Abstract
Attention deficit hyperactivity disorder (ADHD) is one of the most common neurobehavioral disorders. We carried out this comparison of multiple treatments based on sufficient data in attempt to evaluate the efficacy and safety of ADHD medication for children and adolescents. PubMed, Embase and the Cochrane Database were used to search for relevant articles. Changes in the ADHD Rating Scale (ADHD-RS) scores and the Conners’ Parent Rating Scale-Revised (CPRS) scores were used as outcomes for efficacy. Withdrawals due to all-cause, adverse effects and lack of efficacy were defined as primary outcomes evaluating the safety of such medications. Both pair-wise and network meta-analyses were performed. Efficacy and safety of atomoxetine (ATX), bupropion (BUP), clonidine hydrochloride (CLON), guanfacine extended release (GXR), lisdexamfetamine dimesylate (LDX), and methylphenidate (MPH) were evaluated. LDX has the highest efficacy and a relatively lower rate of adverse effects compared to BUP, CLON and GXR. MPH has the lowest incidence rate of adverse effects and takes second place concerning ADHD-RS scores and third place concerning CPRS scores. ATX has the lowest incidence rate of all-cause withdrawals. The efficacy of ATX seems, however, to be lower than CLON, GXR, LDX and MPH. Adversely, BUP has the highest incidence rate of withdrawals and the second highest probability of causing adverse effects as well as lack of efficacy; therefore it should not be recommended as a treatment for ADHD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As one of the most common neurobehavioral disorders, attention deficit hyperactivity disorder (ADHD) typically begins in childhood, becomes prevalent during adolescence, and may further persist into adulthood [1]. ADHD is characterized by the development of inattention, hyperactivity and impulsivity, which leads to academic and social function impairment with an early onset [2]. It is reported there are certain habits which have a negative impact on health may increase ADHD risk, such as smoking, substance abuse and so on. ADHD patients, especially children, have great difficulty in learning how to control their own emotion and behavior [2]. According to a systematic review, ADHD prevalence in children worldwide reached 5.29 %, with an incidence rate of 5 % in Europe and 6.0 % to 6.5 % in North America [3]. A study conducted by the National Survey of Children’s Health (NSCH) indicated that the percentage of children and adolescence (4–17 years) with ADHD, according to the parents, witnessed a 21.8 % increase from 2003 to 2007 in the United States [2]. In other countries, Slovenia for instance, prevalence of ADHD in 2010 is estimated to reach 1 % in children and adolescents, implying an anticipated 6.3 fold increase compared to the 1997 figures [2]. Although there may have been some bias and errors made in this estimation, it is nevertheless evident that there is an increasing trend of ADHD in children. It was also found that males were more susceptible to ADHD than females, with a difference in ratio of 2:1 and 9:1 [2]. Therefore, treating ADHD in children and adolescents is pivotal.
Current treatments for ADHD in children and adolescents include psychological and behavioral interventions such as training for parents, as well as medical therapy [4]. Thanks to the more recent attention brought to ADHD, as well as an early diagnosis, the rate of ADHD patients benefiting from medical treatment has increased rapidly [3]. Licensed medications for ADHD treatment of children and adolescents in the United Kingdom consist of methylphenidate (MPH) and atomoxetine (ATX). Among which ATX is most popular used. Its primary advantage over the standard stimulant treatments for ADHD is that it has little known abuse potential. While it has been shown to significantly reduce inattentive and hyperactive symptoms, the responses were lower than the response to stimulants. Additionally, 40 % of participants who were treated with Atomoxetine experienced significant residual ADHD symptoms [5]. Besides, lisdexamfetamine (LDX), guanfacine (GXR) are also drugs recently approved for ADHD treatment. For patients who do not respond to the drugs mentioned above, medications including bupropion (BUP), catecholamine, amphetamine, clonidine (CLON) are adopted as alternatives.
Multiple systematic reviews have been performed to evaluate the efficacy of ADHD treatment in children and adolescents. However, considering the limitations in trial numbers and the drawback of traditional meta-analysis that can only utilize pair-wised trials, a comprehensive evaluation and analysis is needed to compare and rank the efficacy and safety of medications for ADHD in children and adolescents. The comparison of multiple treatments, or network meta-analysis, is a credible method in calculating the effectiveness of various treatments and in ranking them by direct and indirect comparison. Several network meta-analyses have been performed to evaluate the treatments for ADHD. King et al. conducted a study to evaluate the cost-effectiveness of ADHD treatments [2], he found LDX showed significantly high efficacy than GXR, ATX and MPH [6]. Additional research also validated that LDX was an effective option over ATX and MPH, although the safety of these treatments remained inconclusive [3]. Although network meta-analyses have been performed before, there is still room for improvement in network meta-analysis. King did not measure the safety of ADHD treatments [2]. Furthermore Roskell’s study results were limited by the number of trials involved and the limited outcomes, which only focus on the short-term efficacy of treatments in patients without comorbid disorders [7]. Moreover, the number of randomized clinical trials (RCTs) included in the researches mentioned above was limited, Joseph (29 RCTs) [6], Roskell (32 RCTs) [3]. Hence, we performed this comparison of multiple treatments based on a sufficient amount of data, with the objective to measure the efficacy and safety of ADHD medication for children and adolescents.
Materials and Methods
Search Strategy
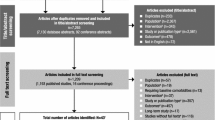
The following databases were used to collect data: (1) PubMed; (2) Embase; (3) The Cochrane Central Register of Controlled Trials (CENTRAL). The search conducted included literature published up until April 2016, with no language restrictions applied. The search strategy was formed by four items: health condition (ADHD and hyperkinetic disorder), population, study type and medication (ATX, BUP, CLON, GXR, LDX, MPH). In addition, a manual search was conducted through the reference list of selected articles.
Inclusion Criteria
Two independent reviewers screened articles separately, any disagreements were openly discussed until a consensus was reached. Certain/specific criteria were included in the search terms: firstly, the patients in studies should be accurately diagnosed with ADHD; secondly, studies were conducted based on children and adolescents (4–17 years old); thirdly, studies had to be randomized, controlled clinical trials (RCTs); finally, active medications had to be involved, which compared the drugs used in combination or singly, or with placebo (PBO). An output of validated and sufficient data was also needed for credible evaluation.
Data Extraction and Outcome Measurements
Information from each enrolled study were collected in descriptive statistics, including first author, year of publication, study design, study duration, diagnostic criteria, treatments, sample size, mean age, and ADHD scores.
According to the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria and relevant studies, the primary outcome measurements were the scores of ADHD, which contained various types such as SNAP-IV, ADHD-RS, CPRS, CPRS-RL, SWAN, IOWA-IO, CADS-T, CGI, and ASQ-T. ADHD Rating Scale-IV (ADHD-RS) [8] is an 18-item scale based on an interview conducted by an experienced clinician (nurse, psychologist, social worker, or physician) with the parent (or primary care) and child (if the child was available for the interview). Each item corresponds to one of the 18 symptoms in DSM-IV criteria, and severity for each item is scored 0 to 3(0 = never or rarely; 1 = sometimes; 2 = often; 3 = very often). The sum of the scores for 18 items was calculated to create a total score [9]. The Conners’ Parent Rating Scale-Revised (CPRS) [10] includes the 39-item version of the Conners’ Teacher Questionnaire and the 93-item version of the Conners’ Parent Questionnaire. The symptoms were rated by the observer on a 4-score scale (not at all, just a little, pretty much, very much). Three factors were used from the Parent Form (Hyperactive-Immature, Restless-Impulsive, and Conduct Disorder), and two factors were chosen from the Teacher Form (Hyperactivity and Conduct Disorder). In this study, we used ADHD-RS and CPRS as primary outcomes for efficacy. The changes of efficacy variables were calculated between the start and the end of treatment. Withdrawals due to all-cause, or adverse effects and lack of efficacy were also defined as primary outcomes to measure the safety of medications for ADHD.
Two reviewers scanned the full text of all identified studies, and reached a consensus through discussion if results were found to be inconsistent. All data including information regarding the studies (publication year, author), participants (sample size, age), medications (treatment duration, type of drug, dose) and outcome measurements were extracted.
Statistical Analysis
To start with, a pair-wise meta-analysis was conducted to evaluate the efficacy and safety of medications in ADHD treatment. Weighted mean differences, odd ratios and their corresponding 95 % confidence interval (CI) were calculated based on the results of a heterogeneity test. The heterogeneity was checked with Q statistics and I 2 test, with P < 0.05 or I 2 > 50 % indicating the existence of heterogeneity. We used a fixed-effects model (Mantel-Haenszel method) for studies without significant heterogeneity and a random-effects model (DerSimonian-Laird method) for studies with significant heterogeneity.
The multiple treatments comparison (MTC), under the Bayesian model, was performed on various comparator groups, which not only included direct comparisons from head-to-head trials but also indirect comparisons between two comparators. Due to the advantage of incorporating direct and indirect evidence, MTC could measure the efficacy and safety of medications for ADHD globally with the maximum statistical power by comparing these multiple treatments. We ranked the efficacy and safety of the medications based on the surface under the cumulative ranking curve (SUCRA). WinBUGS 1.4.3 and R 3.2.3 software were used to perform the MTC. Pair-wise meta-analyses were also conducted based on direct comparisons with STATA 12.1. The consistency was then assessed between direct and indirect comparisons, and the inconsistency of MTC was defined as the variability between the results of MTC analysis and the pair-wise meta-analyses.
Results
Study Characteristics
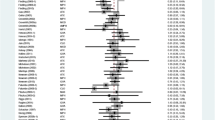
The data collected from a total of 12,930 patients from 62 studies was used in this meta-analysis [4, 11–71]. ATX, BUP, CLON, GXR, LDX, MPH were identified as widely used drugs for ADHD treatment. The main characteristics of the studies included are summarized in Table 1. A network plot of the 62 studies used is illustrated in Fig. 1 to show the comparisons in network meta-analysis. The width of the lines is proportional to the number of studies comparing treatment pairs. As can be seen from the plot, most of the trials we input into the analysis investigated the efficacy and safety of drugs on ADHD treatment compared with placebo; not many trials had been conducted on the direct comparison between drugs. Therefore, a network meta-analysis that enables indirect comparisons based on current data is greatly needed.
Results of Pair-Wise Meta-Analysis
In the traditional pair-wise meta-analysis, most of the results were based on comparisons between ATX, BUP, CLON, GXR, LDX, MPH and the placebo separately. As illustrated in Table 2, the efficacy of ATX, CLON, GXR, LDX, MPH was proved by changes in ADHD-RS compared to PBO (ATX vs. PBO: WMD = −0.80, 95%CI = [−1.01; −0.58] CLON vs. PBO: WMD = −0.52, 95%CI = [−0.88; −0.17]; GXR vs. PBO: WMD = −0.60, 95%CI = [−0.75; −0.44]; LDX vs. PBO: WMD = −1.39, 95%CI = [−1.80; −0.98]; MPH vs. PBO: WMD = −0.87, 95%CI = [−1.14; −0.60]). Furthermore, ATX, BUP, CLON, LDX, MPH have a better performance than the placebo in the CPRS, meanwhile only the comparison of ATX vs. MPH and BUP vs. PBO has no statistical significance (ATX vs. MPH: WMD = −0.01, 95%CI = [−0.23; 0.20]; BUP vs. PBO: WMD = −0.13, 95%CI = [−0.52; 0.27]).
Considering the safety of the drugs involved in the analysis, in the comparisons of the all-cause withdrawals, the safety of CLON, GXR, LDX and MPH were validated compared to the placebo (CLON vs. PBO: OR =0.53, 95%CI = [0.35; 0.82]; GXR vs. PBO: OR =0.82, 95%CI = [0.70; 0.97]; LDX vs. PBO: OR =0.41, 95%CI = [0.21; 0.80]; MPH vs. PBO: OR =0.45, 95%CI = [0.30; 0.68]). It is worthy to note, in the traditional pair-wise meta-analysis, we observed that GXR had a higher likelihood of producing withdrawal symptoms due to adverse effect compared to the placebo (OR =3.09, 95%CI = [1.80; 5.28]). Regarding withdrawal due to lack of efficacy, ATX, CLON, GXR, LDX and MPH proved to have better performance than the placebo, whereas for BUP we did not obtain a significant result (OR =1.57, 95%CI: [0.16; 15.59]).
Results of Network Meta-Analysis
In this network meta-analysis, efficacy of different drugs on ADHD treatment was evaluated by ADHD-RS and CPRS. Due to the lack of sufficient clinical trials, the efficacy of BUP was only evaluated by CPRS, and the efficacy of GXR was only evaluated by ADHD-RS. Results from the network meta-analysis are plotted in Figs. 2-6 and Table 3-5 with corresponding probability of different treatment ranks. As can be observed, when compared with the placebo, the efficacy of ATX, CLON, GXR, LDX, MPH was validated if we took ADHD-RS as a primary outcome (ATX: MD = −7.10, 95%CI: [−8.60, −5.7]; CLON: MD = −6.50, 95%CI: [−11.0, −1.9]; GXR: MD = −7.60, 95%CI: [−10.0, −5.2]; LDX: MD = −14.0, 95%CI: [−17.0, −12.0]; MPH: MD = −9.10, 95%CI: [−12.0, −6.4], Fig. 2, Table 3). In the analysis of CPRS changes, BUP did not illustrate a significant therapeutic value compared to the placebo (MD = −0.9, 95%CI: [−7.1, 5.2], Fig. 3, Table 4).
LDX was proved to be the most efficient medication for ADHD in our results. LDX has a significantly higher efficacy than other drugs considering its change in the ADHD-RS (ATX: MD = 7.1, 95%CI: [4.1, 10.0]; CLON: MD = 7.8, 95%CI: [2.6, 13.0]; GXR: MD = 6.7, 95%CI: [2.9, 10.0]; MPH: MD = 5.2, 95%CI: [1.8, 8.5], Fig. 2, Table 3). Regarding the change of CPRS, LDX also manifested to be significantly more efficient than ATX, BUP and MPH (ATX: MD = 12.0, 95%CI: [6.4, 17.0]; BUP: MD = 17.0, 95%CI: [8.8, 25.0]; MPH: MD = 8.1, 95%CI: [3.2, 13.0], Fig. 3, Table 3). In addition to this, based on the cumulative ranking probabilities, LDX had the highest probability to rank first in terms of efficacy concerning both CPRS and ADHD-RS (Table 5).
When it came to evaluation of safety, as presented in Fig. 4, Table 4, BUP, LDX and MPH had a significantly higher incidence rate of all-cause withdrawals compared with the placebo (BUP: OR =0.05, 95%CI: [0.0012, 0.74]; LDX: OR =0.44, 95%CI: [0.29, 0.66]; MPH: OR =0.58, 95%CI: [0.41, 0.82]). Meanwhile, it was observed that BUP had a more than 90 % probability to rank highest in all-cause withdrawals, which means it ranked lowest in terms of safety compared to the other drugs. ATX and GXR are two drugs that had a relatively low probability of withdrawal (ATX: 22.83 %; GXR: 28.00 %, for the cumulative ranking probabilities see Table 5).
Considering withdrawals due to adverse effect, patients using GXR were more likely to suffer from severe adverse effects than if they were to take the other drugs (87.17 % for cumulative ranking probabilities see Fig. 5, Table 4). Meanwhile, MPH could be considered as the drug with the least adverse effect (20.00 % in cumulative ranking probabilities). Besides, when we analyzed the results of patients’ withdrawals due to lack of efficacy, we observed that LDX had the highest ranking among the drugs (91.50 % for cumulative ranking probabilities, Fig. 6, Table 4), combining LDX being confirmed as the drug with the highest efficacy.
Discussion
In this analysis, we investigated the efficacy and safety of widely used medications for ADHD, including ATX, BUP, CLON, GXR, LDX and MPH. According to our results, LDX has the highest efficacy compared to the other drugs as well as a relatively lower rate of causing adverse effects than BUP, CLON and GXR. MPH has the lowest incidence rate of withdrawals due to adverse effects. Further in regard to MPH, it performed well in terms of efficacy and thus takes second place in correspondence with ADHD-RS and third place in CPRS. ATX is considered the safest drug for ADHD treatment based on our analysis. It has the lowest incidence rate of all-cause withdrawals as well as withdrawals due to lack to efficacy. However, the efficacy of ATX seems to be lower than CLON, GXR, LDX and MPH. Despite this, the use of CLON may be controversial considering its high efficacy but relatively low safety rate. Taking GXR into account, the high incidence rate of withdrawal due to adverse effects may also be taken into consideration when using it as an ADHD treatment. The results that LDX has the best performance and no significant difference between ATX and MPH are consistent with former NMA [72, 7]. According to previous papers, ATX is claimed by Mark E. Bangs to be statistically unrelated to suicidal behaviors in 2014 [2]. Besides, LDX has a proven long-lasting efficacy in long-term treatment among children and adolescence diagnosed with ADHD [4, 3]. In 2015, a study was carried out to further confirm the relatively high performance of MPH in treating ADHD [3]. All in all, results from our study are greatly consistent with those concluded before.
As demonstrated in the results, traditional meta-analysis, which only facilitates direct comparisons based on clinical trials, has huge limitations. In our results, limited by sample size, we did not acquire significant results on the efficacy and safety variables of BUP in treating ADHD in pair-wise meta-analysis; whereas in network meta-analysis, which combined direct evidence and indirect evidence, we observed that BUP had a higher rank than the placebo using CPRS, all-cause withdrawals, as well as withdrawals due to lack of efficacy. Moreover, using network meta-analysis, we were able to make comparisons between different drugs via indirect evidence.
In previous guidelines for ADHD [1], stimulant medications, selective norepinephrine- reuptake inhibitor and selective α2-adrenergic agonists were shown to have high efficacy in reducing symptoms. In this study, drugs from all the three categories were covered in the analysis concerning efficacy and safety. Of the six drugs involved in this study, ATX and BUP are a selective norepinephrine-reuptake inhibitor; CLON and GXR are selective α2-adrenergic agonists; LDX and MPH are stimulants.
From the results, we observed that the efficacy of selective norepinephrine-reuptake inhibitors ATX and BUP might not be as high as other medications. As reported previously by the Texas Children’s Medication Algorithm Project, BUP was listed as a fourth-line medication for ADHD after initial attempts to use two stimulants and then ATX [73]. Concerning the adverse effects, the application of BUP was found to be related to increased risk of epileptic seizures [74]. In regards to ATX, the most common adverse effects were nausea, xerostomia, appetite loss, insomnia, fatigue, headache and coughs. Due to its strong effect on the cardiovascular system, the application of ATX is not allowed on patients with symptomatic cardiovascular disease. Conversely, a significant advantage of ATX over α2-adrenergic agonists is that the use of ATX can be stopped abruptly without causing significant withdrawal effects. CLON and GXR were primarily used as medications for hypertension. Although they were also proven to be effective for ADHD patients, being used for such treatment gave rise to common adverse effects such as hypotension, dizziness, somnolence, xerostomia and fatigue. Stimulant medications, including LDX and MPH were observed to have the highest efficacy and safety in our analysis. The most common adverse effects of these stimulant medications are appetite loss, abdominal pain, headaches, and sleep disturbance. Despite the positive outcomes for LDX and MPH, it is nonetheless important to note their potential to cause drug dependence and withdrawal symptoms. It is reported that 87.6 % of chronic, high-dose users had withdrawal symptoms including anxiety, drug craving, depressed mood, fatigue, increased appetite and dizziness during the first week of drug withdrawal [75].Some drawbacks in our NMA should be mentioned. Although our NMA included 62 publications and studies, not all outcomes in this NMA can form a network, which decreases the credibility of these comparisons. Besides, although several reviews reviewed the RS application, studies related to sensitivity, specificity and diagnose OR analysis are limited. Therefore, ongoing researches are encouraged to overcome these limitations.
Admittedly, there are still some notable flaws lying in our study. For example, the criteria to measure the severity of ADHD: ADHD-RS is reported to be bias in some cases because the parents or teachers may sometimes go subjective and ignore the concrete context of the symptoms when giving rates. Still, the amount of the data concerning ATX unexpectedly outnumbered others and thus may have a chance to cause inaccuracy in our study. Moreover, the dose of MPH involved in our study ranged significantly from 0.52 mg/d to 54 mg/d, the effect of which is believed to be related with the reliability of our results.
In summary, in this NMA we investigated the efficacy and safety of drugs used for ADHD treatment. According to the results, LDX, MPH, CLON and GXR have a high efficacy when ADHD-RS and CPRS were applied as variables, among which LDX has the highest efficacy together with safety ranking the fourth place and MPH is the second safest treatment with efficacy ranking the forth. Still, the high incidence of withdrawals should be taken in to consideration when BUP, CLON, GXR and LDX are used on ADHD patients.
References
Limited ELAP (2013) STRATTERA® (atomoxetine hydrochloride)
Bangs ME, Wietecha LA, Wang S, Buchanan AS, Kelsey DK (2014) Meta-analysis of suicide-related behavior or ideation in child, adolescent, and adult patients treated with atomoxetine. J Child Adolesc Psychopharmacol 24(8):426–434. doi:10.1089/cap.2014.0005
Stuhec M, Munda B, Svab V, Locatelli I (2015) Comparative efficacy and acceptability of atomoxetine, lisdexamfetamine, bupropion and methylphenidate in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta-analysis with focus on bupropion. J Affect Disord 178:149–159. doi:10.1016/j.jad.2015.03.006
Coghill DR, Banaschewski T, Lecendreux M, Johnson M, Zuddas A, Anderson CS, Civil R, Dauphin M et al (2014) Maintenance of efficacy of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder: randomized-withdrawal study design. J Am Acad Child Adolesc Psychiatry 53(6):647–657 . doi:10.1016/j.jaac.2014.01.017e641
Ghuman JK, Hutchison SL (2014) Atomoxetine is a second-line medication treatment option for ADHD. Evid Based Ment Health 17(4):108. doi:10.1136/eb-2014-101805
Joseph A, Ayyagari R, Bischof M, Cai S, Xie M, Zhanabekova Z, Sikirica V (2014) Systematic literature review and mixed treatment comparison of GXR versus other treatments in children and adolescents with attention deficit hyperactivity disorder (ADHD). Value Health 17(7):A454
Roskell NS, Setyawan J, Zimovetz EA, Hodgkins P (2014) Systematic evidence synthesis of treatments for ADHD in children and adolescents: indirect treatment comparisons of lisdexamfetamine with methylphenidate and atomoxetine. Curr Med Res Opin 30(8):1673–1685
Conners CK, Erhardt D, Sparrow EP Conners’ adult ADHD rating scales (CAARS): technical manual. In, 1999. MHS North Tonawanda
Faries DE, Yalcin I, Harder D, Heiligenstein JH (2001) Validation of the ADHD rating scale as a clirlician administered and scored instrument. J Atten Disord 5(2):107–115
Conners CK (2001) Conners’ rating scales revised. Multi-Health Systems, Incorporated
Abikoff HB, Vitiello B, Riddle MA, Cunningham C, Greenhill LL, Swanson JM, Chuang SZ, Davies M et al (2007) Methylphenidate effects on functional outcomes in the preschoolers with attention-deficit/hyperactivity disorder treatment study (PATS). J Child Adolesc Psychopharmacol 17(5):581–592. doi:10.1089/cap.2007.0068
Allen AJ, Kurlan RM, Gilbert DL, Coffey BJ, Linder SL, Lewis DW, Winner PK, Dunn DW et al (2005) Atomoxetine treatment in children and adolescents with ADHD and comorbid tic disorders. Neurology 65(12):1941–1949. doi:10.1212/01.wnl.0000188869.58300.a7
Bangs ME, Emslie GJ, Spencer TJ, Ramsey JL, Carlson C, Bartky EJ, Busner J, Duesenberg DA et al (2007) Efficacy and safety of atomoxetine in adolescents with attention-deficit/hyperactivity disorder and major depression. J Child Adolesc Psychopharmacol 17(4):407–420. doi:10.1089/cap.2007.0066
Bangs ME, Hazell P, Danckaerts M, Hoare P, Coghill DR, Wehmeier PM, Williams DW, Moore RJ et al (2008) Atomoxetine for the treatment of attention-deficit/hyperactivity disorder and oppositional defiant disorder. Pediatrics 121(2):e314–e320. doi:10.1542/peds.2006-1880
Biederman J, Krishnan S, Zhang Y, McGough JJ, Findling RL (2007) Efficacy and tolerability of lisdexamfetamine dimesylate (NRP-104) in children with attention-deficit/hyperactivity disorder: a phase III, multicenter, randomized, double-blind, forced-dose, parallel-group study. Clin Ther 29(3):450–463
Biederman J, Melmed RD, Patel A, McBurnett K, Konow J, Lyne A, Scherer N (2008) A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics 121(1):e73–e84. doi:10.1542/peds.2006-3695
Biederman J, Quinn D, Weiss M, Markabi S, Weidenman M, Edson K, Karlsson G, Pohlmann H et al (2003) Efficacy and safety of Ritalin LA, a new, once daily, extended-release dosage form of methylphenidate, in children with attention deficit hyperactivity disorder. Paediatric drugs 5(12):833–841
Block SL, Kelsey D, Coury D, Lewis D, Quintana H, Sutton V, Schuh K, Allen AJ et al (2009) Once-daily atomoxetine for treating pediatric attention-deficit/hyperactivity disorder: comparison of morning and evening dosing. Clin Pediatr 48(7):723–733. doi:10.1177/0009922809335321
Buitelaar JK, Michelson D, Danckaerts M, Gillberg C, Spencer TJ, Zuddas A, Faries DE, Zhang S et al (2007) A randomized, double-blind study of continuation treatment for attention-deficit/hyperactivity disorder after 1 year. Biol Psychiatry 61(5):694–699. doi:10.1016/j.biopsych.2006.03.066
Coghill D, Banaschewski T, Lecendreux M, Soutullo C, Johnson M, Zuddas A, Anderson C, Civil R et al (2013) European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology 23(10):1208–1218. doi:10.1016/j.euroneuro.2012.11.012
Coghill DR, Banaschewski T, Lecendreux M, Zuddas A, Dittmann RW, Otero IH, Civil R, Bloomfield R et al (2014) Efficacy of lisdexamfetamine dimesylate throughout the day in children and adolescents with attention-deficit/hyperactivity disorder: results from a randomized, controlled trial. Eur Child Adolesc Psychiatry 23(2):61–68. doi:10.1007/s00787-013-0421-y
Conners CK, Casat CD, Gualtieri CT, Weller E, Reader M, Reiss A, Weller RA, Khayrallah M et al (1996) Bupropion hydrochloride in attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry 35(10):1314–1321. doi:10.1097/00004583-199610000-00018
Connor DF, Findling RL, Kollins SH, Sallee F, Lopez FA, Lyne A, Tremblay G (2010) Effects of guanfacine extended release on oppositional symptoms in children aged 6-12 years with attention-deficit hyperactivity disorder and oppositional symptoms: a randomized, double-blind, placebo-controlled trial. CNS drugs 24(9):755–768. doi:10.2165/11537790-000000000-00000
Cutler AJ, Brams M, Bukstein O, Mattingly G, McBurnett K, White C, Rubin J (2014) Response/remission with guanfacine extended-release and psychostimulants in children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 53(10):1092–1101. doi:10.1016/j.jaac.2014.08.001
De Jong CGW, Van De Voorde S, Roeyers H, Raymaekers R, Allen AJ, Knijff S, Verhelst H, Temmink AH et al (2009) Differential effects of atomoxetine on executive functioning and lexical decision in attention-deficit/hyperactivity disorder and reading disorder. J Child Adolesc Psychopharmacol 19(6):699–707
Dell’Agnello G, Maschietto D, Bravaccio C, Calamoneri F, Masi G, Curatolo P, Besana D, Mancini F et al (2009) Atomoxetine hydrochloride in the treatment of children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder: a placebo-controlled Italian study. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology 19(11):822–834. doi:10.1016/j.euroneuro.2009.07.008
Dittmann RW, Schacht A, Helsberg K, Schneider-Fresenius C, Lehmann M, Lehmkuhl G, Wehmeier PM (2011) Atomoxetine versus placebo in children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder: a double-blind, randomized, multicenter trial in Germany. J Child Adolesc Psychopharmacol 21(2):97–110. doi:10.1089/cap.2009.0111
Findling RL, Bukstein OG, Melmed RD, Lopez FA, Sallee FR, Arnold LE, Pratt RD (2008) A randomized, double-blind, placebo-controlled, parallel-group study of methylphenidate transdermal system in pediatric patients with attention-deficit/hyperactivity disorder. The Journal of clinical psychiatry 69(1):149–159
Findling RL, Childress AC, Cutler AJ, Gasior M, Hamdani M, Ferreira-Cornwell MC, Squires L (2011) Efficacy and safety of lisdexamfetamine dimesylate in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 50(4):395–405. doi:10.1016/j.jaac.2011.01.007
Findling RL, McBurnett K, White C, Youcha S (2014) Guanfacine extended release adjunctive to a psychostimulant in the treatment of comorbid oppositional symptoms in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 24(5):245–252. doi:10.1089/cap.2013.0103
Garg J, Arun P, Chavan BS (2014) Comparative short term efficacy and tolerability of methylphenidate and atomoxetine in attention deficit hyperactivity disorder. Indian Pediatr 51(7):550–554
Gau SS, Huang YS, Soong WT, Chou MC, Chou WJ, Shang CY, Tseng WL, Allen AJ et al (2007) A randomized, double-blind, placebo-controlled clinical trial on once-daily atomoxetine in Taiwanese children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 17(4):447–460. doi:10.1089/cap.2007.0091
Geller D, Donnelly C, Lopez F, Rubin R, Newcorn J, Sutton V, Bakken R, Paczkowski M et al (2007) Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. J Am Acad Child Adolesc Psychiatry 46(9):1119–1127. doi:10.1097/chi.0b013e3180ca8385
Greenhill LL, Findling RL, Swanson JM (2002) A double-blind, placebo-controlled study of modified-release methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics 109(3):E39
Handen BL, Aman MG, Arnold LE, Hyman SL, Tumuluru RV, Lecavalier L, Corbett-Dick P, Pan X et al (2015) Atomoxetine, parent training, and their combination in children with autism Spectrum disorder and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 54(11):905–915. doi:10.1016/j.jaac.2015.08.013
Harfterkamp M, van de Loo-Neus G, Minderaa RB, van der Gaag RJ, Escobar R, Schacht A, Pamulapati S, Buitelaar JK et al (2012) A randomized double-blind study of atomoxetine versus placebo for attention-deficit/hyperactivity disorder symptoms in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 51(7):733–741. doi:10.1016/j.jaac.2012.04.011
Hazell PL, Stuart JE (2003) A randomized controlled trial of clonidine added to psychostimulant medication for hyperactive and aggressive children. J Am Acad Child Adolesc Psychiatry 42(8):886–894. doi:10.1097/01.chi.0000046908.27264.00
Hervas A, Huss M, Johnson M, McNicholas F, van Stralen J, Sreckovic S, Lyne A, Bloomfield R et al (2014) Efficacy and safety of extended-release guanfacine hydrochloride in children and adolescents with attention-deficit/hyperactivity disorder: a randomized, controlled, phase III trial. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology 24(12):1861–1872. doi:10.1016/j.euroneuro.2014.09.014
Jain R, Segal S, Kollins SH, Khayrallah M (2011) Clonidine extended-release tablets for pediatric patients with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 50(2):171–179. doi:10.1016/j.jaac.2010.11.005
Kaplan S, Heiligenstein J, West S, Busner J, Harder D, Dittmann R, Casat C, Wernicke JF (2004) Efficacy and safety of atomoxetine in childhood attention-deficit/hyperactivity disorder with comorbid oppositional defiant disorder. J Atten Disord 8(2):45–52
Kelsey DK, Sumner CR, Casat CD, Coury DL, Quintana H, Saylor KE, Sutton VK, Gonzales J et al (2004) Once-daily atomoxetine treatment for children with attention-deficit/hyperactivity disorder, including an assessment of evening and morning behavior: a double-blind, placebo-controlled trial. Pediatrics 114(1):e1–e8
Kollins SH, Jain R, Brams M, Segal S, Findling RL, Wigal SB, Khayrallah M (2011) Clonidine extended-release tablets as add-on therapy to psychostimulants in children and adolescents with ADHD. Pediatrics 127(6):e1406–e1413. doi:10.1542/peds.2010-1260
Kollins SH, Lopez FA, Vince BD, Turnbow JM, Farrand K, Lyne A, Wigal SB, Roth T (2011) Psychomotor functioning and alertness with guanfacine extended release in subjects with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 21(2):111–120. doi:10.1089/cap.2010.0064
Kratochvil CJ, Vaughan BS, Stoner JA, Daughton JM, Lubberstedt BD, Murray DW, Chrisman AK, Faircloth MA et al (2011) A double-blind, placebo-controlled study of atomoxetine in young children with ADHD. Pediatrics 127(4):e862–e868. doi:10.1542/peds.2010-0825
Martenyi F, Zavadenko NN, Jarkova NB, Yarosh AA, Soldatenkova VO, Bardenstein LM, Kozlova IA, Neznanov NG et al (2010) Atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder: a 6-week, randomized, placebo-controlled, double-blind trial in Russia. Eur Child Adolesc Psychiatry 19(1):57–66. doi:10.1007/s00787-009-0042-7
Michelson D, Allen AJ, Busner J, Casat C, Dunn D, Kratochvil C, Newcorn J, Sallee FR et al (2002) Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 159(11):1896–1901
Michelson D, Faries D, Wernicke J, Kelsey D, Kendrick K, Sallee FR, Spencer T (2001) Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 108(5):E83
Nagy P, Hage A, Coghill DR, Caballero B, Adeyi B, Anderson CS, Sikirica V, Cardo E (2016) Functional outcomes from a head-to-head, randomized, double-blind trial of lisdexamfetamine dimesylate and atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder and an inadequate response to methylphenidate. Eur Child Adolesc Psychiatry 25(2):141–149. doi:10.1007/s00787-015-0718-0
Newcorn JH, Harpin V, Huss M, Lyne A, Sikirica V, Johnson M, Ramos-Quiroga JA, van Stralen J et al (2016) Extended-release guanfacine hydrochloride in 6-17-year olds with ADHD: a randomised-withdrawal maintenance of efficacy study. Journal of child psychology and psychiatry, and allied disciplines. doi:10.1111/jcpp.12492
Newcorn JH, Kratochvil CJ, Allen AJ, Casat CD, Ruff DD, Moore RJ, Michelson D, Bailey CE et al (2008) Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. Am J Psychiatr 165(6):721–730
Newcorn JH, Stein MA, Childress AC, Youcha S, White C, Enright G, Rubin J (2013) Randomized, double-blind trial of guanfacine extended release in children with attention-deficit/hyperactivity disorder: morning or evening administration. J Am Acad Child Adolesc Psychiatry 52(9):921–930. doi:10.1016/j.jaac.2013.06.006
Rugino TA (2014) Effect on primary sleep disorders when children with ADHD are administered guanfacine extended release. J Atten Disord. doi:10.1177/1087054714554932
Sallee FR, McGough J, Wigal T, Donahue J, Lyne A, Biederman J (2009) Guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry 48(2):155–165. doi:10.1097/CHI.0b013e318191769e
Sangal RB, Owens J, Allen AJ, Sutton V, Schuh K, Kelsey D (2006) Effects of atomoxetine and methylphenidate on sleep in children with ADHD. Sleep 29(12):1573–1585
Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, Arnsten AF, Cohen DJ et al (2001) A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry 158(7):1067–1074
Shang CY, Pan YL, Lin HY, Huang LW, Gau SS (2015) An open-label, randomized trial of methylphenidate and atomoxetine treatment in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 25(7):566–573. doi:10.1089/cap.2015.0035
Soutullo C, Banaschewski T, Lecendreux M, Johnson M, Zuddas A, Anderson C, Civil R, Higgins N et al (2013) A post hoc comparison of the effects of lisdexamfetamine dimesylate and osmotic-release oral system methylphenidate on symptoms of attention-deficit hyperactivity disorder in children and adolescents. CNS drugs 27(9):743–751. doi:10.1007/s40263-013-0086-6
Spencer T, Heiligenstein JH, Biederman J, Faries DE, Kratochvil CJ, Conners CK, Potter WZ (2002) Results from 2 proof-of-concept, placebo-controlled studies of atomoxetine in children with attention-deficit/hyperactivity disorder. The Journal of clinical psychiatry 63(12):1140–1147
Stein MA, Sikirica V, Weiss MD, Robertson B, Lyne A, Newcorn JH (2015) Does guanfacine extended release impact functional impairment in children with attention-deficit/hyperactivity disorder? Results from a randomized controlled trial. CNS drugs 29(11):953–962. doi:10.1007/s40263-015-0291-6
Sumner CR, Schuh KJ, Sutton VK, Lipetz R, Kelsey DK (2006) Placebo-controlled study of the effects of atomoxetine on bladder control in children with nocturnal enuresis. J Child Adolesc Psychopharmacol 16(6):699–711. doi:10.1089/cap.2006.16.699
Svanborg P, Thernlund G, Gustafsson PA, Hägglöf B, Poole L, Kadesjö B (2009) Efficacy and safety of atomoxetine as add-on to psychoeducation in the treatment of attention deficit/hyperactivity disorder: a randomized, double-blind, placebo-controlled study in stimulant-naïve Swedish children and adolescents. Eur Child Adolesc Psychiatry 4:240–249. doi:10.1007/s00787-008-0725-5
Svanborg P, Thernlund G, Gustafsson PA, Hägglöf B, Schacht A, Kadesjö B (2009) Atomoxetine improves patient and family coping in attention deficit/hyperactivity disorder: a randomized, double-blind, placebo-controlled study in Swedish children and adolescents. Eur Child Adolesc Psychiatry 12:725–735. doi:10.1007/s00787-009-0031-x
Takahashi M, Takita Y, Yamazaki K, Hayashi T, Ichikawa H, Kambayashi Y, Koeda T, Oki J et al (2009) A randomized, double-blind, placebo-controlled study of atomoxetine in Japanese children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 19(4):341–350. doi:10.1089/cap.2008.0154
Thurstone C, Riggs PD, Salomonsen-Sautel S, Mikulich-Gilbertson SK (2010) Randomized, controlled trial of atomoxetine for attention-deficit/hyperactivity disorder in adolescents with substance use disorder. J Am Acad Child Adolesc Psychiatry 49(6):573–582. doi:10.1016/j.jaac.2010.02.013
Wang Y, Zheng Y, Du Y, Song D, Shin YJ, Cho S, Kim B, Ahn D et al (2007) Atomoxetine versus methylphenidate in paediatric outpatients with attention deficit hyperactivity disorder: a randomized, double-blind comparison trial. Aust N Z J Psychiatry 41(3):222–230
Wehmeier PM, Schacht A, Wolff C, Otto WR, Dittmann RW, Banaschewski T (2011) Neuropsychological outcomes across the day in children with attention-deficit/hyperactivity disorder treated with atomoxetine: results from a placebo-controlled study using a computer-based continuous performance test combined with an infra-red motion-tracking device. J Child Adolesc Psychopharmacol 21(5):433–444. doi:10.1089/cap.2010.0142
Weiss M, Tannock R, Kratochvil C, Dunn D, Velez-Borras J, Thomason C, Tamura R, Kelsey D et al (2005) A randomized, placebo-controlled study of once-daily atomoxetine in the school setting in children with ADHD. J Am Acad Child Adolesc Psychiatry 44(7):647–655. doi:10.1097/01.chi.0000163280.47221.c9
Wigal SB, Nordbrock E, Adjei AL, Childress A, Kupper RJ, Greenhill L (2015) Efficacy of methylphenidate hydrochloride extended-release capsules (Aptensio XR) in children and adolescents with attention-deficit/hyperactivity disorder: a phase III, randomized. Double-Blind Study CNS drugs. doi:10.1007/s40263-015-0241-3
Wilens TE, Bukstein O, Brams M, Cutler AJ, Childress A, Rugino T, Lyne A, Grannis K et al (2012) A controlled trial of extended-release guanfacine and psychostimulants for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 51(1):74–85 . doi:10.1016/j.jaac.2011.10.012e72
Wilens TE, McBurnett K, Bukstein O, McGough J, Greenhill L, Lerner M, Stein MA, Conners CK et al (2006) Multisite controlled study of OROS methylphenidate in the treatment of adolescents with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med 160(1):82–90
Wilens TE, Robertson B, Sikirica V, Harper L, Young JL, Bloomfield R, Lyne A, Rynkowski G et al (2015) A randomized, placebo-controlled trial of guanfacine extended release in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 54(11):916–925 . doi:10.1016/j.jaac.2015.08.016e912
King S, Griffin S, Hodges Z, Weatherly H, Asseburg C, Richardson G, Golder S, Taylor E et al. (2006) A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents. Health technology assessment (Winchester, England) 10 (23):iii-iv, xiii-146
Pliszka SR, Crismon ML, Hughes CW, Corners CK, Emslie GJ, Jensen PS, McCracken JT, Swanson JM et al (2006) The Texas Children’s medication algorithm project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry 45(6):642–657
Alper K, Schwartz KA, Kolts RL, Khan A (2007) Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry 62(4):345–354
Shoptaw SJ, Kao U, Heinzerling K, Ling W (2009) Treatment for amphetamine withdrawal. The Cochrane database of systematic reviews 2
Acknowledgments
We thank our hospital for its great effort and all the colleagues of department for their mutual cooperation.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Ying Li and Jie Gao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, Y., Gao, J., He, S. et al. An Evaluation on the Efficacy and Safety of Treatments for Attention Deficit Hyperactivity Disorder in Children and Adolescents: a Comparison of Multiple Treatments. Mol Neurobiol 54, 6655–6669 (2017). https://doi.org/10.1007/s12035-016-0179-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0179-6