Abstract
Objectives
This study investigated the influence of calcium hydroxide intracanal medications on the levels of metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in apical periodontitis (AP).
Materials and methods
Twenty primarily infected root canals with AP were randomly divided into two groups: Ca(OH)2 + sterile saline solution (SSL) group and Ca(OH)2 + 2% chlorhexidine gel (CHX gel) group. We collected samples from the periradicular tissue fluid (PTF) before (s1) and after 14 days of intracanal medication (s2). MMP-1, MMP-2, MMP-9, TIMP-1, and TIMP-2 were measured by ELISA assay.
Results
MMP-1, MMP-2, MMP-9, TIMP-1, and TIMP-2 were detected in all PTF samples at s1 and s2 (20/20). At s1, MMP-2 and MMP-9 were detected at higher levels than MMP-1 (p < .05). Higher levels of TIMP-1 than TIMP-2 were found in AP (p < .05). Additionally, we detected higher MMP-1, MMP-2, and MMP-9 over TIMP-1 and TIMP-2 levels in AP (p < .05). At s2, Ca(OH)2 + SSL was as effective as Ca(OH)2 + 2% CHX gel in lowering the levels of MMP-1, MMP-2, and MMP-9 after 14 days of intracanal medication, with no significant difference between them (p > .05). Both Ca(OH) 2 intracanal medications had no significant impact on the levels of TIMP-1 and TIMP-2 (both p > .05). At s2, TIMP-1 levels were higher than TIMP-2 (p < .05). Moreover, there were positive correlations between the levels of MMP-1 and TIMP-1 and MMP-1 and TIMP-2 (p < .05).
Conclusions
Calcium hydroxide medications effectively lowered the levels of MMP-1, MMP-2, and MMP-9 in periapical tissues after 14 days of treatment, with no difference between them. Moreover, the calcium hydroxide intracanal medications tested here had no impact in TIMP-1 and TIMP-2 in periapical tissues.
Clinical relevance
MMPs and TIMPs play an essential role in the degradation of the extracellular matrix. The imbalance MMPs and TIMPs can cause periapical tissue destruction. Therefore, the reestablishment of the balance between activated MMPs and TIMPs with root canal therapy is essential to restore tissue homeostasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Matrix metalloproteinases (MMPs), calcium-dependent zinc endopeptidases, play an essential role in the degradation of the extracellular matrix (ECM) [1]. MMPs are produced by various cells, including lymphocytes, granulocytes, and periodontal fibroblasts [2, 3]. They are detected in bodily fluids, such as gingival crevicular, saliva, serum, and urine [4,5,6,7,8]. MMPs are classified into several families depending on the specificity of their substrate—collagenase (MMP-1), gelatinases (MMP-2 and MMP-9), stromelysin (MMP-3), and matrylisins (MMP-7 and MMP-26) [9].
MMPs participate in the development and establishment of apical periodontitis (AP) [10,11,12,13,14,15,16,17,18,19,20,21,22]. They are involved in the ECM degradation in periapical tissues in response to root canal infection. Different MMPs, including MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, and MMP-13 are reported in AP [10,11,12,13,14,15,16,17,18,19,20,21,22,23]. Among them, MMP-1, MMP-2, and MMP-9 have been detected both in primary and secondary/persistent AP and primary periodontal lesions with secondary endodontic involvement [10, 17, 23, 24]. Elevated levels of MMPs are correlated with nonhealing AP [10, 25]. Previous studies demonstrated that MMPs are key enzymes in promoting periapical bone resorption [26, 27]. High levels of MMP-1, MMP-2, and MMP-9 are reported in larger periapical lesions [17] and frequently recovered from symptomatic and asymptomatic AP [10, 15, 17, 23, 24]. Moreover, MMP-1 has been positively correlated with MMP-2 and MMP-9 levels in AP [17].
The proteolytic activities of MMPs are counteracted by tissue inhibitors of metalloproteinases (TIMPs) [28, 29]. TIMPs restrict ECM breakdown by proteinase inhibition and by blockage of autocatalytic MMP activation [30]. The balance between activated MMPs and TIMPs is essential to maintain healthy tissue integrity and restore tissue homeostasis [8]. The TIMP family at present comprises four members (TIMP-1 to TIMP-4) of relative molecular mass (Mr) ranging from 22 to 30 K, with a 40–50% sequence identity [31]. TIMP-1 and TIMP-2 have been reported in AP [17, 27, 32, 33]. Torres et al. [33], in a systematic review, indicated that MMP and TIMP levels in periapical lesions were significantly higher than those in normal tissue. TIMP-1 and TIMP-2 play a multifunctional role as proteins [34]. All TIMPs inhibit active MMPs with relatively low selectivity [1]. Previous investigations revealed that MMP-1 and MMP-2 are directly related to TIMP-1 and TIMP-2 in AP [17, 35]. Additionally, MMP-1, MMP-2, TIMP-1, and TIMP-2 expressions indicate significant differences between AP and healthy periapical tissues [19, 35]. TIMP-1 has been suggested to be an enhancer or inhibitor of bone resorption depending on whether TIMP-1 concentrations are low or high, respectively [36]. An elevated level of TIMP-1 is a strong indicator of AP’s chronic stage [16, 17, 32, 37]. Moreover, TIMP-1 seems to be a predictor of poor wound healing in AP [16].
Currently, there is limited clinical evidence of the effect of root canal therapy on the levels of MMPs [10, 16, 23] and TIMPs in AP. To the best of our knowledge, this is the first clinical study to compare the effectiveness of Ca(OH)2 + SSL to Ca(OH)2 + 2% chlorhexidine gel (CHX gel) intracanal medications on MMP-1, MMP-2, MMP-9, TIMP-1, and TIMP-2 in AP. To help understand the impact of intracanal medications on MMPs and TIMPs, this study investigated the influence of calcium hydroxide intracanal medications on the levels of metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in AP.
The null hypothesis tested is that there is no difference between Ca(OH)2 and Ca(OH)2 with 2% CHX gel in lowering the levels of MMP-1, MMP-2, MMP-9, TIMP-1, and TIMP-2 in AP.
Materials and methods
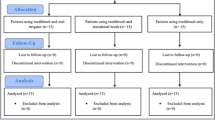
This study included patients referred to the clinic for endodontic therapy between April 2014 and September 2015. Patients were screened for inclusion and exclusion criteria. A total of 103 patients were assessed for eligibility (Fig. 1). We obtained the medical and dental history from each patient. Patients that received antibiotic treatment in the preceding 3 months or who presented any systemic disease that might influence the inflammatory response were excluded. The local Human Research Ethics Committee of the School of Dentistry of Sao Jose dos Campos, Sao Paulo State University, approved this research under protocol # 754.621. We registered it at REBEC (# RBR-9xhgs6). For the procedures, we followed the Helsinki declaration. All patients signed written informed consent to participate. All the single-rooted teeth were maxillary teeth with necrotic pulp and AP. Teeth exhibiting 1 root canal and the absence of periodontal pockets deeper than 4 mm were included here. None of the patients reported any type of systemic disease. We took a periapical radiograph to verify the periapical lesion. All teeth included in this study had a size of AP > 3 mm and < 6 mm.
According to a previous investigation [16], the sample size calculation was determined using a Sealed Envelope (www.sealedenvelope.com/power). The calculation with α-type error = 0.05 and power β = 0.80 indicated that the sample size to be ten individuals per group. The twenty patients selected were randomly assigned and allocated into two groups according to the intracanal medication to be tested: Ca(OH)2 (Biodinâmica Química e Farmacêutica Ltda., Ibiporã, PR, Brazil) powder + sterile saline solution (SSL) [(0.9% Sodium Chloride (NaCl)] (Eurofarma, São Paulo, SP, Brazil) group and Ca(OH)2 + 2% chlorhexidine gel (2% CHX gel) (Terapêutica Farmácia de Manipulação, São José dos Campos, SP, Brazil) group.
Following the randomized order of intervention, an independent researcher concealed the intracanal medications in sequentially numbered sealed envelopes. Another investigator opened the envelope before the treatment and informed the operator of the group assigned. The patient and the outcome assess investigator was not aware of the intervention.
Sampling procedures
The patients were administered local anesthesia with 2% lidocaine with 1:200,000 epinephrine. The tooth was isolated with a rubber dam, and a two-stage access cavity was prepared. The first stage was the removal of lesions and restoration to eliminate significant removal of contaminants. In the second stage, we completed the access cavity after obtaining straight-line access to the canal. The root canal was explored with a size 10 K-file (Dentsply Maillefer, Ballaigues, Switzerland). We estimated the root canal length in the preoperative radiograph and then confirmed with an apex locator (RomiApex A-15; Romidan Dental Solution, Kiryat Ono, Israel). The root canals were instrumented with Mtwo files (VDW, Munich, Germany). We used the files adapted to an electric motor (VDW). The instrumentation was performed within the working length in a gentle in-and-out motion. The sequence for root canal instrumentation was #10/0.04, #15/0.05, #20/0.06, #25/0.06, #30/0.05, #35/0.05, #40/0.04, and #25/0.07. We rinsed the canals with syringes and 30-G NaviTip needles (Ultradent, South Jordan, UT) with 5-mL 2.5% NaOCl solution between the files.
After root canal instrumentation, apical patency verified with 20/0.02 K-file to secure that the paper points extended the sampling site. A first sample (s1) was obtained from the periradicular tissue fluid (PTF) in the AP as reported by Martinho et al. [38]. We placed three sterile paper points (size #15) inside the root canal and approximately 2 mm through the root apex. The paper points were kept in position for the 60 s for sampling the PTF. Then, we cut the paper points 4 mm from the tip, dropped them into a 1.5-mL sterile plastic tube, and stored them at − 80 °C for future analysis of MMPs and TIMPs.
Subsequently, we randomly divided teeth into 2 groups: Ca(OH)2 + SSL (n = 10) and Ca(OH)2 + 2% CHX gel (n = 10). Prior the placement of intracanal medication, the canals were irrigated with 17% ethylenediaminetetraacetic acid (EDTA) (Inodon Industrial Editora Exportação de Produtos Odontológicos Ltda., Porto Alegre, RS, Brazil) for 5 min. We rinsed the canal with 10 mL of sterile saline solution and dried it with paper points. The Ca(OH)2 paste was newly prepared and placed into the canal with Lentulo Spiral Fillers (Dentsply Maillefer). We condensed the paste at the canal orifice level with a sterile cotton pellet. The pulp chamber walls were cleaned with a sterile cotton pellet moistened in alcohol. We filled the access cavities with two layers of Cavit (ESPE, Seefeld, Germany) and light-cured resin (Z-250; 3 M Dental Products, St Paul, MN).
After 14 days of intracanal medication, we accessed the canals after rubber dam isolation and disinfected the operative field, as described above. We removed the Ca(OH)2 paste with a 5-mL SSL and thoroughly instrumented the canal with the master apical file. The root canals were flushed with 10-mL SSL. A second PTF sample (s2) was performed as described above. We rinsed the root canal with 10 mL of 2.5% NaOCl and inundated with 17% EDTA for 5 min. Lastly, we obturated the root canals using a single-cone technique with gutta-percha and AH-Plus sealer (Dentsply DeTrey GmbH, Konstanz, Germany). The access cavities were sealed with light-cured resin (Z-250; 3 M Dental Products).
Quantification of metalloproteinases (MMPs) and TIMPS
The levels of MMP-1 (Cat #DY901), MMP-2 (Cat#DY902), MMP-9 (Cat #DY911), TIMP-1 (Cat# DY970), and TIMP-2 (Cat#DY971) from PTF samples collected before and after the use of Ca(OH)2 intracanal medications were quantified with enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Inc., Minneapolis, MN). We measured the levels of MMPs and TIMPs following the manufacturer’s instructions. Briefly, we added the standard sample solutions to the ELISA well plate pre-coated with specific monoclonal capture antibodies for MMP-1, MMP-2, MMP-9, TIMP-1, and TIMP-2. The ELISA well plate was lightly shaken for 3 h at room temperature. We added the anti-MMP-1, anti-MMP-2, anti-MMP-9, anti-TIMP-1, and anti-TIMP-2 polyclonal antibodies conjugated with horseradish peroxidase to the solution and incubated for 1-h room temperature. Afterward, a substrate solution containing hydrogen peroxidase and chromogen was inoculated to react for 20 min. We measured the levels of MMPs and TIMPs with a micro-ELISA reader at 450 nm and normalized with the standard solution. Each densitometric value was obtained from 3 independent experiments. The values were expressed as mean ± standard deviation.
Statistical analysis
The levels of MMPs (MMP-1, MMP-2, and MMP-9) and TIMPs (TIMP-1 and TIMP-2) for each case were tabulated in duplicate on a spreadsheet and statistically analyzed by STATA 12.0 software (StataCorp, College Station, TX). First, we performed a descriptive analysis using the Bartlett test to explore data distribution. Second, the Pearson correlation test was used to correlate the levels of MMP-1/MMP-2/MMP-9 and TIMP-1/TIMP-2 found in AP. Third, we compared the MMPs and TIMPs levels at s1 and s2 using a paired t test. Lastly, we investigate for differences between the Ca(OH)2 + SSL and Ca(OH)2 + 2% CHX gel group applying one-way analysis of variance (post hoc Bonferroni). All statistical analyses were performed at a significance level of 5%.
Results
MMP-1, MMP-2, MMP-9, TIMP-1, and TIMP-2 were detected in all PTF samples at s1 and s2 (20/20) (Table 1). At s1, MMP-2 and MMP-9 were detected at higher levels than MMP-1 (p < 0.05). Higher TIMP-1 than TIMP-2 were found in AP (p < 0.05) (Table 1). Additionally, we found higher MMP-1, MMP-2, and MMP-9 over TIMP-1 and TIMP-2 in AP (p < 0.05) (Table 1). At s2, Ca(OH)2 + SSL was as effective as Ca(OH)2 + 2% CHX gel in lowering the levels of MMP-1, MMP-2, and MMP-9 after 14 days, with no difference between them (p > 0.05) (Table 1). In contrast, both Ca(OH)2 intracanal medications had no significant impact on TIMP-1 and TIMP-2 (both, p > 0.05). At s2, TIMP-1 levels were higher than TIMP-2 (p < 0.05) (Table 1). There were positive correlations between the levels of MMP-1 and TIMP-1 and MMP-1 and TIMP-2 (p < 0.05). Table 1 shows the mean values of MMP-1, MMP-2, MMP-9, TIMP-1, and TIMP-2 detected in PTF samples collected from AP at s1 and s2.
Discussion
Data obtained in this study showed that MMPs and TIMPs participate in the inflammation of periapical tissues present in teeth with primary endodontic infection and AP. We found higher MMP-1, MMP-2, and MMP-9 over TIMP-1 and TIMP-2 levels in AP. Both Ca(OH)2 medications effectively lowered the levels of MMP-1, MMP-2, and MMP-9 after 14 days of treatment. In contrast, Ca(OH)2 + SSL and Ca(OH)2 + 2% CHX gel had no significant impact on the levels of TIMP-1 and TIMP-2.
The PTF sampling method applied in this study has been widely used in endodontics to investigate inflammatory mediators [22, 23, 39]. Lately, in a systematic review, Virdee et al. [39] noted that although different methods have been applied to sample PTF, paper points endure the most commonly used approach. Furthermore, paper points are efficient at absorbing low amounts of fluid [40] and of clinical relevance, allow longitudinal sampling of PTF.
In this study, MMP-1, MMP-2, and MMP-9 were recovered from all PTF samples collected from AP. MMPs in AP have been reported in the literature [10, 11, 13, 14, 17, 18, 20, 23, 41]. At the baseline samples (s1), higher MMP-2 and MMP-9 were detected in AP compared to MMP-1. MMP-2 (gelatinase A) and MMP-9 (gelatinase B) are synthesized by fibroblasts and pulp cells, which participate in the degradation of IV collagens/gelatins and denatured lamins, elastins, and fibronectins [18].
The detection of TIMP in AP here aligns with previous investigations [16, 17, 32, 37]. Higher levels of TIMP-1 than those in TIMP-2 were detected in PTF samples collected from teeth with primary endodontic infection and AP. Such findings agree with previous studies showing significantly increased expression of TIMP-1 in AP [16, 17, 32, 37]. In particular, TIMP-1 has been indicated as an enhancer or inhibitor of bone resorption depending on whether TIMP-1 concentrations are low or high, respectively [36].
In the present study, we found significantly higher MMP-1, MMP-2, and MMP-9 compared to TIMP-1 and TIMP-2 in AP. Such overactivity of MMPs over TIMPs may be indicative of periapical tissue destructive activity. A disturbed balance between MMPs and TIMPs, usually with relative overactivity of MMPs, has been shown to lead to tissue destruction in various pathologic conditions [8, 42, 43]. The biological effect of a shift in MMP and/or TIMP levels results from their overexpression or reduced expression of TIMPs or vice versa [8].
Here, we investigated the influence of two different Ca(OH)2 intracanal medications on MMP-1, MMP-2, MMP-9, TIMP-1, and TIMP-2 in AP. To date, Ca(OH)2 remains the most frequently intracanal medicament because of its antimicrobial activity [44,45,46]. Ca(OH)2 exhibits many desired properties [46,47,48,49,50]. Besides its well-known antimicrobial activity, Ca(OH)2 exhibits high detoxifying activity against endotoxins present in root canal infections [46, 49, 50], dissolves organic material [48], and stimulates mineralization. It acts as a chemical and physical coronal and apical barrier [47]. Additionally, Ca(OH)2 intracanal medication appears to lower different inflammatory mediators, including IL-1 beta, TNF-alpha, PGE2, INF-gamma, IL-2, IL-4, IL-5, and IL-13 present in AP [10, 23, 38, 51].
In this study, we tested Ca(OH)2 with an inert vehicle (SSL) and in combination with 2% CHX gel. Chlorhexidine (CHX) has broad-spectrum activity on gram-positive and gram-negative bacteria, fungi, and viruses [52,53,54]. One advantage of CHX is its capacity to adhere to dentin and exhibit prolonged antimicrobial activity [54]. Additionally, CHX has broad-spectrum antiproteolytic activity that can inhibit the collagenolytic activity of MMPs [55]. Furthermore, previous in vitro studies have demonstrated the ability of CHX to inhibit the proteolytic activity of MMP-2, MMP-8, and MMP-9 under direct contact conditions [55, 56].
Both calcium hydroxide intracanal medications tested here effectively lowered the levels of MMP-1, MMP-2, and MMP-9 in apical tissues. Such findings are consistent with previous investigations [10, 16, 23]. Paula-Silva et al. [15] showed in an animal study that teeth submitted to Ca(OH)2 medication for 15 days have a lower inflammatory index along with lower expression of MMP-2, MMP-8, and MMP-9 than teeth treated in a single visit with no intracanal medication. More recently, Barbosa-Ribeiro et al. [10] and Duque et al. [23] revealed the effectiveness of Ca(OH)2 intracanal medication to lower MMP-2, MMP-3, MMP-8, and MMP-9 in periapical tissues from teeth with post-treatment in AP. Despite limited clinical evidence, it is not unreasonable to connect this study with previous investigations [10, 16, 23] to suggest that Ca(OH)2 intracanal medications can lower the levels of MMPs in periapical tissues from teeth with primary endodontic infections and AP.
Despite the in vitro ability of CHX to inhibit the proteolytic activity of MMPs [55, 56], Ca(OH)2 was as effective as its combination with 2% CHX gel in lowering MMP-1, MMP-2, and MMP-9 in AP. It is important to highlight that both Ca(OH)2 and CHX exhibit their properties by direct contact. Therefore, we can speculate that the reduction of MMPs found in periapical tissues may not be attributed only to the antiproteolytic activity of the intracanal medications tested inhibiting the collagenolytic activity of MMPs but also to their antimicrobial activity against bacteria and toxins present in respectively root canal infection.
Although the calcium hydroxide intracanal medications tested here had no direct impact on the levels of TIMP-1 and TIMP-2 in periapical tissues, the reduction of MMP-1, MMP-2, and MMP-9 indirectly favor the balance between MMPs and TIMPs, which seems to be essential to restore tissue homeostasis [8]. Overactivity of MMPs results from their overexpression or reduced expression of TIMPs or vice versa. The balance between activated MMPs and TIMPs is essential to maintain healthy tissue integrity and restore tissue homeostasis [8]. Indeed, the impact of therapies in MMPs and TIMPs balance has been explored in different fields [57, 58].
At s2, the levels of TIMP-1 remained higher than those of TIMP-2 irrespective of the Ca(OH)2 medication tested. It is worth pointing out that after intracanal medications, Pearson’s correlation test indicated a positive correlation between MMP-1 and TIMP-1 as well as TIMP-2 in both groups tested.
Using paper points to collected PTF from periapical tissues represents a limitation of this investigation. While there are well-known benefits of using this technique, such as longitudinal sampling of crevicular fluid, acquiring adequate periapical fluid through the root canal is challenging. To hedge against this, we performed an apical debridement before sampling.
Overall, this clinical study demonstrated that Ca(OH)2 + SSL and Ca(OH)2 + 2% CHX gel were effective in lowering the levels of MMP-1, MMP-2, and MMP-9 in periapical tissue after 14 days of treatment with no statistically significant difference between them. This is the first clinical study to compare the impact of two different intracanal medications on selected MMPs and TIMPs present in AP. Besides the well-known antimicrobial activity of Ca(OH)2 intracanal medications, detoxifying activity against LPS, as well as their anti-inflammatory activity, this study revealed the ability of Ca(OH)2 intracanal medications to lower the levels of MMPs in periapical tissues. Further research is needed to unravel the actions and interactions of MMPs and TIMPs in AP.
Conclusions
Calcium hydroxide medications effectively lowered the levels of MMP-1, MMP-2, and MMP-9 in periapical tissues after 14 days of treatment, with no difference between them. Moreover, the calcium hydroxide intracanal medications tested here had no impact in TIMP-1 and TIMP-2 in periapical tissues.
References
Gomis-Rüth F-X, Maskos K, Betz M et al (1997) Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 by TIMP-1. Nature 389:77–81. https://doi.org/10.1038/37995
Goetzl EJ, Banda MJ, Leppert D (1996) Matrix metalloproteinases in immunity. J Immunol 156:1–4
Lisboa RA, Andrade MV, Cunha-Melo JR (2013) Toll-like receptor activation and mechanical force stimulation promote the secretion of matrix metalloproteinases 1, 3 and 10 of human periodontal fibroblasts via p38, JNK and NF-kB. Arch Oral Biol 58:731–739. https://doi.org/10.1016/j.archoralbio.2012.12.009
Fujimoto N, Zhang J, Iwata K et al (1993) A one-step sandwich enzyme immunoassay for tissue inhibitor of metalloproteinases-2 using monoclonal antibodies. Clin Chim Acta 220:31–45. https://doi.org/10.1016/0009-8981(93)90004-N
Ingman T, Tervahartiala T, Ding Y et al (1996) Matrix metalloproteinases and their inhibitors in gingival crevicular fluid and saliva of periodontitis patients. J Clin Periodontol 23:1127–1132. https://doi.org/10.1111/j.1600-051X.1996.tb01814.x
Durkan GC, Nutt JE, Marsh C et al (2003) Alteration in urinary matrix metalloproteinase-9 to tissue inhibitor of metalloproteinase-1 ratio predicts recurrence in nonmuscle-invasive bladder cancer. Clin Cancer Res 9:2576–2582
Asatsuma M, Ito S, Watanabe M et al (2004) Increase in the ratio of matrix metalloproteinase-9 to tissue inhibitor of metalloproteinase-1 in saliva from patients with primary Sjögren’s syndrome. Clin Chim Acta 345:99–104. https://doi.org/10.1016/j.cccn.2004.03.006
Verstappen J, Von den Hoff JW (2006) Tissue inhibitors of metalloproteinases (TIMPs): their biological functions and involvement in oral disease. J Dent Res 85:1074–1084. https://doi.org/10.1177/154405910608501202
Nagase H, Visse R, Murphy G (2006) Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69:562–573. https://doi.org/10.1016/j.cardiores.2005.12.002
Barbosa-Ribeiro M, Arruda-Vasconcelos R, De-Jesus-Soares A et al (2019) Effectiveness of calcium hydroxide-based intracanal medication on infectious/inflammatory contents in teeth with post-treatment apical periodontitis. Clin Oral Investig 23:2759–2766. https://doi.org/10.1007/s00784-018-2719-0
Buzoglu HD, Unal H, Ulger C et al (2009) The zymographic evaluation of gelatinase (MMP-2 and -9) levels in acute and chronic periapical abscesses. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108:e121–e126. https://doi.org/10.1016/j.tripleo.2009.07.014
Pattamapun K, Handagoon S, Sastraruji T et al (2017) Decreased levels of matrix metalloproteinase-2 in root-canal exudates during root canal treatment. Arch Oral Biol 82:27–32. https://doi.org/10.1016/j.archoralbio.2017.05.019
Takahama A, Rôças IN, Faustino ISP et al (2018) Association between bacteria occurring in the apical canal system and expression of bone-resorbing mediators and matrix metalloproteinases in apical periodontitis. Int Endod J 51:738–746. https://doi.org/10.1111/iej.12895
Matsui H, Yamasaki M, Nakata K et al (2011) Expression of MMP-8 and MMP-13 in the development of periradicular lesions. Int Endod J 44:739–745. https://doi.org/10.1111/j.1365-2591.2011.01880.x
Paula-Silva FWG, da Silva LAB, Kapila YL (2010) Matrix metalloproteinase expression in teeth with apical periodontitis is differentially modulated by the modality of root canal treatment. J Endod 36:231–237. https://doi.org/10.1016/j.joen.2009.10.030
Letra A, Ghaneh G, Zhao M et al (2013) MMP-7 and TIMP-1, new targets in predicting poor wound healing in apical periodontitis. J Endod 39:1141–1146. https://doi.org/10.1016/j.joen.2013.06.015
Martinho FC, Teixeira FFC, Cardoso FGR et al (2016) Clinical investigation of matrix metalloproteinases, tissue inhibitors of matrix metalloproteinases, and matrix metalloproteinase/tissue inhibitors of matrix metalloproteinase complexes and their networks in apical periodontitis. J Endod 42:1082–1088. https://doi.org/10.1016/j.joen.2016.04.001
de Cassanta LT, C, Rodrigues V, Violatti-Filho JR, et al (2017) Modulation of matrix metalloproteinase 14, tissue inhibitor of metalloproteinase 3, tissue inhibitor of metalloproteinase 4, and inducible nitric oxide synthase in the development of periapical lesions. J Endod 43:1122–1129. https://doi.org/10.1016/j.joen.2017.02.020
Menezes-Silva R, Khaliq S, Deeley K et al (2012) Genetic susceptibility to periapical disease: conditional contribution of MMP2 and MMP3 genes to the development of periapical lesions and healing response. J Endod 38:604–607. https://doi.org/10.1016/j.joen.2012.02.009
Ahmed GM, El-Baz AA, Hashem AAR, Shalaan AK (2013) Expression levels of matrix metalloproteinase-9 and gram-negative bacteria in symptomatic and asymptomatic periapical lesions. J Endod 39:444–448. https://doi.org/10.1016/j.joen.2012.11.009
Shin S-J, Lee J-I, BAEK S-H, Lim S-S, (2002) Tissue levels of matrix metalloproteinases in pulps and periapical lesions. J Endod 28:313–315. https://doi.org/10.1097/00004770-200204000-00013
Belmar MJ, Pabst C, Martínez B, Hernández M (2008) Gelatinolytic activity in gingival crevicular fluid from teeth with periapical lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 105:801–806. https://doi.org/10.1016/j.tripleo.2007.12.002
Duque TM, Prado M, Herrera DR, Gomes BPFA (2019) Periodontal and endodontic infectious/inflammatory profile in primary periodontal lesions with secondary endodontic involvement after a calcium hydroxide-based intracanal medication. Clin Oral Investig 23:53–63. https://doi.org/10.1007/s00784-018-2401-6
Fernández A, Cárdenas AM, Astorga J et al (2019) Expression of toll-like receptors 2 and 4 and its association with matrix metalloproteinases in symptomatic and asymptomatic apical periodontitis. Clin Oral Investig 23:4205–4212. https://doi.org/10.1007/s00784-019-02861-9
Beidler SK, Douillet CD, Berndt DF et al (2008) Multiplexed analysis of matrix metalloproteinases in leg ulcer tissue of patients with chronic venous insufficiency before and after compression therapy. Wound Repair Regen 16:642–648. https://doi.org/10.1111/j.1524-475X.2008.00415.x
Hong C-Y, Lin S-K, Kok S-H et al (2004) The role of lipopolysaccharide in infectious bone resorption of periapical lesion. J Oral Pathol Med 33:162–169. https://doi.org/10.1111/j.0904-2512.2004.00045.x
Lin S-K, Kok S-H, Kuo MY-P et al (2002) Sequential expressions of MMP-1, TIMP-1, IL-6, and COX-2 genes in induced periapical lesions in rats. Eur J Oral Sci 110:246–253. https://doi.org/10.1034/j.1600-0447.2002.11227.x
Edwards DR, Beaudry PP, Laing TD et al (1996) The roles of tissue inhibitors of metalloproteinases in tissue remodelling and cell growth. Int J Obes Relat Metab Disord 20(Suppl 3):S9-15. https://doi.org/10.7748/en.4.3.9.s3
Lu L, Gunja-Smith Z, Woessner JF et al (2000) Matrix metalloproteinases and collagen ultrastructure in moderate myocardial ischemia and reperfusion in vivo. Am J Physiol Circ Physiol 279:H601–H609. https://doi.org/10.1152/ajpheart.2000.279.2.H601
Shibata Y, Takiguchi H, Abiko Y (1999) Antisense oligonucleotide of tissue inhibitor of metalloproteinase-1 induces the plasminogen activator activity in periodontal ligament cells. J Periodontol 70:1158–1165. https://doi.org/10.1902/jop.1999.70.10.1158
Greene J, Wang M, Liu YE et al (1996) Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem 271:30375–30380. https://doi.org/10.1074/jbc.271.48.30375
Garlet GP, Horwat R, Ray HL et al (2012) Expression analysis of wound healing genes in human periapical granulomas of progressive and stable nature. J Endod 38:185–190. https://doi.org/10.1016/j.joen.2011.09.011
Torres AFC, Antunes LS, de Oliveira NF et al (2020) Genetic polymorphism and expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in periapical lesions: systematic review. J Endod 46:3-11.e1. https://doi.org/10.1016/j.joen.2019.10.011
Nagase H, Woessner JF (1999) Matrix metalloproteinases. J Biol Chem 274:21491–21494. https://doi.org/10.1074/jbc.274.31.21491
Hadziabdic N, Kurtovic-Kozaric A, Pojskic N et al (2016) Gene-expression analysis of matrix metalloproteinases 1 and 2 and their tissue inhibitors in chronic periapical inflammatory lesions. J Oral Pathol Med 45:224–230. https://doi.org/10.1111/jop.12347
Sobue T, Hakeda Y, Kobayashi Y et al (2001) Tissue inhibitor of metalloproteinases 1 and 2 directly stimulate the bone-resorbing activity of isolated mature osteoclasts. J Bone Miner Res 16:2205–2214. https://doi.org/10.1359/jbmr.2001.16.12.2205
de Paula-Silva FWG, D’Silva NJ, da Silva LAB, Kapila YL (2009) High matrix metalloproteinase activity is a hallmark of periapical granulomas. J Endod 35:1234–1242. https://doi.org/10.1016/j.joen.2009.06.008
Martinho FC, Nascimento GG, Leite FRM et al (2015) Clinical influence of different intracanal medications on Th1-type and Th2-type cytokine responses in apical periodontitis. J Endod 41:169–175. https://doi.org/10.1016/j.joen.2014.09.028
Virdee SS, Butt K, Grant M et al (2019) A systematic review of methods used to sample and analyse periradicular tissue fluid during root canal treatment. Int Endod J 52:1108–1127. https://doi.org/10.1111/iej.13104
Hartroth B, Seyfahrt I, Conrads G (1999) Sampling of periodontal pathogens by paper points: evaluation of basic parameters. Oral Microbiol Immunol 14:326–330. https://doi.org/10.1034/j.1399-302X.1999.140510.x
Shin S-J, Lee W, Lee J-I et al (2011) Matrix metalloproteinase-8 and substance P levels in gingival crevicular fluid during endodontic treatment of painful, nonvital teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 112:548–554. https://doi.org/10.1016/j.tripleo.2011.04.026
Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP (1997) Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol 74:111–122
Lambert E, Dassé E, Haye B, Petitfrère E (2004) TIMPs as multifacial proteins. Crit Rev Oncol Hematol 49:187–198. https://doi.org/10.1016/j.critrevonc.2003.09.008
Siqueira JF, Guimarães-Pinto T, Rôças IN (2007) Effects of chemomechanical preparation with 2.5% sodium hypochlorite and intracanal medication with calcium hydroxide on cultivable bacteria in infected root canals. J Endod 33:800–805. https://doi.org/10.1016/j.joen.2006.11.023
Rôças IN, Siqueira JF (2010) Identification of bacteria enduring endodontic treatment procedures by a combined reverse transcriptase-polymerase chain reaction and reverse-capture checkerboard approach. J Endod 36:45–52. https://doi.org/10.1016/j.joen.2009.10.022
Xavier ACC, Martinho FC, Chung A et al (2013) One-visit versus two-visit root canal treatment: effectiveness in the removal of endotoxins and cultivable bacteria. J Endod 39:959–964. https://doi.org/10.1016/j.joen.2013.04.027
Weisenseel JA, Hicks ML, Pelleu GB (1987) Calcium hydroxide as an apical barrier. J Endod 13:1–5. https://doi.org/10.1016/S0099-2399(87)80084-5
Hasselgren G, Olsson B, Cvek M (1988) Effects of calcium hydroxide and sodium hypochlorite on the dissolution of necrotic porcine muscle tissue. J Endod 14:125–127. https://doi.org/10.1016/S0099-2399(88)80212-7
Safavi KE, Nichols FC (1993) Effect of calcium hydroxide on bacterial lipopolysaccharide. J Endod 19:76–78. https://doi.org/10.1016/S0099-2399(06)81199-4
Martinho FC, Gomes BPFA (2008) Quantification of endotoxins and cultivable bacteria in root canal infection before and after chemomechanical preparation with 2.5% sodium hypochlorite. J Endod 34:268–272. https://doi.org/10.1016/j.joen.2007.11.015
Martinho FC, Gomes CC, Nascimento GG et al (2018) Clinical comparison of the effectiveness of 7- and 14-day intracanal medications in root canal disinfection and inflammatory cytokines. Clin Oral Investig 22:523–530. https://doi.org/10.1007/s00784-017-2143-x
Punathil S, Moyin S, Bhat SS et al (2020) Comparison of antibacterial effect of calcium hydroxide combined with chlorhexidine and povidone-iodine against Enterococcus faecalis in dentinal tubules of human incisors: an in vitro comparative study. J Pharm Bioallied Sci 12:S448–S452. https://doi.org/10.4103/jpbs.JPBS_134_20
Ferreira NS, Martinho FC, Cardoso FGR et al (2015) Microbiological profile resistant to different intracanal medications in primary endodontic infections. J Endod 41:824–830. https://doi.org/10.1016/j.joen.2015.01.031
Mohammadi Z, Abbott PV (2009) The properties and applications of chlorhexidine in endodontics. Int Endod J 42:288–302. https://doi.org/10.1111/j.1365-2591.2008.01540.x
Scaffa PMC, Vidal CMP, Barros N et al (2012) Chlorhexidine inhibits the activity of dental cysteine cathepsins. J Dent Res 91:420–425. https://doi.org/10.1177/0022034511435329
Gendron R, Grenier D, Sorsa T, Mayrand D (1999) Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine. Clin Diagn Lab Immunol 6:437–439
Mouzakiti E, Pepelassi E, Fanourakis G et al (2012) Expression of MMPs and TIMP-1 in smoker and nonsmoker chronic periodontitis patients before and after periodontal treatment. J Periodontal Res 47:532–542. https://doi.org/10.1111/j.1600-0765.2011.01465.x
Mouzakiti E, Pepelassi E, Fanourakis G et al (2011) The effect of smoking on the mRNA expression of MMPs and TIMP-1 in untreated chronic periodontitis patients: a cross-sectional study. J Periodontal Res 46:576–583. https://doi.org/10.1111/j.1600-0765.2011.01375.x
Funding
This study is supported by the Brazilian agencies Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP FAPESP (2015/03697–8; 201219536–5).
Author information
Authors and Affiliations
Contributions
FM conceived the experiment(s); FT, FC, NF, AG, BC, MV, and FM conducted the experiment(s) and data analysis. FM and BC wrote the article, and all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the local Institute Review Board (São Paulo State University (Unesp), Institute of Science and Technology, São José dos Campos, Brazil) (#1.734.879) and registered at Brazilian Clinical Trials Registry (REBEC) (#RBR-9xhgs6). The procedures were conducted following the Helsinki Declaration.
Consent to participate
All individual participants included in the study signed an informed consent form.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Teixeira, F.F.C., Cardoso, F.G.R., Ferreira, N.S. et al. Clinical influence of calcium hydroxide intracanal medications on matrix metalloproteinases and tissue inhibitors of metalloproteinases in apical periodontitis. Clin Oral Invest 26, 643–650 (2022). https://doi.org/10.1007/s00784-021-04042-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-021-04042-z