85].
As expected, the methodological quality of included trials was considered overall good, revealing that studies were well designed, conducted, and reported. The comparison of HTH DBRCT can provide high level and clear evidence and may support clinical decisions. Baseline characteristics of ADHD patients were similar among the included trials, being the diagnostic of combined symptoms of the disorder the most common, along with ODD comorbidity.
Despite psychostimulant drugs such as MPH and amphetamines (DEX, LDX, and MAS) being considered as the first-line pharmacological agents [86], other important factors including adverse effects, toxicity, personal preference, the presence of a psychiatric problem, and cost should be taken into account during therapeutic decisions [75]. Currently, several alternatives such as ATX, BUP, BSP, EDX, MOD, PDL, GXR, RBX, SLG, and VEN are being studied to improve treatment alternatives, both in children or adolescents and adults [87]. Our results showed significant differences in one psychometric scale for efficacy (CGI-I), which revealed that MPH was more effective than GXR and ATX. Another similar study [32] concluded that the best clinical response measured on the same scale was from LDX, followed by MPH. Among the non-stimulants, the previous studies also concluded that GXR has more probability of being effective than ATX [32]. Moreover, NMA associated with the previous pairwise meta-analyses addressing the efficacy and tolerability of drugs used in ADHD and other psychiatric disorders [25, 32, 85], demonstrated only few statistical differences among drugs that were similarly highlighted in our results. Researchers who used other psychometric scales showed that ATX and MPH have no significant difference in the clinical profile [85]. Thus, because a few significant differences among drug’s efficacy exist, safety outcomes are paramount for decision-making.
The drugs BSP, PDL, and RBX were, in general, safer than the others. BSP, a anxiolytic agent with an antidepressant action [72], was approved by the FDA (US Food and Drug Administration) in 1986 and is indicated for generalized anxiety disorder [88], but, because of its dopaminergic effects, it was supposed that BSP might also be effective in treating ADHD (off-label use). In our study, BSP and SLG, an antidepressant agent (type B monoamine oxidase inhibitor) that is metabolized to amphetamine and MPH stimulant compounds, were probably the best options to avoid sleep disorder reactions, especially when compared to ATX and MPH. We evidenced significant differences among these last two drugs, being MPH more responsible for cases of insomnia than ATX. Despite a few significant differences have been found in the network regarding CGI-I by Guy 1976 favoring MPH over ATX, one should consider that this NMA was built with only two studies, providing large credibility intervals for this comparison. Hence, taking into account the efficacy data of a previous study [85], we may suggest that ATX would be as effective as MPH and less likely to cause sleep disturbances. However, neither drug was considered the best option for this outcome.
According to clinical experience, stimulants like MPH often adversely affect sleep, either due to a direct drug effect or due to a secondary “rebound” effect. On the other hand, ATX might have fewer negative effects on sleep, because it is a non-stimulant that has highly selective inhibition of the presynaptic norepinephrine transporter with a little affinity for other neurotransmitter transporters or receptors [64]. Despite ATX having been approved in USA and Australia, as well as in South America, the Middle East, Africa, Asia, and Europe [66], there is still a need for better quality evidence about this agent that support its use in clinical practice [63]. As well, despite MPH being the reference drug for the treatment of ADHD [75], our results revealed worse safety profiles compared with other options, mainly regarding sleep disturbances, loss of appetite, and behavior events.
GXR was revealed to be safer than ATX, LDX, MPH, and MAS against loss of appetite. In another study, researchers found a greater probability of GXR being discontinued than ATX [32] due to adverse events. However, further study is needed to confirm this outcome in ADHD treatment.
PDL was the safest option in behavior events, causing less crying and irritability. On the other hand, LDX was more likely to cause disturbances of sleep, loss of appetite, and behavioral problems. Some researchers [78] compared LDX with ATX and reported adverse events such as decreased appetite, restlessness, drowsiness, excoriations, tics, indifference, nausea, and irritability, leading to LDX discontinuation in DBRCT. The study by Coghill [75, 89] comparing LDX with MPH also reported adverse events such as anorexia, loss of weight and appetite, nausea, and insomnia in 72.1% of the 196 participants who were treated with LDX. This drug became the first long-acting, amphetamine-based medication to be approved in Europe and is one of the drugs used as a first-line treatment for ADHD in USA, Canada, and Brazil. Several European countries use LDX for the treatment of children and adolescents with ADHD who have had a clinically inadequate response to MPH [78]. However, our results discourage the use of LDX, given its safety profile, and we recommend that other above-mentioned drugs are taken into account for further investigation.
Finally, RBX presented promising results in the NMA. This is a selective norepinephrine reuptake inhibitor [90,91,92,93] used to treat depression. It has been approved and marketed in the United Kingdom and Germany since 1997, besides Italy, Spain, Sweden, Denmark, Ireland, Austria, and Finland [91]. However, some clinical studies conducted in USA and Canada, prompted by the FDA, resulted in a letter of non-approval, and this drug was ultimately rejected after preliminary acceptance [92]. In May 2001, the FDA declined a new drug application because of a lack of compelling evidence of efficacy. At present, it is unclear whether further investment will be made to seek sufficient evidence of efficacy to gain marketing approval for RBX for ADHD treatment [91, 93, 94]. The previous non-controlled studies and controlled trials support the promising efficacy of RBX for treating ADHD in patients without psychiatric comorbidities [95].
The major limitations of the present study include few comparisons that were able to be drawn for efficacy outcomes. Moreover, NMA was not sufficiently sensitive to provide solid conclusions for some other safety outcomes. The reasons for this weakness are mainly the lack of enough studies properly reporting outcomes and the absence of a core outcome set (COS) for ADHD. COS are useful methodologies to standardize the outcomes that should be reported to evaluate a clinical condition such as ADHD in clinical trials [96]. This may ensure evidence gathering for future studies and standardize the reporting of results, avoiding selective bias and loss of information. Given the wide range of psychometric scales and symptom measures in psychiatry, we reinforce the need for a COS construction to allow future evidence gathering on efficacy outcomes. Furthermore, other studies with the drugs that showed promising results (i.e. PDL and RBX) should be conducted to place these drugs in clinical practice. We have evaluated “any adverse event” as an overall safety outcome (as reported by the authors of the original trials), but further evaluation on specific adverse events is paramount to draw conclusions about drug’s profile.
NMA has the advantage of producing information that cannot be obtained only with the conventional pairwise meta-analysis of HTH DBRCT. All available therapeutic options seem to present a similar efficacy profile. First- and second-line therapies (MPH and ATX) were not the safer options among treatments used for ADHD. Other drugs such as BSP, PDL, and RBX may provide safer alternatives with a certain equivalent efficacy. Our results can guide future studies, reinforcing the need for the development of a COS for ADHD to support evidence generating for clinical practice.
References
Polanczyk GV et al (2014) ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol 43(2):434–442
Austerman J (2015) ADHD and behavioral disorders: assessment, management, and an update from DSM-5. Cleve Clin J Med 82(11 Suppl 1):S2–S7
Bruxel EM et al (2014) ADHD pharmacogenetics across the life cycle: new findings and perspectives. Am J Med Genet B Neuropsychiatr Genet 165B(4):263–282
Mohammadi MR, Mohammadzadeh S, Akhondzadeh S (2015) Memantine versus methylphenidate in children and adolescents with attention deficit hyperactivity disorder: a double-blind, randomized clinical trial. Iran J Psychiatry 10(2):106–114
Root RW II, Resnick RJ (2003) An update on the diagnosis and treatment of attention-deficit/hyperactivity disorder in children. Prof Psychol Res Pract 34(1):34
Lopresti AL (2015) Oxidative and nitrosative stress in ADHD: possible causes and the potential of antioxidant-targeted therapies. Atten Defic Hyperact Disord 7(4):237–247
Stevens JR, Wilens TE, Stern TA (2013) Using stimulants for attention-deficit/hyperactivity disorder: clinical approaches and challenges. Prim Care Companion CNS Disord 15(2). https://doi.org/10.4088/PCC.12f01472
Stahl SM (2010) Mechanism of action of stimulants in attention-deficit/hyperactivity disorder. J Clin Psychiatry 71(1):12–13
Spiller HA, Hays HL, Aleguas A Jr (2013) Overdose of drugs for attention-deficit hyperactivity disorder: clinical presentation, mechanisms of toxicity, and management. CNS Drugs 27(7):531–543
Nakanishi Y et al (2017) Differential therapeutic effects of atomoxetine and methylphenidate in childhood attention deficit/hyperactivity disorder as measured by near-infrared spectroscopy. Child Adolesc Psychiatry Ment Health 11:26
Storebo OJ, et al (2015) Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). Cochrane Database Syst Rev (11):Cd009885
Rostain A et al (2015) Toward quality care in ADHD: defining the goals of treatment. J Atten Disord 19(2):99–117
Procyshyn RM, Bezchlibnyk-Butler KZ, Jeffries JJ (2015) Clinical handbook of psychotropic drugs. Hogrefe Publishing Corporation/Hogrefe Publishing GmbH, Boston/Göttingen
Banaschewski T et al (2004) Non-stimulant medications in the treatment of ADHD. Eur Child Adolesc Psychiatry 13(Suppl 1):I102–I116
Hennissen L et al (2017) Cardiovascular effects of stimulant and non-stimulant medication for children and adolescents with ADHD: a systematic review and meta-analysis of trials of methylphenidate, amphetamines and atomoxetine. CNS Drugs 31:1–17
Jafarinia M et al (2012) Bupropion versus methylphenidate in the treatment of children with attention-deficit/hyperactivity disorder: randomized double-blind study. Hum Psychopharmacol 27(4):411–418
Akhondzadeh S et al (2003) Selegiline in the treatment of attention deficit hyperactivity disorder in children: a double blind and randomized trial. Prog Neuropsychopharmacol Biol Psychiatry 27(5):841–845
Arabgol F, Panaghi L, Hebrani P (2009) Reboxetine versus methylphenidate in treatment of children and adolescents with attention deficit-hyperactivity disorder. Eur Child Adolesc Psychiatry 18(1):53–59
Mohammadi MR et al (2010) Amantadine versus methylphenidate in children and adolescents with attention deficit/hyperactivity disorder: a randomized, double-blind trial. Hum Psychopharmacol 25(7–8):560–565
Zarinara AR et al (2010) Venlafaxine versus methylphenidate in pediatric outpatients with attention deficit hyperactivity disorder: a randomized, double-blind comparison trial. Hum Psychopharmacol 25(7–8):530–535
Song F et al (2009) Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. BMJ 338:b1147
Song F et al (2011) Inconsistency between direct and indirect comparisons of competing interventions: meta-epidemiological study. BMJ 343:d4909
Tonin FS et al (2015) Adverse events and treatment failure leading to discontinuation of recently approved antipsychotic drugs in schizophrenia: a network meta-analysis. Schizophr Res 169(1):483–485
Tonin FS et al (2017) Network meta-analysis: a technique to gather evidence from direct and indirect comparisons. Pharm Pract 1(1):943
Cortese S et al (2017) Comparative efficacy and tolerability of pharmacological interventions for attention-deficit/hyperactivity disorder in children, adolescents and adults: protocol for a systematic review and network meta-analysis. BMJ Open 7(1):e013967
Punja S et al (2016) To meta-analyze or not to meta-analyze? A combined meta-analysis of N-of-1 trial data with RCT data on amphetamines and methylphenidate for pediatric ADHD. J Clin Epidemiol 76:76–81
Camporeale A et al (2015) Safety and tolerability of atomoxetine in treatment of attention deficit hyperactivity disorder in adult patients: an integrated analysis of 15 clinical trials. J Psychopharmacol 29(1):3–14
Chan E, Fogler JM, Hammerness PG (2016) Treatment of attention-deficit/hyperactivity disorder in adolescents: a systematic review. JAMA J Am Med Assoc 315(18):1997–2008
Cohen SC et al (2015) Meta-analysis: risk of tics associated with psychostimulant use in randomized, placebo-controlled trials. J Am Acad Child Adolesc Psychiatry 54(9):728–736
Maneeton B et al (2015) Comparative efficacy, acceptability, and tolerability of lisdexamfetamine in child and adolescent ADHD: a meta-analysis of randomized, controlled trials. Drug Des Dev Ther 9:1927–1936
Maneeton N et al (2015) A systematic review of dexmethylphenidate versus placebo in child and adolescent ADHD: a meta-analysis of randomized, controlled trials. Eur Neuropsychopharmacol 25:S642
Joseph A et al (2017) Comparative efficacy and safety of attention-deficit/hyperactivity disorder pharmacotherapies, including guanfacine extended release: a mixed treatment comparison. Eur Child Adolesc Psychiatry 26:1–23
Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0. Cochrane
Hutton B et al (2015) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 162(11):777–784
Moher D et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62(10):1006–1012
Jadad AR et al (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17(1):1–12
Jansen JP et al (2011) Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health 14(4):417–428
Dias S et al (2013) Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Mak 33(5):641–656
Weiss G et al (1971) Comparison of the effects of chlorpromazine, dextroamphetamine and methylphenidate on the behaviour and intellectual functioning of hyperactive children. Can Med Assoc J 104(1):20–25
Arnold LE et al (1972) Levoamphetamine and dextroamphetamine: comparative efficacy in the hyperkinetic syndrome. Assessment by target symptoms. Arch Gen Psychiatry 27(6):816–822
Huestis RD, Arnold LE, Smeltzer DJ (1975) Caffeine versus methylphenidate and d-amphetamine in minimal brain dysfunction: a double-blind comparison. Am J Psychiatry 132(8):868–870
Garfinkel BD, Webster CD, Sloman L (1975) Methylphenidate and caffeine in the treatment of children with minimal brain dysfunction. Am J Psychiatry 132(7):723–728
Arnold LE et al (1978) Methylphenidate vs dextroamphetamine vs caffeine in minimal brain dysfunction: controlled comparison by placebo washout design with Bayes’ analysis. Arch Gen Psychiatry 35(4):463–473
Arnold LE et al (1976) Levoamphetamine vs dextroamphetamine in minimal brain dysfunction. Replication, time response, and differential effect by diagnostic group and family rating. Arch Gen Psychiatry 33(3):292–301
Conners CK, Taylor E (1980) Pemoline, methylphenidate, and placebo in children with minimal brain dysfunction. Arch Gen Psychiatry 37(8):922–930
Butter HJ et al (1983) A comparative study of the efficacy of ACTH4-9 analog, methylphenidate, and placebo on attention deficit disorder with hyperkinesis. J Clin Psychopharmacol 3(4):226–230
Garfinkel BD et al (1983) Tricyclic antidepressant and methylphenidate treatment of attention deficit disorder in children. J Am Acad Child Psychiatry 22(4):343–348
Donnelly M et al (1989) Fenfluramine and dextroamphetamine treatment of childhood hyperactivity. Clinical and biochemical findings. Arch Gen Psychiatry 46(3):205–212
Zametkin A et al (1985) Treatment of hyperactive children with monoamine oxidase inhibitors. I. Clinical efficacy. Arch Gen Psychiatry 42(10):962–966
Pelham WE Jr et al (1990) Relative efficacy of long-acting stimulants on children with attention deficit-hyperactivity disorder: a comparison of standard methylphenidate, sustained-release methylphenidate, sustained-release dextroamphetamine, and pemoline. Pediatrics 86(2):226–237
Elia J et al (1993) Classroom academic performance: improvement with both methylphenidate and dextroamphetamine in ADHD boys. J Child Psychol Psychiatry 34(5):785–804
Barrickman LL et al (1995) Bupropion versus methylphenidate in the treatment of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 34(5):649–657
Buitelaar JK et al (1996) Pindolol and methylphenidate in children with attention-deficit hyperactivity disorder. Clinical efficacy and side-effects. J Child Psychol Psychiatry 37(5):587–595
Efron D, Jarman F, Barker M (1997) Methylphenidate versus dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics 100(6):E6
Pelham WE et al (1999) A comparison of ritalin and adderall: efficacy and time-course in children with attention-deficit/hyperactivity disorder. Pediatrics 103(4):e43
Pelham WE et al (1999) A comparison of morning-only and morning/late afternoon adderall to morning-only, twice-daily, and three times-daily methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics 104(6):1300–1311
Pliszka SR et al (2000) A double-blind, placebo-controlled study of adderall and methylphenidate in the treatment of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 39(5):619–626
James RS et al (2001) Double-blind, placebo-controlled study of single-dose amphetamine formulations in ADHD. J Am Acad Child Adolesc Psychiatry 40(11):1268–1276
Overtoom CC et al (2003) Effects of methylphenidate, desipramine, and l-dopa on attention and inhibition in children with attention deficit hyperactivity disorder. Behav Brain Res 145(1–2):7–15
Mohammadi MR et al (2004) Selegiline in comparison with methylphenidate in attention deficit hyperactivity disorder children and adolescents in a double-blind, randomized clinical trial. J Child Adolesc Psychopharmacol 14(3):418–425
Mohammadi MR et al (2004) Efficacy of theophylline compared to methylphenidate for the treatment of attention-deficit hyperactivity disorder in children and adolescents: a pilot double-blind randomized trial. J Clin Pharm Ther 29(2):139–144
Wigal S et al (2004) A double-blind, placebo-controlled trial of dexmethylphenidate hydrochloride and d,l-threo-methylphenidate hydrochloride in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 43(11):1406–1414
Wigal SB et al (2005) A laboratory school comparison of mixed amphetamine salts extended release (Adderall XR) and atomoxetine (Strattera) in school-aged children with attention deficit/hyperactivity disorder. J Atten Disord 9(1):275–289
Sangal RB et al (2006) Effects of atomoxetine and methylphenidate on sleep in children with ADHD. Sleep 29(12):1573–1585
Biederman J et al (2007) Lisdexamfetamine dimesylate and mixed amphetamine salts extended-release in children with ADHD: a double-blind, placebo-controlled, crossover analog classroom study. Biol Psychiatry 62(9):970–976
Wang Y et al (2007) Atomoxetine versus methylphenidate in paediatric outpatients with attention deficit hyperactivity disorder: a randomized, double-blind comparison trial. Aust N Z J Psychiatry 41(3):222–230
Amiri S et al (2008) Modafinil as a treatment for attention-deficit/hyperactivity disorder in children and adolescents: a double blind, randomized clinical trial. Prog Neuropsychopharmacol Biol Psychiatry 32(1):145–149
Newcorn JH et al (2008) Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. Am J Psychiatry 165(6):721–730
Daviss WB et al (2008) Clonidine for attention-deficit/hyperactivity disorder: II. ECG changes and adverse events analysis. J Am Acad Child Adolesc Psychiatry 47(2):189–198
Palumbo DR et al (2008) Clonidine for attention-deficit/hyperactivity disorder: I. Efficacy and tolerability outcomes. J Am Acad Child Adolesc Psychiatry 47(2):180–188
Nair V, Mahadevan S (2009) Randomised controlled study-efficacy of clonidine versus carbamazepine in children with ADHD. J Trop Pediatr 55(2):116–121
Davari-Ashtiani R et al (2010) Buspirone versus methylphenidate in the treatment of attention deficit hyperactivity disorder: a double-blind and randomized trial. Child Psychiatry Hum Dev 41(6):641–648
Stein MA et al (2011) Dose effects and comparative effectiveness of extended release dexmethylphenidate and mixed amphetamine salts. J Child Adolesc Psychopharmacol 21(6):581–588
Mohammadi MR et al (2012) Buspirone versus methylphenidate in the treatment of children with attention- deficit/hyperactivity disorder: randomized double-blind study. Acta Med Iran 50(11):723–728
Coghill D et al (2013) European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol 23(10):1208–1218
Santisteban JA et al (2014) Effect of extended-release dexmethylphenidate and mixed amphetamine salts on sleep: a double-blind, randomized, crossover study in youth with attention-deficit hyperactivity disorder. CNS Drugs 28(9):825–833
Lin DY et al (2014) A randomized trial of edivoxetine in pediatric patients with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 24(4):190–200
Dittmann RW et al (2013) Efficacy and safety of lisdexamfetamine dimesylate and atomoxetine in the treatment of attention-deficit/hyperactivity disorder: a head-to-head, randomized, double-blind, phase IIIb study. CNS Drugs 27(12):1081–1092
Nagy P et al (2016) Functional outcomes from a head-to-head, randomized, double-blind trial of lisdexamfetamine dimesylate and atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder and an inadequate response to methylphenidate. Eur Child Adolesc Psychiatry 25(2):141–149
Arabgol F, Panaghi L, Nikzad V (2015) Risperidone versus methylphenidate in treatment of preschool children with attention-deficit hyperactivity disorder. Iran J Pediatr 25(1):e265
McCracken JT et al (2016) Combined stimulant and guanfacine administration in attention-deficit/hyperactivity disorder: a controlled, comparative study. J Am Acad Child Adolesc Psychiatry 55(8):657.e1–666.e1
Huss M et al (2015) Efficacy and safety of extended-release guanfacine hydrochloride in children and adolescents with attention-deficit/hyperactivity disorder: a randomized, double-blind, multicentre, placebo- and active-reference phase 3 study. Aust N Z J Psychiatry 49:111
Efron D, Jarman F, Barker M (1997) Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics 100(4):662–666
ATTENTION-DEFICIT SO (2011) ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 2011–2654
Rezaei G et al (2016) Comparative efficacy of methylphenidate and atomoxetine in the treatment of attention deficit hyperactivity disorder in children and adolescents: a systematic review and meta-analysis. Med J Islam Repub Iran 30:325
Thapar A, Cooper M (2016) Attention deficit hyperactivity disorder. Lancet 387(10024):1240–1250
Chierrito de Oliveira D, et al (2017) Safety of treatments for ADHD in adults: pairwise and network meta-analyses. J Atten Disord. https://doi.org/10.1177/1087054717696773
Apter JT, Allen LA (1999) Buspirone: future directions. J Clin Psychopharmacol 19(1):86–93
Coghill DR et al (2014) Efficacy of lisdexamfetamine dimesylate throughout the day in children and adolescents with attention-deficit/hyperactivity disorder: results from a randomized, controlled trial. Eur Child Adolesc Psychiatry 23(2):61–68
Cohen-Yavin I et al (2009) Efficacy of reboxetine in the treatment of attention-deficit/hyperactivity disorder in boys with intolerance to methylphenidate: an open-label, 8-week, methylphenidate-controlled trial. Clin Neuropharmacol 32(4):179–182
Page ME (2003) The promises and pitfalls of reboxetine. CNS Drug Rev 9(4):327–342
Eyding D et al (2010) Reboxetine for acute treatment of major depression: systematic review and meta-analysis of published and unpublished placebo and selective serotonin reuptake inhibitor controlled trials. BMJ 341:c4737
Preskorn SH (2004) Reboxetine: a norepinephrine selective reuptake pump inhibitor. J Psychiatr Pract 10(1):57–63
Sepede G et al (2012) Reboxetine in clinical practice: a review. Clin Ter 163(4):e255–e262
Ghanizadeh A (2015) A systematic review of reboxetine for treating patients with attention deficit hyperactivity disorder. Nord J Psychiatry 69(4):241–248
Kirkham JJ et al (2013) Can a core outcome set improve the quality of systematic reviews?–a survey of the Co-ordinating Editors of Cochrane Review Groups. Trials 14:21
Author information
Authors and Affiliations
Contributions
SV and RP conceived and designed the study; SCOSP, FST, and HHLB acquired and analyzed data; SCOSP, FST, and HHLB drafted the manuscript; SV and RP read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Padilha, S.C.O.S., Virtuoso, S., Tonin, F.S. et al. Efficacy and safety of drugs for attention deficit hyperactivity disorder in children and adolescents: a network meta-analysis. Eur Child Adolesc Psychiatry 27, 1335–1345 (2018). https://doi.org/10.1007/s00787-018-1125-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-018-1125-0