Abstract
Objective
This randomized clinical trial aimed to compare the efficacy of an oral irrigator and an interdental brush in patients with peri-implant mucositis clinically and biochemically at different time points (at baseline and at the 2nd, 4th, and 12th weeks).
Materials and methods
Forty-five patients with at least one implant with peri-implant mucositis were included in the present study (n = 45). The patients were divided into three groups: oral irrigator + toothbrush (OI group, n = 15), interdental brush + toothbrush (IB group, n = 15), and toothbrush only (control) (C group, n = 15). The modified plaque index (mPlI), modified sulcus bleeding index (mSBI), probing pocket depth (PPD), probing attachment level (PAL), and bleeding on probing (BOP) were recorded at baseline and at the 2nd, 4th, and 12th weeks. The levels of interleukin 1 beta (IL-1β), transforming growth factor-beta (TGF-β), tissue-type plasminogen activator (t-PA), and plasminogen activator inhibitor-1 (PAI-1) were also determined in the peri-implant crevicular fluid samples biochemically.
Results
The mSBI and t-PA at the 2nd week (p = 0.003; p = 0.003); the mPlI, mSBI, BOP, t-PA, and PAI-1 at the 4th week (p < 0.05; p < 0.001; p < 0.001; p = 0.015; p = 0.011); and the mPlI, mSBI, IL-1β, t-PA, and PAI-1 at the 12th week (p < 0.05; p < 0.001; p = 0.013; p < 0.001; p = 0.002) were significantly lower in the OI group compared with those in the C group. Meanwhile, PAI-1 at the 2nd week, mSBI at the 4th week, and t-PA at the 12th week were significantly lower in the OI group compared with those in the IB group (p < 0.001; p = 0.011; p = 0.003). At the 2nd, 4th, and 12th weeks, all other parameters were not statistically different in the three groups.
Conclusion
The clinical indexes (such as mSBI and BOP) that play an important role in the diagnosis of peri-implant mucositis showed the lowest means (although limited) in the OI group at all evaluation time points. Moreover, when the clinical and biochemistry results were interpreted altogether, it became apparent that the OI group exhibited similar or more effective results than the IB group in resolving peri-implant mucositis. In light of the foregoing, this study concluded that the use of an oral irrigator can be as effective as an interdental brush in interdental cleaning.
Clinical relevance
In this study, it is suggested that the regular use of an oral irrigator along with a toothbrush could be an appropriate alternative to other oral hygiene products such as dental floss and interdental brush for the management of peri-implant mucositis by preventing the accumulation of dental plaque (NCT03844035).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peri-implant diseases are characterized by the inflammation of the tissues around the implant; similar to periodontal diseases, they consist of the complex interactions of the oral biofilm deposited on the host and dental implants [1, 2]. To address these dental concerns, regular removal of the microbial biofilm from the teeth and implant surfaces prevents the occurrence of possible peri-implant disease and preserves oral health [1]. Based on the literature, manual or electric toothbrushes and interdental cleaning devices are well-documented devices for individual daily oral hygiene procedures [3]. Dental floss, toothpicks, and interdental brushes are the most widely used tools in interdental cleaning. However, evidence regarding the most efficacious interdental cleaning tool remains equivocal [4,5,6,7].
The dental water jet, also named as water flosser or oral irrigator, is an electric device that delivers pulsating fluid via controlled pressure [6]. Notably, the working principle of oral irrigators differs with variations in vibration and pressure [8]. This device is used both in the office and at home for the removal of interdental and subgingival plaque biofilm and bacteria from the tooth surfaces. It also stimulates the gingiva with the pressure and vibration it creates [9]. There are also some types of this oral care supply on the market that create microbubbles by mixing the air with water and make it easier to deal with bacteria [10]. Oral irrigators have been shown to be effective in reducing oral biofilm, clinical periodontal indexes, and host inflammatory mediators with regular usage [11,12,13]. It was also reported that the use of oral irrigators in addition to tooth brushing can result in the gain of clinical attachment level (CAL), reduction in pocket probing depth (PPD), bleeding on probing (BOP), gingival index (GI) and plaque index (PI) scores, and levels of host inflammatory mediators when compared to the use of dental floss in addition to brushing [6, 12,13,14]. Moreover, it was proven that these devices do not cause soft tissue (periodontal and mucosal) trauma and additional risk of bacteremia and, therefore, are quite safe for cleaning the tooth surface [9]. However, there is a dearth of research showing that the application of oral irrigation to implant patients can reduce the presence and severity of peri-implant disease [15,16,17]. Thus, more detailed studies are needed on this subject.
The microbial biofilm is instrumental in the initiation and progression of periodontal and peri-implant diseases, but the consequent tissue destruction, wound healing, and tissue remodeling are mediated predominantly by the host inflammatory response [12, 18]. During tissue destruction, tissue repair, and remodeling, the local inflammatory reactions, as well as the synthesis of specific extracellular matrix molecules by fibroblasts, angiogenesis, reepithelization, and remodeling, are all regulated by growth factors such as transforming growth factor-beta (TGF-β), cytokines such as interleukin 1 beta (IL-1β), and enzymes such as tissue plasminogen activator (t-PA) and plasminogen activator inhibitor 1 (PAI-1) [19, 20]. In addition, the plasminogen-activating (PA) system remains balanced through the activities of plasminogen activators such as urokinase (u-PA) and tissue-type PA (t-PA) and plasminogen activator inhibitors such as PAI-1 and PAI-2 [21]. These components of the PA system have been shown to contribute to periodontal connective tissue degradation and cell migration, as well as to the entire process of periodontal wound healing [21]. There are studies showing that these markers, which are thought to be effective in both the inflammatory and healing processes of periodontal increases in periodontal disease [22,23,24,25] and peri-implant diseases [26,27,28].
Based on the current data, there is still no specific standard of care for implants. In light of all the foregoing information, this study aimed to examine the effectiveness of the use of oral irrigators along with routine brushing in disease management and determine its limitations in patients with peri-implant mucositis. Thereafter, its effectiveness was compared with the use of interdental brushes. Our hypothesis is that oral irrigators used regularly can be an alternative device for interdental cleaning in patients with implant-supported prostheses based on both the clinical parameters and biochemical markers (IL-1β, TGF-β, t-PA, PAI-1).
Materials and methods
Subjects
Forty-five patients with peri-implant mucositis (age range between 45 and 60) who were screened in the Department of Periodontology and Oral Diagnosis and Radiology in the Ondokuz Mayıs University Faculty of Dentistry were enrolled in the study. This study was designed as a non-inferiority study. The least number of patients for each group was determined as 10 for 95% power, 5% type 1 error, 0.05 significance level, and 1.06675 effect size [14]. The definitions and diagnoses of the peri-implant conditions were based on the declared criteria according to the “2017 World Workshop on the Classification of Periodontal and Peri-implant Diseases and Conditions” [29] and “Case definitions and diagnostic considerations” [30]. The diagnosis of peri-implant mucositis required the following: (a) visual inspection demonstrating the presence of peri-implant signs of inflammation: red as opposed to pink, swollen tissues as opposed to no swelling, soft as opposed to firm tissue consistency; (b) presence of profuse (line or drop) bleeding and/or suppuration on probing; (c) less than 2-mm increase in probing depths compared to baseline; (d) absence of bone loss beyond crestal bone level changes resulting from the initial remodeling [30].
The inclusion criteria were 18 years of age, no systemic diseases, and no professional cleaning for at least 6 months prior to baseline examination and having implants (Straumann, Waldenburg, Switzerland) functioning at least 24 months before the study. If a subject had more than one implant, one of the implants was chosen for inclusion in the study using a computer-generated randomization scheme. Subjects not cooperative, having disease associated with bacteremia (such as tonsillitis, pneumonia, and urinary infections), taking medications influencing gingival health, and using long-term antibiotic and anti-inflammatory agents, smokers, and women who were pregnant or in lactation period, were excluded from the study. The study protocol was carried out according to the Helsinki Declaration of 1975, as revised in 2002 and also approved by the Human Ethical Committee of Ondokuz Mayis University (protocol no: 2014/644). Informed consent was obtained from all individuals recruited for the study.
Study design
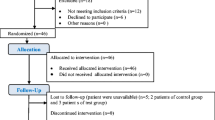
The subjects with peri-implant mucositis in this randomized, single-blinded, parallel designed, and non-inferiority study were divided into three equal groups (Fig. 1).
Participant flow diagram and study design. Oral irrigator group, OI group; interdental brush group, IB group; control group, C group; control session, CS (clinical index records and biochemistry samples were taken from the patients); full mouth instrumentation; FMI (full mouth instrumentation were performed and oral hygiene procedures were described according to the groups)
Oral irrigator group (OI Group) (n = 15)
The patients used a toothbrush and an oral irrigator (Oral-B® Professional Care MD20 Oxyjet Oral Irrigator, Germany) as home care products. The patients in the OI group were recommended to use an oral irrigator (rated voltage: 100–240 V, 50–60 Hz, power consumption: 18 W) that has the technology of mixing air and water and creating microbubbles with 100 ml of warm water (distilled) every evening after brushing according to the manufacturer’s instructions. There are five options in the device for liquid pressure adjustment, ranging from 1 (gentle) to 5 (strong). In order to standardize the use of the patients and to gain maximum benefit from the device, the patients were informed to use the device in the fifth mode (strong mode). However, it was also mentioned that if any discomfort (such as pain, irritation) occurs while using the device, the pressure can be reduced in order not to cause mucosal injuries (however, the feedbacks were that all patients used the device in the fifth mode without any problems and did not need a mode change). In addition, it was recommended to use only standard water jet nozzles (subragingival) for all applications.
Interdental brush group (IB Group) (n = 15)
The patients used a toothbrush and an interdental brush (Oral-B® Pro-Expert Clinic Line Interdental starter kit, Germany) as home care products. The recommended kit included a brush handle that allowed the angle of the brush to be altered and brush heads that could be easily changed during use. Standard interdental brushing instructions were given to this group, and the patients were asked to clean all interdental spaces with an interface brush every evening after brushing their teeth.
Control group (C Group) (n = 15)
The patients used only toothbrush.
The subjects were randomly assigned to one of the groups by way of a computer-generated randomization scheme. A research coordinator also enrolled participants and assigned them to randomized groups. Examiners were blinded to the subjects’ treatment group. All patients in the study used an American Dental Association (ADA)-standard manual toothbrush (Oral-B® Soft Compact 35, Procter & Gamble, Gross-Gerau, Germany) and an ADA-standard dentifrice (Colgate Total 12, Colgate-Palmolive Company, Sao Paulo, Brazil). In addition, all patients were told to apply the modified Bass method of brushing. The subjects used all of the products under the direction of the research coordinator before they were allowed to take them home. They were also given written instructions to follow. The subjects in all groups were instructed to brush twice a day (morning and evening) for 2 min each time. Subjects in the IB and OI groups used the products in the evening. The patients were informed not to use other dental devices or oral care products throughout the duration of the study.
The modified plaque index (mPlI) [31], modified sulcus bleeding index (mSBI) [31], BOP [32], PPD (measurement of the distance between the mucosal margin and the peri-implant pocket base), and probing attachment level (PAL) (measurement of the distance between the implant shoulder and the peri-implant pocket base) were assessed at baseline and at the 2nd, 4th, and 12th weeks. All clinical parameters were evaluated at six sites of the implant (mesio-buccal, mid-buccal, disto-buccal, mesio-lingual, mid-lingual, disto-lingual) using routine protocols. The highest measurement values were accepted as the score of each implant. In addition, the BOP scores recorded from four sides of each implant were averaged. The presence of bleeding on probing in the peri-implant tissues of each participant was determined as a percentage (as “0%, 25%, 50%, 57%, and 100%”). PPD and PAL measurements were conducted using an automated periodontal probe (Florida Probes, version FP 32/7.2.2, Florida Probe Corporation) with implant grade titanium (diameter of the probe tip, 0.45 mm), which applies constant force (15 g) during probing and has a sensitivity of 0.1 mm. Following the initial clinical records, full mouth instrumentation was performed and oral hygiene procedures were described according to the groups.
Peri-implant crevicular fluid sampling
Peri-implant crevicular fluid (PICF) was collected 1 day after the clinical examinations from the same sites in order to eliminate possible quantitative and qualitative alterations (due to bleeding, suppuration, and plaque) in the PICF. Prior to PICF sampling, a supragingival plaque was removed by sterile curets and, after air-drying, the surfaces were isolated by cotton rolls. Filter paper strips (Periopaper, ProFlow Inc., Amityville, NY, USA) were placed in peri-implant sulcus to a depth of 1 mm or mild resistance was felt and left in place for 30 s. Care was taken not to avoid mechanical trauma and strips contaminated with blood or saliva were discarded. The absorbed PICF volume was estimated by a calibrated instrument (Periotron 8000, ProFlow Inc., Amityville, NY, USA). Then, the strips were sealed into sterile tubes before freezing at – 80 °C. The readings were converted to an actual volume (μl) by reference to the standard curve.
Biochemical analysis
The concentrations of IL-1β, TGF-β, t-PA, and PAI-1 in PICF were measured using commercially available sandwich enzyme-linked immunosorbent assay (ELISA) kits (Assaymax Human IL-1β ELISA kit, Assaypro LLC Co., Cat No. EI2200-1, St.Charles, MO, USA; Human TGF-β1 Platinum ELISA kit, eBioscience An Affymetrix Company, Cat No. BMS249/4/BMS249/TEN, Vienna, Austria; IMUBIND t-PA ELISA kit, Sekisui Diagnostics LLC Company, REF860, Stamford, Connecticut, USA; IMUBIND PAI-1 ELISA kit, Sekisui Diagnostics LLC Company, REF822, Stamford, Connecticut, USA). The enzymatic reactions were quantified in an automatic microplate photometer. The levels of the parameters were expressed as picograms per milliliter.
Statistical analysis
Statistical analyses were performed using a statistical software package (SPSS 15.0 Software Package Programme, Inc., Chicago, IL). Compliance with the normal distribution of data was evaluated by the Shapiro–Wilk test. The one-way analysis of variance (ANOVA) parametric test was used for the comparisons of data having a normal distribution. The non-parametric Pearson chi-square, Kruskal Wallis, and Mann–Whitney U tests were used for the data with non-normal distribution. Correlations between variables were evaluated by the Spearman correlation analysis. Statistical significance level was considered as p < 0.05 Data are expressed as means ± standard deviations and median (minimum–maximum).
Results
Clinical findings
No significant differences were observed in the mean age and sex distribution between the study groups (p > 0.05) (Table 1).
When the clinical periodontal indices were considered, no statistically significant differences between the groups were found with regard to the mPlI values (secondary outcome) at baseline and at the 2nd week (p > 0.05), whereas the mPlI values at the 4th and 12th weeks were statistically significantly lower in the OI group compared with the C group (p < 0.05) (Table 2, Fig. 2A).
A Intergroup comparisons in plaque index at different time points (baseline, 2 weeks, 4 weeks, 12 weeks). B Intergroup comparisons in the gingival index at different time points (baseline, 2 weeks, 4 weeks, 12 weeks). C Intergroup comparisons in bleeding on probing at different time points (baseline, 2 weeks, 4 weeks, 12 weeks). D Intergroup comparisons in PICF volume at different time points (baseline, 2 weeks, 4 weeks, 12 weeks). Superscript letters “a” and “b”: There were no differences between groups with the same superscript letter for each parameter compared to the groups at the same times (p < 0.05). Peri-implant crevicular fluid, PICF; oral irrigator group, OI; interdental brush group, IB; control group, C
The mSBI values (primary outcome) showed lower means over the previous time point in all study groups throughout the study period. No statistically significant differences were found in the baseline mSBI values (p > 0.05), whereas mSBI levels at the 2nd week evaluation in the C group were statistically higher compared with those in the OI group (p = 0.03). Furthermore, mSBI levels at the 4th week in the OI group were statistically lower compared with those in the IB and C groups, respectively (p = 0.011, p < 0.001). In addition, this parameter was statistically significantly lower in the OI group compared with the C group at the 12th week evaluation (p < 0.001) (Table 2, Fig. 2B).
The BOP levels (primary outcome) exhibited no significant differences at baseline and at the 2nd and 12th week observations between the groups (p > 0.05). However, the BOP levels at the 4th week evaluation in the OI and IB groups were significantly lower compared with those in the C group, respectively (p < 0.001, p = 0.011) (Table 2, Fig. 2C). Also, the distribution of the patients in the groups according to the percentages of bleeding on probing in different time point is shown in Fig. 3.
There were no statistically significant differences in the PPD and PAL levels between the groups in any of the intervals as the patients had no clinical attachment and bone loss (p > 0.05) (Table 2).
Biochemical findings
The mean interassay coefficient of variation (CV) % and intraassay CV% for IL-1β were 8.4% and 3.5%, respectively. The mean interassay CV% and intraassay CV% for TGF-β were 4.9% and 3.2%, respectively. The mean interassay CV% and intraassay CV% for t-PA were 8.2% and 4.9%, respectively. The mean interassay CV% and intraassay CV% for PAI-1 were 9.0% and 6.6%, respectively. The samples which showed higher concentrations were measured in duplicate.
The PICF IL-1β, TGF-β, t-PA, and PAI-1 total volume values in all groups at all time points are shown in Fig. 2D. The PICF volumes showed no significant differences between the groups (p > 0.05).
Statistically significant differences were found in the IL-1β total volume between the OI and C groups only at the 12th week (p = 0.013), while there were no significant differences in this parameter between other groups at any other time points (p > 0.05) (Table 3, Fig. 4A).
A Intergroup comparisons in total IL-1β levels at different time points (baseline, 2 weeks, 4 weeks, 12 weeks). B Intergroup comparisons in total TGF-β levels at different time points (baseline, 2 weeks, 4 weeks, 12 weeks). C Intergroup comparisons in total t-PA levels at different time points (baseline, 2 weeks, 4 weeks, 12 weeks). D Intergroup comparisons in total PAI-1 levels at different time points (baseline, 2 weeks, 4 weeks, 12 weeks). Superscript letters “a,” “b,” and “c”: There were no differences between groups with the same superscript letter for each parameter compared to the groups at the same times (p < 0.05). Interleukin 1 beta, IL-1β; transforming growth factor-beta, TGF-β; tissue-type plasminogen activator, t-PA; plasminogen activator inhibitor 1, PAI-1; oral irrigator group, OI; interdental brush group, IB; control group, C
No significant differences were found in the TGF-β total volume between the groups at any of the time points (p > 0.05) (Table 3, Fig. 4B). When the T-PA total values are analyzed, the results in the OI group were significantly lower than those in the C group at the 2nd (p = 0.003), 4th (p = 0.015), and 12th week (p < 0.001) evaluations. No significant differences in this parameter were observed between the IB and C groups at the 2nd and 4th weeks (p > 0.05), whereas the t-PA levels in the IB group were found to be significantly lower compared with those in the C group at the 12th week observation (p = 0.006). When the two test groups were compared, this parameter was statistically significantly lower in the OI group at the 12th week compared with the IB group (p = 0.003) (Table 3, Fig. 4C).
The PAI-1 total values showed a statistically significant difference between the OI group and the C group at the 4th (p = 0.011) and 12th weeks (p = 0.002). Test group comparisons showed that the PAI-1 total values in the OI group were significantly lower compared with those in the IB group at the 2nd week (p < 0.001) (Table 3, Fig. 3D).
When the correlation between clinical and biochemical findings were analyzed; Spearman’s correlation analysis revealed statistically significant positive correlations between mSBI and IL-1β values at the 2nd week (r2 = 0.422; p = 0.004) and between BOP and IL-1β values at the 12th week (r2 = 0.458; p = 0.002). Also, correlation analysis between biochemical finding showed that significant positive correlations between IL-1β and t-PA and IL-1β and PAI-1 were found at the 2nd (r2 = 0.463; p = 0.001 and r2 = 0.555; p < 0.001 respectively), 4th (r2 = 0.630; p < 0.001 and r2 = 0.579; p < 0.001 respectively), and 12th (r2 = 0.607; p < 0.001 and r2 = 0.579; p < 0.001 respectively) week observations.
Discussion
In the present study, clinical recordings include mPlI, mSBI, BOP, PPD, and PAL and biochemical recordings include IL-1β, TGF-β, t-PA, and PAI-1 in the PICF samples in patients with peri-implant mucositis to compare the efficacy of oral irrigators to interdental brush. To the best of our knowledge, this study is the first to investigate the effectiveness of the use of oral irrigators in the management of peri-implant diseases in light of biochemical and clinical data.
The dexterity and additional time requirement for using interdental cleaning devices are known to affect mechanical plaque control, particularly the effective removal of oral biofilm from the interproximal areas [4, 33]. To date, there is no standard of care for the interdental cleaning of implants and the search for alternative methods that are easier would increase patient comfort. Based on this idea, this study was planned to evaluate the success of oral irrigators which are a more controlled and easy-to-use application than other protocols in the management of peri-implant mucositis. The study was planned as 12 weeks in which the clinical signs of peri-implant disease could be minimized through the improvement of oral hygiene based on recent literature [15, 34].
The results of the study showed that the patients who used oral irrigators had better clinical and biochemical recordings (even if limited) compared with the IB and C groups. The OI group had significantly lower mPlI scores compared with the C group at the 4th and 12th weeks, whereas there was no statistically significant difference in this parameter between the C and IB groups. Our results are supported by previous studies which reported that the feeling of cleanliness associated with the use of the oral irrigator is very satisfying [12,13,14]. In another study evaluating the effectiveness of two different oral irrigator devices, 57.4% of the patients were observed to have continued using the oral irrigator at the end of 1 year [35]. Moreover, a 6-month study [36] noted a 90.6% compliance rate in patients using the oral irrigator. In accordance with these studies, the lower mPlI levels in the OI group can be attributed to the fact that the product was more effective in removing plaque or was more successful in terms of patient motivation/adaptation. When the mPlI results of the present study were examined, the values of all study groups in the 2nd week were observed to be quite close to the baseline, whereas the values at the 4th and 12th weeks were lower than the values in the baseline and the 2nd week in all groups. This can be attributed to the limited manual dexterity of the patients while using the related products in the first weeks of the experimental protocol. In other words, the development of patients’ hand manipulations in the following weeks might have led to gradually lower plaque means in all groups. Another explanation might be the possibility of increased patient motivations during the control sessions.
In this study, it was observed that the mean mSBI was lower in the patients using oral irrigators compared with the patients in the C group. Upon comparison of the two test groups, the mSBI values were found to be lower in patients using oral irrigators at the 4th and 12th weeks. In a clinical study evaluating the efficacy of oral irrigators in individuals with gingivitis, it was shown that using oral irrigators in combination with a toothbrush improved gingival health compared with using only a toothbrush [36]. Similarly, in another clinical study, the subjects using oral irrigators had better gingival index values compared with those using routine oral care supplies [12]. In light of our findings, the usage of an oral irrigator seems more effective compared to the interdental brush to control gingival inflammation. Although no significant difference was found in the mPlI values among the three groups at the 2nd week, there were statistically significant differences in the mSBI values between the C and OI groups at the same time points. As a result, our findings support recent reports that oral irrigators are beneficial in controlling gingival and peri-implant inflammation [15, 36,37,38,39,40]. It can, therefore, be concluded that supragingival irrigation might reduce gingival inflammation without affecting the supra and subgingival plaque formation. The limited effect on supra and subgingival plaque might be related to either a reduction in specific bacteria within the plaque and/or a reduction in the quantity of toxic products produced by plaque. Another explanation for this finding might be the additional healing effect caused by supragingival irrigation and/or a mechanical stimulation of the gingiva or any combination of these factors [36, 40]. Notably, these situations can stimulate specific antibodies against periodontal pathogens, which may result in decreased inflammation.
The BOP index is considered to be the response of the gingival sulcus or periodontal pocket against the stimulus. In the present study, the OI group showed lower values for this parameter in the 2nd and 4th weeks. Our results are in agreement with the literature showing better clinical results with water flossing compared with the use of string floss on natural teeth [6, 12,13,14, 40]. Furthermore, in another clinical study involving patients with peri-implant mucositis, lower BOP values were found in the OI group compared with those using dental floss [41]. When the 12th week findings were examined, both interdental brush and oral irrigator seemed to have similar mean values in the peri-implant mucositis patients. This result indicates that the oral irrigator may limit inflammation in peri-implant mucositis at least as much as the interface brush.
In many studies related to periodontal disease, high levels of IL-1β in both gingival crevicular fluid [42, 43] and gingiva [44, 45] were associated with chronic periodontitis. A correlation between the levels of IL-1β and peri-implantitis was also reported [18, 46,47,48,49]. Our biochemical findings showed that this marker was statistically lower in patients using oral irrigators at the 12th week compared with the C group, while it was similar in the C and IB groups. Indeed, a positive correlation was observed between the total value of IL-1β and mSBI at the 2nd week. Our biochemical findings indicate that the use of oral irrigators yielded successful results in resolving peri-implant mucositis, in accordance with clinical findings.
TGF-β is a growth factor that is expressed in both the inflammatory and proliferative phases. In the inflammatory phase, TGF-β increases neutrophil and monocyte chemotaxis, while in the proliferative phase, this cytokine increases the proliferation of the fibroblasts and stimulates the synthesis of the extracellular matrix [50]. Although not statistically significant, lower levels of TGF-β at the 2nd week and higher levels of TGF-β at the 4th and 12th weeks supported the fact that growth factors are closely related to both inflammation and proliferation phases.
The PA system is known to be associated with fibrinolysis and thrombolysis. Therefore, it is of central importance in ECM degradation and remodeling. t-PA is the plasminogen activator that has been detected to be at high levels in gingival inflammation [51]. Higher t-PA levels have been associated with gingivitis [52, 53] and periodontitis [52]. The authors reported that t-PA may play an important role in tissue remodeling and that the t-PA level in the gingival crevicular fluid can be used as a marker for the clinical evaluation and efficacy of periodontal treatment. Moreover, Kinnby et al. [51] compared the t-PA and PAI-1 levels before and after periodontal treatment and found that the t-PA levels decreased after treatment without any changes in the PAI-1 levels. A clinical study of plasminogen activator activity in gingival tissue in dogs with gingivitis and periodontitis also reported that t-PA increased significantly in the inflamed gingiva compared with the healthy regions of the gingiva, whereas PAI-1 was not detected in either normal or inflamed gingiva [54]. Bizzarro et al. [55] indicated that periodontitis progression may be associated with increased PAI-1 plasma levels. In our study, it was observed that the t-PA expression levels in the OI group were consistently lower at all times than the levels evaluated at the previous time point. Furthermore, as a result of the statistical analysis of the t-PA values in our study, it was found that the t-PA concentration and total values in the OI group were statistically significantly lower in each period (2nd, 4th, and 12th weeks) compared with those in the C group. In an earlier study, Yin et al. [52] evaluated this marker in periodontal diseases. Similar to the OI group in our study, they reported that the levels of the marker changed with the improvement in clinical parameters. Contrary to this situation, although clinical parameters improved at different time points in the C group of the present study, t-PA showed close stable values at all time points. It is worth mentioning that Yin et al. [52] previously reported that even in patients in the same group, t-PA progresses in very wide ranges. Thus, this parameter may not reflect the true biochemical reactions of the disease. Therefore, based on these findings, the usability of the parameter in determining the condition of peri-implant patients should be questioned and examined in detail in future studies. There has been no clinical or experimental study detecting PAI expression in peri-implant inflammation. This is the first report evaluating the PAI-1 levels in PICF. Given that our biochemical correlation reports also showed a correlation between plasminogen activator system members and IL-1β, all biochemical findings that are in agreement with the clinical results support better improvement in patients using oral irrigators in daily oral hygiene.
Although the treatment recommendations for peri-implant infections remain unclear, improving oral hygiene plays a pivotal role in the management of peri-implant diseases. Peri-implant mucositis can be successfully treated by reinforcing oral hygiene and focusing on proper cleaning of the implants [3]. Oral irrigators are effective in removing bacterial plaque from all dental areas regardless of the location [3]. Our investigation compared two different hygiene methods to treat peri-implant mucositis and unveiled potential cleaning methods that could be implemented in daily oral home care protocols. Although the sample size was large enough to detect a statistically significant difference between the groups based on the results of the power analysis, one of the limitations of the present study may be the relatively small patient number. Further studies are needed to confirm our clinical and biochemical findings in larger sample sizes.
Conclusion
In the present study, the role of the oral irrigators in controlling disease and restoring peri-implant health in patients with peri-implant mucositis was biochemically and clinically evaluated. It was observed that the clinical disease symptoms of the patients in the OI group were significantly lower than those in the control group. Moreover, the biochemical markers associated with inflammation showed results that are consistent with the clinical data. In fact, mSBI, which is one of the recorded parameters (one of the most utilized clinical parameters in the diagnosis of peri-implant mucocitis), showed lower mean values in the OI group compared to the IB group at different time points. We interpreted this finding as a manifestation of the superiority of oral irrigators and bring to mind the idea that these devices, which are more practical to use and easier to manipulate, can play an active role in patient motivation and compliance in maintaining long-term oral care. The oral irrigators showed promising results within a follow-up period of 12 weeks, in accordance with our hypothesis. Nonetheless, we believe that this device should be tested with the longer follow-up studies in peri-implant diseases and with more comprehensive parameters.
References
Klinge B, Klinge A, Bertl K, Stavropoulos A (2018) Peri-implant diseases. Eur J Oral Sci 126 Suppl 1:88–94. https://doi.org/10.1111/eos.12529
Belibasakis GN (2014) Microbiological and immuno-pathological aspects of peri-implant diseases. Arch Oral Biol 59(1):66–72. https://doi.org/10.1016/j.archoralbio.2013.09.013
Ioannidis A, Thurnheer T, Hofer D, Sahrmann P, Guggenheim B, Schmidlin PR (2015) Mechanical and hydrodynamic homecare devices to clean rough implant surfaces - an in vitro polyspecies biofilm study. Clin Oral Implants Res 26(5):523–528. https://doi.org/10.1111/clr.12436
Noorlin I, Watts TL (2007) A comparison of the efficacy and ease of use of dental floss and interproximal brushes in a randomised split mouth trial incorporating an assessment of subgingival plaque. Oral Health Prev Dent 5(1):13–18
Rasines G (2009) The use of interdental brushes along with toothbrushing removes most plaque. Evid Based Dent 10(3):74. https://doi.org/10.1038/sj.ebd.6400666
Rosema NA, Hennequin-Hoenderdos NL, Berchier CE, Slot DE, Lyle DM, van der Weijden GA (2011) The effect of different interdental cleaning devices on gingival bleeding. J Int Acad Periodontol 13(1):2–10
Kotsakis GA, Lian Q, Ioannou AL, Michalowicz BS, John MT, Chu H (2018) A network meta-analysis of interproximal oral hygiene methods in the reduction of clinical indices of inflammation. J Periodontol 89(5):558–570. https://doi.org/10.1002/jper.17-0368
Lyle DM (2011) Use of a water flosser for interdental cleaning. Compend Contin Educ Dent 32(9):78 80–72
Jahn CA (2010) The dental water jet: a historical review of the literature. J Dent Hyg 84(3):114–120
Sharma PK, Gibcus MJ, van der Mei HC, Busscher HJ (2005) Influence of fluid shear and microbubbles on bacterial detachment from a surface. Appl Environ Microbiol 71(7):3668–3673
Newman MG, Flemmig TF, Nachnani S, Rodrigues A, Calsina G, Lee YS, de Camargo P, Doherty FM, Bakdash MB (1990) Irrigation with 0.06% chlorhexidine in naturally occurring gingivitis. II. 6 months microbiological observations. J Periodontol 61(7):427–433. https://doi.org/10.1902/jop.1990.61.7.427
Cutler CW, Stanford TW, Abraham C, Cederberg RA, Boardman TJ, Ross C (2000) Clinical benefits of oral irrigation for periodontitis are related to reduction of pro-inflammatory cytokine levels and plaque. J Clin Periodontol 27(2):134–143. https://doi.org/10.1034/j.1600-051x.2000.027002134.x
Barnes CM, Russell CM, Reinhardt RA, Payne JB, Lyle DM (2005) Comparison of irrigation to floss as an adjunct to tooth brushing: effect on bleeding, gingivitis, and supragingival plaque. J Clin Dent 16(3):71–77
Al-Mubarak S, Ciancio S, Aljada A, Mohanty P, Ross C, Dandona P (2002) Comparative evaluation of adjunctive oral irrigation in diabetics. J Clin Periodontol 29(4):295–300. https://doi.org/10.1034/j.1600-051x.2002.290404.x
Bunk D, Eisenburger M, Häckl S, Eberhard J, Stiesch M, Grischke J (2020) The effect of adjuvant oral irrigation on self-administered oral care in the management of peri-implant mucositis: a randomized controlled clinical trial. Clin Oral Implants Res 31(10):946–958. https://doi.org/10.1111/clr.13638
Salles MM, Oliveira VC, Macedo AP, do Nascimento C, Silva-Lovato CH, Paranhos HFO (2021) Brushing associated with oral irrigation in maintaining implants and overdentures hygiene - a randomized clinical trial. Odontology 109(1):284–294. https://doi.org/10.1007/s10266-020-00543-7
Salles MM, Oliveira VC, Macedo AP, Silva-Lovato CH, Oliveira Paranhos HF (2020)Effectiveness of brushing associated with oral irrigation in maintenance of peri-implant tissues and overdentures: clinical parameters and patient satisfaction. J Oral Implantol.https://doi.org/10.1563/aaid-joi-D-19-00092
Aboyoussef H, Carter C, Jandinski JJ, Panagakos FS (1998) Detection of prostaglandin E2 and matrix metalloproteinases in implant crevicular fluid. Int J Oral Maxillofac Implants 13(5):689–696
Buduneli N, Buduneli E, Kardeşler L, Lappin D, Kinane DF (2005) Plasminogen activator system in smokers and non-smokers with and without periodontal disease. J Clin Periodontol 32(4):417–424. https://doi.org/10.1111/j.1600-051X.2005.00694.x
Aykol G, Baser U, Maden I, Kazak Z, Onan U, Tanrikulu-Kucuk S, Ademoglu E, Issever H, Yalcin F (2011) The effect of low-level laser therapy as an adjunct to non-surgical periodontal treatment. J Periodontol 82(3):481–488. https://doi.org/10.1902/jop.2010.100195
Sarajlic J, Agis H, Kandler B, Watzek G, Gruber R (2007) Plasminogen activation by fibroblasts from periodontal ligament and gingiva is not directly affected by chemokines in vitro. Arch Oral Biol 52(7):663–668. https://doi.org/10.1016/j.archoralbio.2006.12.020
Tatakis DN (1993) Interleukin-1 and bone metabolism: a review. J Periodontol 64(5 Suppl):416–431
Gürkan A, Emingil G, Cinarcik S, Berdeli A (2006) Gingival crevicular fluid transforming growth factor-beta1 in several forms of periodontal disease. Arch Oral Biol 51(10):906–912. https://doi.org/10.1016/j.archoralbio.2006.04.008
Ghosh AK, Vaughan DE (2012) PAI-1 in tissue fibrosis. J Cell Physiol 227(2):493–507. https://doi.org/10.1002/jcp.22783
Kinnby B (2002) The plasminogen activating system in periodontal health and disease. Biol Chem 383(1):85–92. https://doi.org/10.1515/bc.2002.008
Ghassib I, Chen Z, Zhu J, Wang HL (2019) Use of IL-1 β, IL-6, TNF-α, and MMP-8 biomarkers to distinguish peri-implant diseases: a systematic review and meta-analysis. Clin Implant Dent Relat Res 21(1):190–207. https://doi.org/10.1111/cid.12694
Faot F, Nascimento GG, Bielemann AM, Campão TD, Leite FR, Quirynen M (2015) Can peri-implant crevicular fluid assist in the diagnosis of peri-implantitis? A systematic review and meta-analysis. J Periodontol 86(5):631–645. https://doi.org/10.1902/jop.2015.140603
Hall J, Pehrson NG, Ekestubbe A, Jemt T, Friberg B (2015) A controlled, cross-sectional exploratory study on markers for the plasminogen system and inflammation in crevicular fluid samples from healthy, mucositis and peri-implantitis sites. Eur J Oral Implantol 8(2):153–166
Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, Camargo PM, Chen S, Cochran D, Derks J, Figuero E (2018) Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 89:S313–S318
Renvert S, Persson GR, Pirih FQ, Camargo PM (2018) Peri-implant health, peri-implant mucositis, and peri-implantitis: case definitions and diagnostic considerations. J Periodontol 89 Suppl 1:S304-s312. https://doi.org/10.1002/jper.17-0588
Mombelli A, van Oosten MA, Schurch E Jr, Land NP (1987) The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol 2(4):145–151. https://doi.org/10.1111/j.1399-302x.1987.tb00298.x
Ainamo J, Bay I (1975) Problems and proposals for recording gingivitis and plaque. Int Dent J 25(4):229–235
Ohrn K, Sanz M (2009) Prevention and therapeutic approaches to gingival inflammation. J Clin Periodontol 36 Suppl 10:20–26. https://doi.org/10.1111/j.1600-051X.2009.01418.x
Javed F, BinShabaib MS, Alharthi SS, Qadri T (2017) Role of mechanical curettage with and without adjunct antimicrobial photodynamic therapy in the treatment of peri-implant mucositis in cigarette smokers: a randomized controlled clinical trial. Photodiagnosis Photodyn Ther 18:331–334. https://doi.org/10.1016/j.pdpdt.2017.04.015
Lainson PA, Bergquist JJ, Fraleigh CM (1972) A longitudinal study of pulsating water pressure cleansing devices. J Periodontol 43(7):444–446. https://doi.org/10.1902/jop.1972.43.7.444
Flemmig TF, Newman MG, Doherty FM, Grossman E, Meckel AH, Bakdash MB (1990) Supragingival irrigation with 0.06% chlorhexidine in naturally occurring gingivitis I. 6 month clinical observations. J Periodontol 61(2):112–117. https://doi.org/10.1902/jop.1990.61.2.112
Meklas JF, Stewart JL (1972) Investigation of the safety and effectiveness of an oral irrigating device. J Periodontol 43(7):441–443. https://doi.org/10.1902/jop.1972.43.7.441
Walsh M, Heckman B, Leggott P, Armitage G, Robertson PB (1989) Comparison of manual and power toothbrushing, with and without adjunctive oral irrigation, for controlling plaque and gingivitis. J Clin Periodontol 16(7):419–427. https://doi.org/10.1111/j.1600-051x.1989.tb01670.x
Brownstein CN, Briggs SD, Schweitzer KL, Briner WW, Kornman KS (1990) Irrigation with chlorhexidine to resolve naturally occurring gingivitis A methodologic study. J Clin Periodontol 17(8):588–593
Newman MG, Cattabriga M, Etienne D, Flemmig T, Sanz M, Kornman KS, Doherty F, Moore DJ, Ross C (1994) Effectiveness of adjunctive irrigation in early periodontitis: multi-center evaluation. J Periodontol 65(3):224–229. https://doi.org/10.1902/jop.1994.65.3.224
Magnuson B, Harsono M, Stark PC, Lyle D, Kugel G, Perry R (2013) Comparison of the effect of two interdental cleaning devices around implants on the reduction of bleeding: a 30-day randomized clinical trial. Compend Contin Educ Dent 34 Spec No 8:2–7
Masada MP, Persson R, Kenney JS, Lee SW, Page RC, Allison AC (1990) Measurement of interleukin-1 alpha and -1 beta in gingival crevicular fluid: implications for the pathogenesis of periodontal disease. J Periodontal Res 25(3):156–163. https://doi.org/10.1111/j.1600-0765.1990.tb01038.x
Engebretson SP, Grbic JT, Singer R, Lamster IB (2002) GCF IL-1beta profiles in periodontal disease. J Clin Periodontol 29(1):48–53. https://doi.org/10.1034/j.1600-051x.2002.290108.x
Stashenko P, Jandinski JJ, Fujiyoshi P, Rynar J, Socransky SS (1991) Tissue levels of bone resorptive cytokines in periodontal disease. J Periodontol 62(8):504–509. https://doi.org/10.1902/jop.1991.62.8.504
Engebretson SP, Lamster IB, Herrera-Abreu M, Celenti RS, Timms JM, Chaudhary AG, di Giovine FS, Kornman KS (1999) The influence of interleukin gene polymorphism on expression of interleukin-1beta and tumor necrosis factor-alpha in periodontal tissue and gingival crevicular fluid. J Periodontol 70(6):567–573. https://doi.org/10.1902/jop.1999.70.6.567
Panagakos FS, Aboyoussef H, Dondero R, Jandinski JJ (1996) Detection and measurement of inflammatory cytokines in implant crevicular fluid: a pilot study. Int J Oral Maxillofac Implants 11(6):794–799
Ataoglu H, Alptekin NO, Haliloglu S, Gursel M, Ataoglu T, Serpek B, Durmus E (2002) Interleukin-1β, tumor necrosis factor-α levels and neutrophil elastase activity in peri-implant crevicular fluid: Correlation with clinical parameters and effect of smoking. Clin Oral Implant Res 13(5):470–476
Tsalikis L, Parapanisiou E, Bata-Kyrkou A, Polymenides Z, Konstantinidis A (2002) Crevicular fluid levels of interleukin-1alpha and interleukin-1beta during experimental gingivitis in young and old adults. J Int Acad Periodontol 4(1):5–11
Schierano G, Pejrone G, Brusco P, Trombetta A, Martinasso G, Preti G, Canuto RA (2008) TNF-alpha TGF-beta2 and IL-1beta levels in gingival and peri-implant crevicular fluid before and after de novo plaque accumulation. J Clin Periodontol 35(6):532–538. https://doi.org/10.1111/j.1600-051X.2008.01224.x
Border WA, Noble NA (1994) Transforming growth factor beta in tissue fibrosis. N Engl J Med 331(19):1286–1292. https://doi.org/10.1056/nejm199411103311907
Kinnby B, Matsson L, Lecander I (1994) The plasminogen-activating system in gingival fluid from adults.: An intra-individual study before and after treatment of gingivitis. Eur J Oral Sci 102(6):334–341. https://doi.org/10.1111/j.1600-0722.1994.tb01480.x
Yin X, Bunn CL, Bartold PM (2000) Detection of tissue plasminogen activator (t-PA) and plasminogen activator inhibitor 2(PAI-2) in gingival crevicular fluid from healthy, gingivitis and periodontitis patients. J Clin Periodontol 27(3):149–156. https://doi.org/10.1034/j.1600-051x.2000.027003149.x
Olofsson A, Lindberg P, Lanke J, Matsson L, Kinnby B (2003) Relationship between fibrinolytic activity and gingival inflammatory reaction in young individuals. J Periodontal Res 38(1):104–108. https://doi.org/10.1034/j.1600-0765.2003.01370.x
Papadimitriou S, Tsantarliotou M, Makris G, Papaioannou N, Batzios C, Kokolis N, Dessiris A (2006) A clinical study of plasminogen activator activity in gingival tissue in dogs with gingivitis and periodontitis. Res Vet Sci 80(2):189–193. https://doi.org/10.1016/j.rvsc.2005.06.002
Bizzarro S, Van Der Velden U, Ten Heggeler JM, Leivadaros E, Hoek FJ, Gerdes VE, Bakker SJ, Gans RO, Ten Cate H, Loos BG (2007) Periodontitis is characterized by elevated PAI-1 activity. J Clin Periodontol 34(7):574–580
Funding
This study was supported by the Ondokuz Mayis Research Fund (Project number: PYO.DIS.1904.14.006).
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to the conception and design of the study. S.T. and B.O.C. have been involved in data collection and B.A. has been involved in data analysis. F.P., G.C.K., and S.K.B. have been involved in data interpretation and drafting of the manuscript. M.L. has been involved in revising it critically and has given the final approval of the version to be published.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the ethics committee (Human Ethical Committee of Ondokuz Mayis University, protocol no: 2014/644).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tütüncüoğlu, S., Cetinkaya, B.O., Pamuk, F. et al. Clinical and biochemical evaluation of oral irrigation in patients with peri-implant mucositis: a randomized clinical trial. Clin Oral Invest 26, 659–671 (2022). https://doi.org/10.1007/s00784-021-04044-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-021-04044-x