Abstract
Objective
The objective of this work was to investigate in vivo the effects of calcium hydroxide-based intracanal medication (ICM) on the levels of bacteria, pro-inflammatory cytokines (PICs), and matrix metalloproteinases (MMPs) in root canals and periradicular tissues of teeth with failure of the root canal treatment and apical periodontitis.
Materials and methods
Twenty infected root canals of single-rooted teeth were randomly assigned into two groups according to the irrigant used for chemomechanical preparation (CMP) (n = 10 per group): G1 – 2% chlorhexidine (CHX) gel and G2 – 6% sodium hypochlorite (NaOCl). Root canal contents were taken by using paper points before CMP (S1) and after 30 days of calcium hydroxide-based ICM (S2). Microbial reduction was calculated by means of colony-forming unit count (CFU/mL), with PICs and MMPs (pg/mL) being measured by using enzyme-linked immunosorbent assay (ELISA).
Results
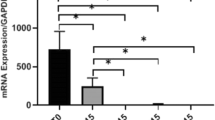
Culturable bacteria (101.2 ± 79.2), PICs (IL-1β 1.2 ± 0.4 and TNF-α 8.8 ± 4.7), MMP-2 (803.7 ± 96.4), MMP-3 (453.9 ± 229.3), MMP-8 (245.9 ± 122.4), MMP-9 (129.4 ± 29.6), and MMP-13 (70.8 ± 12.8) were present in all S1 samples. After 30 days of ICM (S2), a 99.5% microbial reduction was observed, together with a significant reduction of PICs in all groups. Overall, it was observed a decrease in the levels of MMPs (S2), except MMP-13, which was found in increased levels after ICM (P < .05), independently of the groups.
Conclusions
Calcium hydroxide-based intracanal medications have had a positive effect on the microbial reduction by decreasing the levels of PICs and MMPs. Both auxiliary chemical substances (i.e., 2% CHX and 6% NaOCl) presented similar effects when calcium hydroxide was used as intracanal medication.
Clinical relevance
Teeth with failure of the root canal treatment and apical periodontitis, and consequently with high levels of bacteria, PIC, and MMP, may present a better prognosis after a 30 days of a calcium hydroxide-based ICM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The establishment of microorganisms and their by-products inside the root canals is one of the main factors related to failure of endodontic treatment, which is characterized by persistence or emergence of apical periodontitis after root canal filling [1,2,3,4,5,6]. As periodontitis is a dynamic inflammatory process located at the periapical region, the type of immune-inflammatory response is determined by a network of chemical mediators produced by immune cells in response to the stimulus caused by the action of microorganisms and/or virulence factors. This may result in damage to the tissues and in the development of endodontic signs and symptoms [7,8,9].
In infected root canals, high concentrations of tumor necrosis factor alpha (TNF-α) and interleukin-1 beta (IL-1β) can promote tissue destruction [7, 10], mainly by stimulating macrophages to produce matrix metalloproteinases (MMPs), causing destruction of the extracellular matrix (ECM) [11]. The destruction of periapical tissues mediated by TNF-α and IL-1β appears to be directly related to high levels of infectious content present in the root canal [12]. TNF-α and IL-1β have been detected in teeth with periapical lesions [13] and root canals with exudate [14]. Particularly, high levels of IL-1β appear to be related to the presence of clinical signs/symptoms [15] and periapical bone destruction [16]. However, the literature hardly addresses the role of pro-inflammatory cytokines (PICs) in disease processes of periradicular tissues, especially in cases related to failure of endodontic treatment.
MMPs belong to a family of enzymes known as zinc-dependent endopeptidases and are able to degrade ECM components, such as collagens, proteoglycans, fibronectins, laminins, and elastins. Most of the matrix metalloproteinases are initially produced as pro-enzymes and are secreted before conversion into their active form [17, 18]. Additional MMP substrates comprise cytokines, growth factors, binding proteins, chemokines, cell/cell adhesion molecules, and other proteinases. Their classification is specifically based on the following substrates: collagenases, gelatinases, estromelysins, matrilysins, membrane-type MMPs, and others [18,19,20,21,22]. Under physiological conditions, the synthesis and activity of MMPs are severely controlled by the body because the increase in their levels may threaten the integrity of the cells and cause apoptosis [19, 20]. This instability occurs routinely in pathological situations, such as inflammation and tissue invasion [21].
Calcium hydroxide [Ca(OH)2] is the most commonly used intracanal medication (ICM) worldwide [22]. Because of the limitation of calcium hydroxide alone against some resistant microbial species, its association to 2% chlorhexidine (CHX) has been purposed [23, 24]. Recent studies have evaluated the effect of ICM on inflammatory contents of root canals with primary endodontic infections [22] and primary periodontal lesions with secondary endodontic involvement [25].
Knowing the role of bacteria in cases of failure of endodontic treatment and also the role of PICs and MMPs in modulating tissue displacement in periapical pathologies may contribute to the understanding and upper interpretation of clinical manifestations of apical periodontitis. The reduction in the levels of bacteria, PICs, and MMPs by endodontic procedures in teeth with apical periodontitis is a challenge, since these are aimed at providing immune balance and tissue healing. Therefore, the objective of this work was to investigate in vivo the effects of calcium hydroxide-based ICM on the levels of bacteria, PICs, and MMPs in root canals and periradicular tissues of teeth with failure of the root canal treatment and apical periodontitis.
Material and methods
Patients’ selection
Patients’ selection was based on previous study [6].
Based on a previous study [25], a power calculation was performed using G*Power 3.1 software (Heinrich Heine University, Dusseldorf, Germany). The calculation indicated that the sample size must be a minimum of ten individuals per group with α-type error = 0.05 and power β = 0.80. Therefore, 20 volunteers were selected from those who attended the Piracicaba Dental School, State University of Campinas (UNICAMP), Piracicaba, SP, Brazil, with a need for endodontic retreatment.
The Human Research Ethics Committee of the Piracicaba Dental School, State University of Campinas – UNICAMP, São Paulo, SP, Brazil, approved the study (protocol number 018/2014), describing the sample collection for this investigation. All patients signed an informed consent form regarding their participation in the research. The age of the patients ranged from 30 to 60 years. All the selected teeth were single-rooted which had been previously filled and showed radiographic evidence of apical periodontitis.
Failure of root canal treatment was determined based on clinical and radiographic examinations. Presence of persistent periapical radiolucent lesion, voids in or around the root canal filling, persistent symptoms (e.g., pain on palpation, discomfort to percussion), and persistent sinus tract were considered reasons for retreatment [2, 3].
Exclusion criteria comprised subjects who had received antibiotic treatment within the preceding 3 months, reported systemic disease starting with ASA 3 (American Society of Anesthesiology), teeth that could not be isolated with rubber dam, teeth with absence of coronary sealing, and teeth with periodontal pockets deeper than 3 mm.
Sample collection and clinical procedures
Sample collection and clinical procedures were performed according to Barbosa-Ribeiro et al. [6], Martinho and Gomes [26], and Gomes et al. [27]. Briefly, the teeth were isolated with rubber dam before the crown and surrounding structures were disinfected with 30% hydrogen peroxide (volume/volume for 30 s) followed by 2.5% sodium hypochlorite (NaOCl) for the same period of time and then inactivated with 5% sodium thiosulfate. Disinfection of external surfaces of the crown was checked by taking a swab sample from them and streaking it onto blood agar plates, which were then incubated aerobically and anaerobically.
Endodontic treatment was performed under high magnification with dental operating microscope (DF Vasconcellos S/A, São Paulo, Brazil).
After local anesthesia (2% lidocaine with 1:100.000 epinephrine), a two-stage access preparation was performed. The access cavity was made without the use of water spray, but under manual irrigation with sterile saline and by using sterile high-speed diamond bur. This first stage was performed to promote a major removal of contaminants. In the second stage, before entering the pulp chamber, the access cavity was disinfected according to the decontamination protocol described above. Disinfection of the internal surface of the access cavity was checked as previously described, and all procedures were performed aseptically.
Root-filling materials were removed by using Reciproc R25 files (VDW, Munich, Germany) along the working length, as determined by pre-operatory radiography, according to the manufacturer’s instructions in a crown-down technique with no chemical solvent.
Before the first sampling (S1) of the root canal, a K-file #20 (Dentsply Maillefer, Ballaigues, Switzerland) was used to confirm the working length (previously estimated by radiographs) with an apex locator (Novapex, Forum Technologies, Rishon le-Zion, Israel). Three sterile paper points (Dentsply Maillefer, Ballaigues, Switzerland) were consecutively introduced into the full length of the canal and retained in position for 60 s. The paper points were removed and then placed in a sterile tube containing 1 mL Viability Medium Göteborg Agar III (VGMA III) transport medium [6, 27] for microbial culture. Next, they were transported within 15 min to an anaerobic workstation (Don Whitley Scientific, Bradford, UK) for bacterial culture analysis.
Sampling of the periapical content was performed according to Duque et al. [25]. Briefly, a sterile paper point (Cell Pack, Denstply Maillefer) was introduced 2 mm over the apex, reaching the periapical tissues and maintained in position for 60 s. Following, the paper point was gently removed and placed in a sterile tube for enzyme-linked immunosorbent assay (ELISA) and then frozen at − 80 °C for further analysis.
Following, the root canals were then prepared by using Reciproc R40 files (VDW, Munich, Germany) according to the manufacturer’s instructions in a reciprocating motion generated by the motor. The instrument was used in an in-and-out pecking motion of about 3 mm in amplitude with apical pressure. After three pecking motions, the instrument was removed from the canal and cleaned. Next, a K-file #20 was taken into the working length (WL) to check whether the canal was patent. These procedures were repeated until the Reciproc instrument reached the WL (zero point displayed on the apex locator) [6].
Retreatment was considered complete when the Reciproc file reached the working length with no filling material covering the instrument and the canal walls being smooth and free of visible debris. Furthermore, a close inspection under higher magnification showed complete removal of gutta-percha.
The 20 infected root canals of single-rooted teeth with post-treatment apical periodontitis were randomly divided into two groups according to the chemical substances used.
-
Group 1 (n = 10): 2% chlorhexidine gel (CHX)
-
Group 2 (n = 10): 6% sodium hypochlorite (NaOCl)
A freshly prepared calcium hydroxide-based intracanal medication (ICM) was used in all cases for 30 days.
EndoVac System (Discus Dental, Culver, CA, USA) was used as irrigation protocol to both groups (group 1—saline) and (group 2—NaOCl). In group 1, during instrumentation, the root canals were filled with 1 mL of 2% CHX gel (Endogel, Essencial Pharma Ltd. Itapetininga, SP, Brazil) by using a syringe (27-gauge needle) before the use of each instrument and immediately rinsed afterwards with 5 mL of saline solution by using the EndoVac System. In the end of instrumentation, CHX was inactivated with 5 mL of 5% Tween-80 and 0.07% (w/v) lecithin solution during 1-min period, which was removed with 5 mL of saline solution.
In group 2, during instrumentation, the root canals were filled with 1 mL of 6% NaOCl (Drogal, Piracicaba, SP, Brazil) by using a syringe (27-gauge needle) before the use of each instrument and immediately rinsed afterwards with 5 mL of 6% NaOCl by using the EndoVac System. In the end of the instrumentation, NaOCl was inactivated with 5 mL of a solution of 5% sodium thiosulfate (Drogal, Piracicaba, SP, Brazil) for 60 s, which was also removed with 5 mL of saline solution.
After the CMP, the canal was dried with paper points. A calcium hydroxide-based paste was placed over the entire length of the prepared canal by using Lentulo spiral fillers (Dentsply Maillefer, Ballaigues, Switzerland). The access cavity was then temporarily sealed with temporary cement (Coltosol, Coltène/Whaledent, OH, USA) of a 2-mm thickness at least, and a second layer of composite material (Filtek Z250; 3 M ESPE, St. Paul, MN, USA) was applied in combination with a single bond adhesive (3M ESPE). After 30 days, the canal was aseptically accessed and the ICM removed with 5 mL of saline solution and the master apical file (# 40) plus two further files. Further irrigation with 17% EDTA, ultrasonically activated (as mentioned above), was performed under higher magnification to certify the complete removal of ICM. Next, the canal was rinsed with 5 mL of sterile saline [28]. Ca(OH)2 activity was neutralized with 5 mL of 0.5% citric acid for 1 min, which was then removed with 5 mL of saline solution before immediately performing the second sampling (S2), as mentioned earlier.
The canals were then irrigated with 3 mL of 17% EDTA by using ultrasound, as previously described, followed by irrigation with 5 mL of sterile saline. Finally, after the canals were found to be asymptomatic and dried, the teeth were filled with single Reciproc gutta-percha cone and Endométhasone sealer (Septodont, Saint-Maur-des-Fossés, France). Access cavities were restored with Coltosol (Coltène/Whaledent) of 2-mm thickness at least and a second layer of light-cured composite material (Filtek Z250; 3 M ESPE) was applied in combination with single-bond adhesive.
Culture procedure
The method for colony-forming unit (CFU) count has been previously published by Barbosa-Ribeiro et al. [6] and Martinho and Gomes [26]. The tubes containing root canal samples were transported within 15 min to an anaerobic workstation (Don Whitley Scientific, Bradford, UK) for bacterial culture analysis. They were shaken thoroughly for 60 s (Vortex; Marconi, Piracicaba, São Paulo, Brazil), and then serial 10-fold dilutions were made to 10−4 in tubes containing Fastidious Anaerobe Broth (Lab M, Bury, UK). By using sterile plastic spreaders, 50 μL of each serial dilution was plated onto 5% defibrinated sheep blood fastidious anaerobe agar (LAB M) to obtain obligate anaerobes and facultative anaerobes. The plates were incubated at 37 °C in anaerobic atmosphere for up to 14 days. After this period, CFUs were visually quantified for each plate.
Measurements of PICs and MMP levels
Measurements of PICs and MMP levels were based on previous study [25]. Levels of TNF-α, IL-1β, MMP-2, MMP-3, MMP-8, MMP-9, and MMP-13 in the different phases of endodontic procedures were measured by using specific Quantikine® Human ELISA Kit (R&D Systems®, Minneapolis, MN, USA) and quantitative sandwich enzyme immunoassay technique. Next, standard and sample solutions were added to an ELISA well plate (R&D Systems®) which had been precoated with specific immobilized monoclonal antibody supplied by the manufacturer for TNF-α, IL-1β, MMP-2, MMP-3, MMP-8, MMP-9, and MMP-13. After washing away the unbound substances, an enzyme-linked polyclonal antibody specific for each molecule was added to the wells, forming an immune complex. The plate was incubated for 60 min at room temperature on the shaker before washing for removal of unbound enzymes. After another washing, a substrate solution containing hydrogen peroxidase and chromogen was added and allowed to react. The color development was stopped, and its intensity was measured, thus revealing the concentration of PICs and MMPs. The levels of PICs and MMPs were assessed with an ELISA reader (Thermo Fisher Scientific, Waltham, MA, USA) at 450 nm, and the negative control values were normalized. Each densitometric value, expressed as mean and standard deviation, was obtained from two independent experiments.
Statistical analysis
The data collected for concentrations of CFUs, PICs, and MMPs were statistically analyzed by using SAS for Windows (SAS Inc., Cary, NC, USA). Data normality was verified by using Shapiro-Wilk test, with those presenting normal distribution being analyzed with post hoc one-way analysis of variance and Tukey–Kramer method for intragroup analysis before and after the use of calcium hydroxide-based ICM. In all statistical tests, the significance level was set at 5%.
Results
Clinical features
Twenty single root canals were used for sample collection. Among all the teeth, most of them presented tenderness to percussion (12/20), previous pain (6/20), and pain on palpation (4/20). With regard to indications for endodontic retreatment, 8 out of 20 presented periapical lesion and poor coronal restoration, whereas 7 out of 20 presented periapical lesion and unsatisfactory root canal filling. Most of the teeth were restored with resin composite (10/20). The average time between previous treatment of root canal and its retreatment was 9.5 years, ranging from 2 to 35 years. The mean size of the periapical lesion was 3.5 mm, ranging from 1 to 9 mm.
Microbiological findings
The microbiological samples from the teeth surfaces before and after coronal access presented no positive cultures, which means that all procedures were performed under sterile conditions. Culturable bacteria (101.2 ± 79.2) were recorded in all initial samples (S1) (20/20). After ICM, microbial reduction was 99.5% (P < .05). In group 1, it was observed a reduction of 100% of culturable bacteria in S2 (P < 0.05), whereas in group 2, the reduction level was 98.5% after the use of ICM (P < .05).
Cytokines and matrix metalloproteinases
Pro-inflammatory cytokines were present in all initial samples (S1). Overall, it was observed a decrease in the levels of TNF-α (62.5% reduction) and IL-1β (41.7% reduction) after the use of ICM (S2) (P < 0.05). In S2, there was a significant reduction of PICs in all groups.
Matrix metalloproteinases were present in all S1 samples with varying levels between them. Overall, it was observed a decrease in the levels of MMPs after ICM, except MMP-13, which was found in increased levels after ICM (P < 0.05), independently of the groups.
In general, ICM was effective in reducing the levels of culturable bacteria, PICs, and MMPs. Means, standard deviations, and percentages of the overall reductions for each auxiliary chemical substance (ACS) used during endodontic therapy are listed in Table 1.
Discussion
The major objective of this clinical study was to investigate the effects of Ca(OH)2-based ICM on microbial load within the root canals and on the levels of PICs and MMPs present in the periapical region of teeth with failure of the root canal treatment and persistent periapical lesion. It was the first time that this methodology of collecting exsudate from periapical fluids, via root canals, was used for direct quantification of inflammatory contents in teeth with endodontic treatment failure. The quantification of PICs and MMPs from periradicular tissues has been previously described by our group; however, it was performed in teeth with primary periodontal lesions with secondary endodontic involvment [25]. It is important to elucidate the expression profile of inflammatory molecules before performing the endodontic treatment in order to understand the effect of intracanal medication on molecular signals occurring in the apical periodontitis as well as on the host’s response.
As auxiliary chemical substances, 6% sodium hypochlorite (NaOCl) and 2% chlorhexidine gel (CHX) were used during the chemo-mechanical preparation. NaOCl in solution formulation has been chosen, since it is the most used root canal irrigant worldwide due to its satisfactory properties [29]. On the other hand, CHX in gel formulation was used as it is the choice auxiliary chemical substance of the Piracicaba Dental School, which is supported by several studies [4, 6, 8, 25]. Similarly to the solution formulation, CHX gel presents a broad antimicrobial properties and substantivity (i.e., prolonged antimicrobial activity) [23]. The CHX gel presents great lubricating property which is important during retreatment. Moreover, it reduces the smear layer formation, which does not occur with the liquid form [23]. CHX gel maintains most of dentinal tubules open because its viscosity keeps the debris in suspension (rheological action) [23]. Furthermore, due to the gel formulation, CHX may keep the “active principle” of CHX in contact with the microorganisms for a longer time, inhibiting their growth [23].
Calcium hydroxide-based intracanal medication used for 7 or 14 days [8] did not improve the disinfection achieved by CMP in vivo. The 30 days used in the present study and also in previous studies [25, 30] seemed to be important for improving the LPS detoxifying activity of Ca(OH)2 in vivo. Furthermore, Ca(OH)2 has been reported to denature infectious contents of bacteria [31], which will consequently reduce the levels of PIC and MMP.
Microbiological findings
The microbiological results were similar to those reported by Barbosa-Ribeiro et al. [6]. Since bacteria and their by-products play an important role in the initiation of an inflammatory cascade in the periapical tissues, it is important to eliminate them as much as possible during the endodontic treatment, and to avoid their entrance after root canal filling via coronal microleakage [32]. Microorganisms can maintain an infection after the obturation of the root canal and induce an inflammatory process in the periapical tissues [6, 9].
Colony Forming Units count is a reliable technique for assessing the effectiveness of antimicrobial agents to reduce viable bacterial cells [33]. Nevertheless, it is important to highlight that having negative samples does not necessarily mean that the bacteria were absent, as they could be below detection levels [33]. Even considering the limitations of sampling biofilm using paper points, this approach is a well-established protocol based on that all “floating” species present in the main canal are or were members of the endodontic biofilm [25]. In the present study, all the initial root canals samples (20/20) supported microbial growth. CMP performed with 2% CHX reduced 100%, while NaOCl provided a 98.5% reduction; therefore, both ACS were effective in reducing the levels of culturable bacteria within the root canals, agreeing with previous studies [4, 8, 34]. The calcium hydroxide-based intracanal medicament used for 30 days was effective in reducing the level of culturable bacteria in 99.5% of the samples, and consequently of their by-products, which would decrease the inflammatory process in the periapical tissues.
PIC findings
With regard to the levels of TNF-α and IL-1β, CMP performed with CHX or NaOCl was effective in reducing these levels. CHX promoted a reduction of 62.8%, and NaOCl provided a reduction of 62.9% in the TNF-α levels. Lower levels of IL-1β (41.7% and 36.4% reduction) were observed after CMP performed with CHX and NaOCl, respectively.
The benefits of a 30-day ICM were detected by the reduction in all PICs levels, which is corroborated by previous studies [22, 25], showing that calcium hydroxide-based intracanal medication can prevent the increase of PICs levels in the periapical tissues.
The main biological function of TNF-α is to recruit neutrophils and monocytes towards the site of infection and to activate these cells to eradicate the microorganisms [12]. TNF-α is also an important mediator of the inflammatory response to Gram-positive bacteria and other microorganisms [10, 16] and can modulate its own expression as well as of other cytokines, such as IL-1β [7, 10, 16]. In our study, it was observed lower levels of IL-1β (1.2 ± 0.4 pg/mL) compared to TNF-α (8.8 ± 4.7 pg/mL) in the initial samples (S1), which confirmed the regulatory role of the TNF-α in the inflammatory process. In S2, it was observed a reduction of 62.5% in the TNF-α level.
One of the most consistent biological effects of IL-1β is related to the increased synthesis of prostaglandin E2 (PGE2), which presents the ability to sensitize nociceptors [35]. IL-1β has been associated with the presence of clinical signs and/or symptoms and also bone resorption [15], being detected at higher levels in the initial sample and related to the presence of periradicular lesion in all cases. As expected, the levels of IL-1β decreased after ICM, indicating the beginning of the repair process.
MMP findings
MMPs are relevant for the pathogenesis of pulpal and periradicular tissues damage [36] and PICs can directly and indirectly stimulate the release of MMPs in the periradicular tissues, sustaining an inflammatory process in their presence [20].
Different profiles of MMP expression were observed in the initial samples, with MMP-2 (collagenase; 803.7 ± 96.4 pg/mL) and MMP-3 (stromelysin-1; 453.9 ± 229.3 pg/mL) being the most frequently found ones, followed by MMP-8 (245.9 ± 122.4 pg/mL), MMP-9 (129.4 ± 29.6 pg/mL), and MMP-13 (70.8 ± 12.8 pg/mL). All the MMPs participate in the degradation of the extracellular matrix (ECM) with different mechanisms of action and in different times of the inflammatory process. After the use of ICM (S2), it was observed a reduction of MMP-2, MMP-3, MMP-8, and MMP-9, particularly MMP-3 (27.3%).
MMP-2 and MMP-9 are known as gelatinases. MMP-2 (gelatinase A or IV collagenase type/72 kDa) and MMP-9 (gelatinase B or IV collagenase type/92 kDa) degrade denatured collagens (gelatins), elastins, fibronectins, proteoglycans, laminins, and type-IV collagens, with the latter being the main component of the basement membrane, indicating involvement of these enzymes in the invasion process [37]. These gelatinases are particularly expressed in cases of chronic periapical lesions, granulomas, and cysts, being also present in the gingival crevicular fluid of teeth with chronic periapical lesions [38, 39]. High levels of MMP-2 have been detected in teeth with asymptomatic post-treatment periodontitis, indicating that MMP-2 can play a role as a marker of chronic inflammatory process. In addition, a previous study related high levels of MMP-9 to symptomatic apical periodontitis in primary endodontic infections, stimulated by Gram-negative bacteria [17]. In our study, CHX provided greater reduction of MMP-2 and MMP-9 compared to NaOCl; however, ICM was found to be effective in reducing the levels of MMP-2 (758.4 ± 64.1 pg/mL) and MMP-9 (122.9 ± 93.1 pg/mL), irrespective of the ACS used, which explains the absence of symptomatology in all cases.
MMP-3 is an important enzyme in the degradation of the extracellular matrix, being able to degrade proteoglycans (i.e., fibronectin and laminin), whereas ICM is responsible for the reduction of MMP-3 by 27.3% (from 453.9 ± 229.3 to 329.8 ± 183.6). Both CHX (24.6% reduction) and NaOCl (30.9% reduction) promoted similar effects in reducing the levels of MMP-3.
With regard to MMP-8 and MMP-13, known as collagenases, their importance is known in the beginning of the inflammation process.
MMP-8 (neutrophil collagenase) and MMP-13 (collagenase 3) are enzymes responsible for the breakdown of type I and III collagens, which are major organic constituents of the alveolar bone and periodontal ligament [21]. Geijersstam et al. [40] suggested that in cases of endodontic failure, which involves a higher prevalence of Gram-positive bacteria and absence of symptomatology, there is a lower expression of MMP-8. The presence of periradicular lesion in post-treatment cases confirms the role of MMP-8 and MMP-13 in the inflammatory process. After the CMP, irrespective of the ACS used, and after a 30-day ICM, the levels of MMP-8 decreased (223.3 ± 69.0). On the other hand, there was an increase in the level of MMP-13 (79.0 ± 17.4).
In the inflammatory process, IL-1β presents as a potent stimulator of MMP-8 and MMP-13, which are directly connected with the establishment and remodeling of periapical lesions [19, 41]. Wahlgren et al. [42] suggested that the levels of MMP-8 and MMP-13 from the periapical exudate could be used to indicate the status of the inflammatory response in order to predict a successful treatment of teeth with periapical lesions. In this case, the increase of MMP-13 after the use of ICM may be related to the endodontic retreatment performed, which reduced microbial level and consequently stimulated the periapical repair process. In the present study, the overall levels of collagenases (MMP-8 and MMP-13) were lower than those of gelatinases (MMP-2 and MMP-9), probably because the former are usually more active at the early stage of periapical lesion formation.
In general, this in vivo study was important to provide knowledge about the clinical levels of culturable bacteria within the root canals, PICs and MMPs in the periapical region of teeth with post-treatment apical periodontitis, thus allowing further comparisons with different pathological situations involving the pulp and periapical tissues.
In conclusion, calcium hydroxide-based intracanal medications have had a positive effect on the microbial reduction by decreasing the levels of PICs and MMPs. Both auxiliary chemical substances (i.e., 2% CHX and 6% NaOCl) presented similar effects when calcium hydroxide was used as intracanal medication.
References
Gomes BP, Lilley JD, Drucker DB (1996) Associations of endodontic symptoms and signs with particular combinations of specific bacteria. Int Endod J 29:69–75
Pinheiro ET, Gomes BP, Ferraz CC, Sousa EL, Teixeira FB, Souza-Filho FJ (2003a) Microorganisms from canals of root-filled teeth with periapical lesions. Int Endod J 36(1):1–11
Pinheiro ET, Gomes BP, Ferraz CC, Teixeira FB, Zaia AA, Souza-Filho FJ (2003b) Evaluation of root canal microorganisms isolated from teeth with endodontic failure and their antimicrobial susceptibility. Oral Microbiol Immunol 18:100–103
Endo MS, Martinho FC, Zaia AA, Ferraz CC, Almeida JF, Gomes BP (2012) Quantification of cultivable bacteria and endotoxin in post-treatment apical periodontitis before and after chemo-mechanical preparation. Eur J Clin Microbiol Infect Dis 31:2575–2583
Zhang C, Hou BX, Zhao HY, Sun Z (2012) Microbial diversity in failed endodontic root-filled teeth. Chin Med J 125:1163–1168
Barbosa-Ribeiro M, De-Jesus-Soares A, Zaia AA, Ferraz CC, Almeida JF, Gomes BP (2016) Antimicrobial susceptibility and characterization of virulence genes of Enterococcus faecalis isolates from teeth with failure of the endodontic treatment. J Endod 42:1022–1028
Martinho FC, Chiesa WM, Leite FR, Cirelli JA, Gomes BP (2011) Antigenicity of primary endodontic infection against macrophages by the levels of PGE(2) production. J Endod 7:602–607
Endo MS, Ferraz CC, Zaia AA, Almeida JF, Gomes BP (2013) Quantitative and qualitative analysis of microorganisms in root-filled teeth with persistent infection: monitoring of the endodontic retreatment. Eur J Dent 7:302–309
Zhao L, Chen J, Cheng L, Wang X, Du J, Wang F, Peng Z (2013) Effects of Enterococcus faecalis lipoteichoic acid on receptor activator of nuclear factor-κB ligand and osteoprotegerin expression in periodontal ligament fibroblasts. Int Endod J 47:163–172
Hong CY, Lin SK, Kok SH, Cheng SJ, Lee MS, Wang TM, Chen CS, Lin LD, Wang JS (2004) The role of lipopolysaccharide in infectious bone resorption of periapical lesion. J Oral Pathol Med 33:162–169
Pezelj-Ribarić S, Magasić K, Prpić J, Miletić I, Karlović Z (2007) Tumor necrosis factor alpha in periapical tissue exudates of teeth with apical periodontitis. Mediat Inflamm 2007:69416
Martinho FC, Chiesa WM, Leite FR, Cirelli JA, Gomes BP (2012) Correlation between clinical/radiographic features and inflammatory cytokine networks produced by macrophages stimulated with endodontic content. J Endod 8:740–745
Martinho FC, Chiesa WM, Leite FR, Cirelli JA, Gomes BP (2010) Antigenic activity of bacterial endodontic contents from primary root canal infection with periapical lesions against macrophage in the release of interleukin-1beta and tumor necrosis factor alpha. J Endod 36:1467–1474
Sousa EL, Martinho FC, Leite FR, Nascimento GG, Gomes BP (2014) Macrophage cell activation with acute apical abscess contents determined by interleukin-1 Beta and tumor necrosis factor alpha production. J Endod 40:1752–1757
Lim GC, Torabinejad M, Kettering J, Linkhardt TA, Finkelman RD (1994) Interleukin 1-beta in symptomatic and asymptomatic human periradicular lesions. J Endod 20:225–227
Ataoglu H, Alptekin NO, Haliloglu S, Gursel M, Ataoglu T, Serpek B, Durmus E (2002) Interleukin-1beta, tumor necrosis factor-alpha levels and neutrophil elastase activity in peri-implant crevicular fluid. Clin Oral Implants Res 13:470–476
Ahmed GM, El-Baz AA, Hashem AA, Shalaan AK (2013) Expression levels of matrix metalloproteinase-9 and gram-negative bacteria in symptomatic and asymptomatic periapical lesions. J Endod 39:444–448
Sambandam V, Neelakantan P (2014) Matrix metalloproteinases (MMP) in restorative dentistry and endodontics. J Clin Pediatri Dent 39:57–59
Ozeki N, Kawai R, Yamaguchi H (2014) IL-1β-induced matrix metalloproteinase-13 is activated by a disintegrin and metalloprotease-28-regulated proliferation of human osteoblast-like cells. Expl Cell Res 323:165–177
Ozeki N, Hase N, Kawai R, Yamaguchi H, Hiyama T, Kondo A, Nakata K, Mogi M (2015) Unique proliferation response in odontoblastic cells derived from human skeletal muscle stem cells by cytokine-induced matrix metalloproteinase-3. Exp Cell Res 331:105–114
Hannas AR, Pereira JC, Granjeiro JM, Tjäderhane L (2007) The role of matrix metalloproteinases in the oral environment. Acta Odontol Scand 65:1–13
Martinho FC, Gomes CC, Nascimento GG, Gomes APM, Leite FRM (2018) Clinical comparison of the effectiveness of 7- and 14-day intracanal medications in root canal disinfection and inflammatory cytokines. Clin Oral Investig 22:523–530
Gomes BP, Vianna ME, Zaia AA, Almeida JF, Souza-Filho FJ, Ferraz CC (2013) Chlorhexidine in endodontics. Braz Dent J 24:89–102
Gomes BP, Vianna ME, Sena NT, Zaia AA, Ferraz CC, de Souza Filho FJ (2006) In vitro evaluation of the antimicrobial activity of calcium hydroxide combined with chlorhexidine gel used as intracanal medicament. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102:544–550
Duque TM, Prado M, Herrera DR, Gomes BPFA (2018) Periodontal and endodontic infectious/inflammatory profile in primary periodontal lesions with secondary endodontic involvement after a calcium hydroxide-based intracanal medication. Clin Oral Investig
Martinho FC, Gomes BP (2008) Quantification of endotoxins and cultivable bacteria in root canal infection before and after chemomechanical preparation with 2.5% sodium hypochlorite. J Endod 34:268–272
Gomes BP, Berber VB, Kokaras AS, Chen T, Paster BJ (2015) Microbiomes of endodontic-periodontal lesions before and after chemomechanical preparation. J Endod 41:1975–1984
Abi-Rached GP, Herrera DR, Zaia AA, Ferraz CC, Almeida JF, Gomes BP (2014) Efficacy of ethylene-diamine-tetra-acetic acid associated with chlorhexidine on intracanal medication removal: a scanning electron microscopy study. Microsc Res Tech 77:735–739
Zhender M (2006) Root canal irrigants. J Endod 32:389–398
Sousa EL, Martinho FC, Nascimento GG, Leite FR, Gomes BP (2014) Quantification of endotoxins in infected root canals and acute apical abscess exudates: monitoring the effectiveness of root canal procedures in the reduction of endotoxins. J Endod 40:177–181
Marinho ACS, To TT, Darveau RP, Gomes BPFA (2018) Detection and function of lipopolysaccharide and its purified lipid A after treatment with auxiliary chemical substances and calcium hydroxide dressings used in root canal treatment. Int Endod J 51:1118–1129
Henriques LC, de Brito LC, Tavares WL, Teles RP, Vieira LQ, Teles FR, Sobrinho AP (2016) Microbial ecosystem analysis in root canal infections refractory to endodontic treatment. J Endod 42:1239–1245
Herrera DR, Martinho FC, de-Jesus-Soares A, Zaia AA, Ferraz CCR, Almeida JFA, Gomes BPFA (2017) Clinical efficacy of EDTA ultrasonic activation in the reduction of endotoxins and cultivable bacteria. Int Endod J 50:933–940
Zandi H, Rodrigues RC, Kristoffersen AK, Enersen M, Mdala I, Ørstavik D, Rôças IN, Siqueira JF Jr (2016) Antibacterial effectiveness of 2 root canal irrigants in root-filled teeth with infection: a randomized clinical trial. J Endod 42:1307–1313
van Deuren M, Dofferhoff AS, van der Meer JW (1992) Cytokines and the response to infection. J Pathol 168:349–356
Shin SJ, Lee W, Lee JI, Baek SH, Kum KY, Shon WJ, Bae KS (2011) Matrix metalloproteinase-8 and substance P levels in gingival crevicular fluid during endodontic treatment of painful, nonvital teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 112:548–554
Bauvois B (2012) New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: outside-in signaling and relationship to tumor progression. Biochim Biophys Acta 1825:29–36
Carneiro E, Menezes R, Garlet GP, Garcia RB, Bramante CM, Figueira R, Sogayar M, Granjeiro JM (2009) Expression analysis of matrix metalloproteinase-9 in epithelialized and nonepithelialized apical periodontitis lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 107:127–132
Dezerega A, Madrid S, Mundi V, Valenzuela MA, Garrido M, Paredes R, García-Sesnich J, Ortega AV, Gamonal J, Hernández M (2012) Pro-oxidant status and matrix metalloproteinases in apical lesions and gingival crevicular fluid as potential biomarkers for asymptomatic apical periodontitis and endodontic treatment response. J Inflamm (Lond) 9:8
Geijersstam A, Sorsa T, Stackelberg S, Tervahartiala T, Haapasalo M (2005) Effect of E. faecalis on the release of serine proteases elastase and cathepsin G, and collagenase-2 (MMP-8) by human polymorphonuclear leukocytes (PMNs). Int Endod J 8:667–677
Matsui H, Yamasaki M, Nakata K, Amano K, Nakamura H (2011) Expression of MMP-8 and MMP-13 in the development of periradicular lesions. Int Endod J 44:739–745
Wahlgren J, Salo T, Teronen O, Luoto H, Sorsa T, Tjäderhane L (2002) Matrix metalloproteinase-8 (MMP-8) in pulpal and periapical inflammation and periapical root-canal exudates. Int Endod J 35:897–904
Funding
This work was supported by the Brazilian agencies São Paulo Research Foundation (FAPESP, grant no. 2015/23479-5), National Council for Scientific and Technological Development (CNPq, grant no. 308162/2014-5) and Coordination for the Improvement of Higher Education Personnel (CAPES). We are thankful to Maicon R Z Passini, from the Piracicaba Dental School-UNICAMP for the technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The present study was approved by the Research Ethics Committee of the Piracicaba Dental School, State University of Campinas – UNICAMP, São Paulo, SP, Brazil. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Barbosa-Ribeiro, M., Arruda-Vasconcelos, R., de-Jesus-Soares, A. et al. Effectiveness of calcium hydroxide-based intracanal medication on infectious/inflammatory contents in teeth with post-treatment apical periodontitis. Clin Oral Invest 23, 2759–2766 (2019). https://doi.org/10.1007/s00784-018-2719-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2719-0