Abstract
Objective
The aim of this study was to analyze the biological effects of MTA Repair HP and ProRoot MTA on human periodontal ligament stem cells (hPDLSCs) after exposure to acidic and neutral environments.
Materials and methods
Discs of each material (n = 30) were exposed to phosphate buffered saline (pH = 7.4) or butyric acid (pH = 5.2) for 7 days, and biological testing was carried out in vitro on hPDLSCs. Cell viability and apoptosis assays were performed using eluates of each root-end filling material. To evaluate cell attachment to the different materials, hPDLSCs were directly seeded onto the material surfaces and analyzed by scanning electron microscopy. The chemical composition of the root-end filling materials was determined by energy-dispersive x-ray and eluates were analyzed by inductively coupled plasma-mass spectrometry. Statistical differences were assessed by ANOVA and Tukey test (p < 0.05).
Results
Under an acidic environment, both materials displayed similar ion release abilities, with the increased release of Si and Ca ions. Substantial changes in microstructure were observed for both materials after exposure to acidic pH. In addition, material exposure to an acidic environment showed a similar degree of cell adherence, and, surprisingly, MTA Repair HP exhibited higher cell viability rates at pH 5.2 than ProRoot MTA.
Conclusions
Exposure to an acidic environment promoted Si and Ca ion release from ProRoot MTA and MTA Repair HP. Moreover, we observed optimal biological properties of ProRoot MTA and MTA Repair HP in terms of cell viability, cell death, and cell attachment in both environments.
Clinical relevance
These results may suggest that MTA Repair HP and ProRoot exhibited optimal biological properties in terms of cell viability, cell death and cell attachment in acidic environment, being considered as materials for root-end filling and perforations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bioactive cements are widely used to induce mineralization, for cementation, and for filling perforations, among other clinical applications [1,2,3]. These biomaterials are usually composed of hydroxyapatite, calcium-silicate, beta-tricalcium phosphate, and other components. One of the main advantages of these bioceramics is their ability to dissociate calcium ions [4, 5]. The materials differ dramatically in terms of the amount of the calcium they release and also in the kinetics of this process. For instance, Biodentine releases approximate 95 ppm in the first 3 h and the release decreases to 18 ppm at 24 h [6], while MTA releases approximately 5 and 25 ppm at the same time points [7]. Although the values of calcium release may change according to the methodology used, the literature agrees on the potential of these materials to alkalinize the environment and increase the pH to values of around 11 and above [8, 9]. Together, the calcium release and alkaline potential can induce an increase in phosphatase activity and in the expression of several genes involved in mineralization processes (e.g., osteocalcin, bone sialoprotein, dentin sialophosphoprotein) [10, 11]. However, previous reports described that an acidic environment leads to alterations in the setting time and sealing ability of the bioactive cements, weakens their micro hardness and binding strength to root dentin, alters the superficial microstructure and cytocompatibility, and increases their solubility [12,13,14,15].
Recently, new bioactive cements have been introduced in clinical practice [3, 16, 17], among them, MTA Repair HP (Angelus, Londrina, PR, Brazil). MTA Repair HP (MTA “High Plasticity”) was launched in the form of a bioceramic material with high plasticity, aiming to maintain the biological properties of the MTA, while improving its chemical and physical properties. The main differences to its predecessor are the addition of an organic plasticizer to the distilled water and the use of calcium tungstate as radiopacifier [18]. Previous reports have demonstrated that the use of plasticizers leads to improvements in the physicochemical properties and handling of the MTA and the addition of calcium tungstate improve its cytocompatibility [19, 20].
These materials have been used for root-end filling and perforations, so they are in close contact with the surrounding tissues [21] where the setting of cements might also be affected by the surrounding environment. The adjacent tissue near the apex may have normal or acidic pH due to infection and inflammation and induce changes in the biological properties of these cements.
Importantly, the presence of stem cells in periapical tissues and their interaction with the endodontic materials have been considered an important event that favors healing [10]. Indeed, these cells have demonstrated their ability to form alveolar bone, cementum, gingiva, periodontal ligaments, peripheral nerves, and blood vessels in vivo [22]. Due to the presence of periodontal ligament stem cells in the periapical tissues, these cells have been previously used for a variety of in vitro studies into the biological effects of different endodontic cements [23, 24].
To date, there have been no studies evaluating how an acidic environment affects ion release from MTA Repair HP or how such this environment could affect the viability of cells and their levels of attachment to these materials. Considering this background information, the aim of this study was to analyze the biological effects of MTA Repair HP on human periodontal ligament stem cells after exposure to acidic and neutral environments and compare the results with those obtained with ProRoot MTA.
Materials and methods
Cement extracts
MTA Repair HP and ProRoot MTA were mixed according to the manufacturer’s instructions for all the tests. The composition of the evaluated materials is shown in Table 1. Discs of each material were shaped under aseptic conditions in sterile cylindrical rubber molds (1.5 cm in diameter and 0.2 cm in height) and pressed by a glass slide for 6 min at room temperature. The samples were initially set in a humidified 5% CO2 and 95% air atmosphere for 2 h at 37 °C, carefully polished using silicon carbide paper, washed in phosphate-buffered saline (PBS) (Gibco, Gaithersburg, MD, USA), and sterilized by ultraviolet light. Each specimen was then immersed in 2 mL of aseptic PBS (pH = 7.4) or butyric acid (0.025% in PBS, pH = 5.2) (Sigma-Aldrich, St Louis, MO, USA) for 7 days. The PBS or butyric acid was replaced every 24 h. To simulate the clinical situation where cells are in contact with the cement materials, we obtained extracts or eluates of the materials, according to the International Standard ISO 10993-5. The eluates of the different materials were extracted in sterile conditions, using Dulbecco Modified Eagle’s Medium (DMEM) (Gibco) as extraction vehicle. The extraction procedure was as follows: the materials were immersed in the culture medium for 24 h at 37 °C in a humid atmosphere containing 5% CO2. In accordance with the ISO standards, the ratio between the surface of the sample and the volume of the medium was 1.5 cm2/mL. The extraction medium was collected at the end of this period and passed through a 0.22-μm syringe filter (Merck Millipore, Billerica, MA, USA). Then, in order to study the effect of the concentration of each material, various dilutions (1:1, 1:2, and 1:4 v/v) of these extraction media were prepared using fresh complete DMEM medium.
Ion release analysis and pH
Three discs from each material type were immersed in 5 mL of PBS or butyric acid to measure the ion leached over a period of 7 days. The presence of silicon, phosphorus, calcium, and strontium was assessed using inductively coupled plasma-mass spectrometry (ICP-MS Agilent 7900, Stockport, UK). The pH of each extract was determined using a twin pH meter (GLP21+, Cri-son, Barcelona, Spain) and the results are expressed as the mean ± standard deviation.
Isolation, culture, and characterization of hPDLSCs
Human periodontal ligament stem cells (hPDLSCs) were isolated and characterized as described previously [21, 25]. Human periodontal ligaments (hPDL) were obtained from impacted third molars from healthy subjects (n = 10). Donors gave written informed consent according to the guidelines of the Ethics Committee of our Institution (University of Murcia ID: 1528/2017). The donor age was 18–35 years old. The hPDL was scraped from the middle third region of the root surface. After extraction, hPDL was washed with Ca2+/Mg2+-free Hank’s balance salt solution (Gibco), and subjected to collagenase-A digestion (3 mg/mL) (Sigma-Aldrich) for 1 h at 37 °C. Then, cells were seeded in 75-cm2 culture flasks (Corning, NY, USA) containing alpha minimum essential medium (α-MEM) supplemented with 100 U/mL penicillin and streptomycin and 10% fetal bovine serum (FBS) (Gibco) (complete medium) in an incubator at 37 °C and 5% CO2. This study was carried out using cells from passage 4 onward. To analyze cell surface antigen expression, 105 cells per well were seeded on 6-well plates, and allowed to adhere for 24 h. After 72 h, cells were harvested and identified following the guidelines of the International Society of Cellular Therapy (ISCT) to confirm their mesenchymal stem cell phenotype [26]. Surface markers of hPDLSCs were analyzed by flow cytometry using specific fluorescence-conjugated antibodies. The antibodies used were CD73-APC (clone AD2), CD90-FITC (clone DG3), CD105-PE (clone 43A4E1), CD14-PerCP (clone TÜK-4), CD20-PerCP (clone LT20.B4), CD34-PerCP (clone AC136), and CD45-PerCP (clone 5B1) (Human MSC Phenotyping Cocktail, Miltenyi Biotec, Bergisch Gladbach, Germany). The antibodies were diluted following the manufacturer’s specifications (10 μL of the Human MSC Phenotyping Cocktail per 106 cells). Flow cytometry analysis was performed using a BD FACS Canto II flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed with Kaluza and FlowJo software.
Cell viability assays
To determine the metabolic activity of treated hPDLSCs, a colorimetric metabolic activity assay using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT Cell Growth Kit, Chemicon, Rosemont, IL, USA) was used. Briefly, hPDLSCs were seeded in 96-well plates (1 × 103 cells/well) in complete culture medium and left undisturbed for 24 h. The volume of culture medium in each well was 180 μL. After 24 h, the culture medium was replaced by the fresh medium containing the corresponding volume of each eluate in which the discs were previously immersed. As control, cells cultured without eluate were used. At each time point (24, 48, and 72 h after the addition of the different material eluates to cells), MTT was added at a final concentration of 1 mg/mL and cells were incubated for 4 h. Then, the MTT was removed and 100 μL of DMSO was added to release formazan synthesized in the mitochondria. A spectrophotometer (ELx800; Bio-Tek Instruments, Winooski, VT, USA) was used to measure the absorbance values at 570 nm. The experiments were performed in quintuplicates, using cells cultured in complete medium as control.
Analysis of apoptosis and necrosis by flow cytometry (Annexin V/7-AAD staining)
hPDLSCs were cultured with different eluates for 72 h. The eluates from three discs of each material were obtained after immersion in complete culture medium for 24 h. After incubation of the cells with the eluates, double staining with PE-conjugated annexin-V and 7-AAD (Immunostep, Salamanca, Spain) was performed. Percentages of live (annexin-V−/7-AAD−), early apoptotic (annexin-V+/7-AAD−), or late apoptotic and necrotic cells (annexin-V+/7-AAD+ and annexin-V−/7-AAD+) were determined by flow cytometry. Subsequently, the percentages of each population were calculated. These assays were performed in triplicate.
Scanning electronic microscopy, energy-dispersive x-ray analysis
Six discs of both MTA Repair HP and ProRoot MTA were prepared and subdivided into two groups (1.5 cm in diameter and 0.2 cm in height). Three of them were used to analyze the attachment of the cells by SEM. The hPDLSCs were directly seeded onto each disc at 5 × 104 cells/mL. After 72 h of culture, the samples seeded with hPDLSCs were removed from the culture wells and fixed with 3% glutaraldehyde in PBS for 4 h. Then, samples were dehydrated in a graded series of ethanol concentrations, immersed in 100% hexamethyldisilazane, air-dried, mounted on aluminum stubs, and sputter-coated with gold/palladium. Finally, gold/palladium-coated specimens were examined by SEM, using a magnification of ×100, ×300, ×500, and ×1000. For SEM-EDX assays, three discs of each cell-free material were immersed in HBSS medium (Gibco) at 1.5 cm2/mL and stored at 37 °C for 24 h. Discs were coated with carbon in a CC7650 SEM Carbon Coater unit (Quorum Technologies Ltd., East Sussex, UK) and each sample was examined using a scanning electron microscope (SEM Jeol 6100 EDAX, USA) connected to a secondary electron detector for energy-dispersive x-ray analysis (EDX; Oxford INCA 350 EDX, Abingdon, UK), using computer-controlled software (INCA energy version 18) and an accelerating voltage of 20 KV. The full scale for quantification was 8677 cts. The stoichiometric Ca/P molar ratios were calculated taking into account the respective atomic masses of the two elements using the formula: Ca/P = [Ca (% of weight)/40.08 (g/mol)]/[P (% of weight)/30.97 (g/mol)].
Statistical analysis
For the ICP-MS measurements, the equipment measures three times each element. As a result, the average and the relative standard deviation are obtained. Data from the MTT were analyzed using SPSS version 22.0 statistical software (SPSS, Inc., Chicago, IL, USA). Each experiment was performed with five replicates and carried out at least three times. Absorbance values are presented as the mean ± standard deviation (SD). Statistical differences between proliferation in the control conditions and that obtained in the presence of the different material extracts were analyzed. Statistical differences between the proliferation rates of different extracts were also analyzed. In both cases, results were analyzed by ANOVA and Tukey’s test. A p value of <0.05 was considered significant.
Results
Ion release analysis and pH
As shown in Table 2, when MTA Repair HP and ProRoot MTA were immersed in PBS (pH = 7.4) or butyric acid (pH = 5.2) for 7 days, there were appreciable increases in both Si, Sr, and Ca ion concentrations in the surrounding solutions, whereas a significant decrease in P ion concentration was also observed (Table 2). A similar pH was observed in all elutes from materials immersed in PBS. However, when MTA Repair HP and ProRoot MTA were immersed in butyric acid, 1:4 dilution of both materials exhibited higher pH when compared to the other two eluates (1:1, 1:2) (Table 3).
Isolation and characterization of hPDLSCs
Flow cytometry analysis showed that more than 95% of viable hPDLSCs were positive for the typical MSC surface markers CD73, CD90, and CD105 and negative for the hematopoietic markers CD14, CD20, CD34, and CD45 (Fig. 1a).
ahPDLSCs were stained with monoclonal fluorescence-conjugated antibodies against typical MSC surface markers (dark gray) and their respective isotypes (light gray), and analyzed by flow cytometry. Insert numbers represent percentages of positive cells for each marker. The figure shows representative flow cytometry analyses obtained from n = 3 separate experiments. b Metabolic activity of hPDLSCs treated with different eluents was assessed for 3 days. Significant differences were indicated as *p < 0.05, **p < 0.01, and ***p < 0.001
MTT assay
The MTT assay revealed an increased metabolic activity in the presence of ProRoot and MTA Repair HP with different pH values. After 72 h, there was a significant difference in cell viability when ProRoot was compared with MTA Repair HP at pH 7.4, especially when dilutions 1:2 and 1:4 were used (*p < 0.05). Indeed, ProRoot 7.4 showed higher cell viability than MTA Repair HP at all dilutions. Surprisingly, MTA Repair HP at pH 5.2 exhibited higher values of cell viability than that observed in the control group with dilutions 1:2 and 1:4 (***p < 0.001), although there was still no significant difference with dilution 1:1. On the other hand, ProRoot 5.2 only revealed greater cell viability than the control with the 1:4 dilution (**p < 0.01), whereas cell viability was similar or lower than control with dilutions 1:1 and 1:2 (Fig. 1b).
Apoptosis/necrosis of hPDLSCs in presence of eluates
Representative 2-dimensional dot plots of the distribution of live (Annexin-V−/7-AAD), early apoptotic (Annexin-V+/7-AAD−), or late apoptotic and necrotic cells (Annexin−V+/7-AAD+ and Annexin-V−/7-AAD+) in untreated hPDLSCs, or exposed to different dilutions of the eluates of the studied endodontic materials are shown in Fig. 2a. The percentages of live cells cultured for 72 h with the different dilutions and materials were higher than 96% in all conditions and similar to the values obtained with cells cultured in the absence of the eluates (control).
ahPDLSCs were cultured in presence or absence (control) of cement extracts for 72 h, labeled with annexin-V and 7-AAD and analyzed using flow cytometry. Four quadrants are representing: (−): live cells (annexinV−/7-AAD−); (+−): early apoptotic cells (annexinV+/7-AAD−); (−+) and (++): late apoptotic and necrotic cells (annexinV−/7-AAD+ and annexinV+/7-AAD+). b Cell attachment of hPDLSCs onto ProRoot (pH 7.4 and 5.2) and MTA Repair HP (pH 7.4 and 5.2) at 72 h. Specimens were observed by SEM. Scale bar = 100 μm
Cell attachment on materials and characterization of set materials
The scanning electron micrographs of the cells in direct contact with the materials are shown in Fig. 2b. After 72 h of culture, hPDLSCs were seen to be attached to the material, showing prominent cytoplasmic processes interacting with the surface of the studied samples and the neighboring cells in both materials exposed to different environments. No differences were observed between materials or acidic or neutral environments.
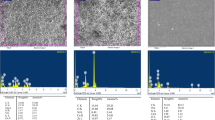
Different surface microstructures were observed in MTA Repair HP and ProRoot according to the setting conditions. In specimens exposed to PBS, slender columns and round crystals composed of these nanosized particles were observed (Fig. 3). The energy-dispersive x-ray results revealed that the precipitates on both materials had high peaks of Ca, C, and O and a weak peak of Si and P. The Ca/P ratio in ProRoot MTA (2.06) was higher than that observed for MTA Repair HP (1.88).
On the other hand, more crystalline structures were observed in ProRoot specimens than with MTA Repair HP when the specimens were exposed to butyric acid. In fact, ProRoot exhibited a large number of petal-like crystallized structures, whereas MTA Repair HP displayed similar surface characteristics to when PBS was used. The peaks and mean weight percentage for every element are listed in Fig. 3.
Discussion
When used as retrograde filling material, bioactive cement is placed in the apical cavity in contact with periapical tissues often exposed to and acidic environment, which may affect its biological properties [13]. The present study evaluates the biological properties of MTA Repair HP and ProRoot MTA in human periodontal ligament stem cells after exposure to acidic and neutral environments.
hPDLSCs were used for our experiments because the materials used for root-end fillings are usually placed in close proximity to the apical and periodontal tissues, and it is widely accepted that the interactions between bioactive cements and dental stem cells are very important [27]. Furthermore, harvesting and isolating this progenitor population of cells is relatively straightforward, and a sufficient number of cells can be obtained in a few weeks [28].
To simulate the conditions of periradicular infections, we used butyric acid was used to simulate acidic conditions because this acid is a product of the bacterial anaerobic metabolism and it is clinically relevant [29]. Furthermore, it has been described that after the resolution of the inflammatory process by the appropriate treatment, the pH of the dental environment returns to normal within 168 h [30].
Bioactive cements release various ions for over a long period of time during storage [31]. The Ca released is beneficial for apatite formation: when tricalcium silicate-based cements were in contact with the tissue fluids, they produced calcium silicate hydrate and calcium hydroxide [5]. The calcium hydroxide releases calcium ions which are essential for cell attachment, migration, differentiation and proliferation of hard tissue-producing cells. Moreover, the Ca released during setting could diffuse into the dentin, increasing in concentration, thus making MTA a potential candidate for use in inflammatory root resorption treatment [32]. In our study, both materials showed Ca release. However, more Ca and Si ions were released with both MTA Repair HP and ProRoot at a pH of 5.2. These results corroborate those of a previous study, which appointed to higher Ca release in the presence of ProRoot at pH 5.4 than at pH 7.4. [13]. Higher concentrations of Si and Ca ions may facilitate osteoblast and cementoblast differentiation, and also inhibit osteoclastogenesis, providing a support for periradicular healing processes [33].
The first step necessary before cells can differentiate and produce an extracellular mineralized matrix on a substrate is that the new materials must be cytocompatible. To evaluate the biological effects of both endodontic cements exposed to different environments, the cell viability and induction of apoptosis of hPDLSCs cultured in the presence of the different extracts was investigated by MTT assay, and the binding of annexin-V and 7-AAD was measured—two-color flow cytometry analyses usually used to determine the stage of cell apoptosis. The results revealed that neither MTA Repair HP nor ProRoot MTA induced apoptosis at pH 5.2 or 7.4, thus preserving cell viability. So, both biomaterials satisfy clinical demands in inflamed acidic environments. This is consistent with previous reports describing that high concentrations of MTA Repair HP in the cell culture medium significantly increase stem cell viability [3, 16]. Moreover, previous studies reported that both ProRoot MTA and iRoot BP Plus displayed good biocompatibility and bioactivity when used as root-end filling materials in an apical periodontitis animal model [34]. However, one limitation of our study in this respect may be the lack of previous reports evaluating the cytocompatibility of MTA Repair HP exposed to an acidic environment.
The quality and quantity of cell attachment onto retrograde filling materials are generally accepted as valid criteria for biologically evaluating biomaterials. Scanning electronic microscopy (SEM) is a useful tool for examining the morphology and cell attachment of primary cultured cells seeded on different biomaterials [35]. The SEM results indicated that each of these materials exhibited a different degree of surface roughness and morphology; ProRoot exhibited a large number of petal-like crystallized structures when exposed to acidic environment, whereas MTA Repair HP displayed a globular precipitates in both environments. These results were consistent with other studies that reported similar prismatic crystalline structures when ProRoot MTA was exposed to butyric acid [13, 36]. Regarding cell attachment, previous studies have reported that spindle-shaped cells observed in contact with biomaterials such as Biodentine or ProRoot MTA are a good indicator of low-toxicity of the materials [23]. Despite acidic conditions, hPDLSCs exhibited adequate fibroblastoid morphology with numerous prolongations anchored to the surface of the cements. These findings are consistent with previous observations for other bioactive cements that maintained their biological properties [13].
For its part, EDX is a chemical microanalysis technique used to study the composition of dental materials, including cements. In fact, the compositions of retrograde filling materials could be related with their cytotoxicity [37]. Using EDX, numerous studies have shown several detectable variations in the elemental composition of MTA [16, 38], but there is little information about variations in the elemental compositions of these bioactive cements after acidic exposition [13]. When using MTA Repair HP at pH 5.2, EDX revealed the presence of Na and Cl (attributable to the PBS medium), traces of Si, and a marked increase in O and Ca. However, at pH 7.4, MTA Repair HP showed higher O, Si, and Ca levels than at 5.2.
We observed the marked presence of Ca ions from both MTA Repair HP and ProRoot MTA in an acidic and a physiological environment, which was consistent with a previous study using ProRoot MTA [13]. Also, EDX showed the presence of bismuth from MTA, which agrees with previous studies, performed using Pro Root MTA [13]. However, MTA Repair HP is bismuth-free. The absence of bismuth oxide reduces the risk of tooth discoloration, although this is not a major clinical problem with root-end filling materials, [18, 39, 40].
In summary, our results revealed that exposure to an acidic environment promoted Si and Ca ion release from ProRoot MTA and MTA Repair HP. Moreover, acceptable biological properties in terms of cell viability, cell death, and cell attachment, were observed for ProRoot MTA and MTA Repair HP in both environments.
References
Alsulaimani RS (2018) Immediate and delayed repair of 2 sizes of furcal perforations in dogs’ teeth using mineral trioxide aggregate cement. J Endod 44:1000–1006. https://doi.org/10.1016/j.joen.2018.02.026
Torabinejad M, Parirokh M, Dummer PMH (2018) Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview—part II: other clinical applications and complications. Int Endod J 51(3):284–317. https://doi.org/10.1111/iej.12843
Tomas-Catala CJ, Collado-Gonzalez M, Garcia-Bernal D, Onate-Sanchez RE, Forner L, Llena C, Lozano A, Moraleda JM, Rodriguez-Lozano FJ (2018) Biocompatibility of new pulp-capping materials NeoMTA Plus, MTA Repair HP, and Biodentine on human dental pulp stem cells. J Endod 44(1):126–132. https://doi.org/10.1016/j.joen.2017.07.017
Camilleri J (2007) Hydration mechanisms of mineral trioxide aggregate. Int Endod J 40(6):462–470. https://doi.org/10.1111/j.1365-2591.2007.01248.x
Camilleri J (2015) Staining potential of Neo MTA Plus, MTA Plus, and Biodentine used for pulpotomy procedures. J Endod 41(7):1139–1145. https://doi.org/10.1016/j.joen.2015.02.032
Gandolfi MG, Siboni F, Botero T, Bossu M, Riccitiello F, Prati C (2015) Calcium silicate and calcium hydroxide materials for pulp capping: biointeractivity, porosity, solubility and bioactivity of current formulations. J Appl Biomater Funct Mater 13(1):43–60. https://doi.org/10.5301/jabfm.5000201
Natu VP, Dubey N, Loke GC, Tan TS, Ng WH, Yong CW, Cao T, Rosa V (2015) Bioactivity, physical and chemical properties of MTA mixed with propylene glycol. J Appl Oral Sci 23(4):405–411. https://doi.org/10.1590/1678-775720150084
Prati C, Gandolfi MG (2015) Calcium silicate bioactive cements: biological perspectives and clinical applications. Dent Mater 31(4):351–370. https://doi.org/10.1016/j.dental.2015.01.004
Dubey N, Rajan SS, Bello YD, Min KS, Rosa V (2017) Graphene nanosheets to improve physico-mechanical properties of bioactive calcium silicate cements. Materials (Basel) 10(6):606. https://doi.org/10.3390/ma10060606
Sultana N, Singh M, Nawal RR, Chaudhry S, Yadav S, Mohanty S, Talwar S (2018) Evaluation of biocompatibility and osteogenic potential of tricalcium silicate-based cements using human bone marrow-derived mesenchymal stem cells. J Endod 44(3):446–451. https://doi.org/10.1016/j.joen.2017.11.016
Bortoluzzi EA, Niu LN, Palani CD, El-Awady AR, Hammond BD, Pei DD, Tian FC, Cutler CW, Pashley DH, Tay FR (2015) Cytotoxicity and osteogenic potential of silicate calcium cements as potential protective materials for pulpal revascularization. Dent Mater 31(12):1510–1522. https://doi.org/10.1016/j.dental.2015.09.020
Agrafioti A, Taraslia V, Chrepa V, Lymperi S, Panopoulos P, Anastasiadou E, Kontakiotis EG (2016) Interaction of dental pulp stem cells with Biodentine and MTA after exposure to different environments. J Appl Oral Sci 24(5):481–486. https://doi.org/10.1590/1678-775720160099
Tian J, Zhang Y, Lai Z, Li M, Huang Y, Jiang H, Wei X (2017) Ion release, microstructural, and biological properties of iRoot BP plus and ProRoot MTA exposed to an acidic environment. J Endod 43(1):163–168. https://doi.org/10.1016/j.joen.2016.10.011
Agrafioti A, Tzimpoulas N, Chatzitheodoridis E, Kontakiotis EG (2016) Comparative evaluation of sealing ability and microstructure of MTA and biodentine after exposure to different environments. Clin Oral Investig 20(7):1535–1540. https://doi.org/10.1007/s00784-015-1638-6
Akhavan H, Mohebbi P, Firouzi A, Noroozi M (2016) X-ray diffraction analysis of ProRoot mineral trioxide aggregate hydrated at different pH values. Iran Endod J 11(2):111–113. https://doi.org/10.7508/iej.2016.02.007
Tomas-Catala CJ, Collado-Gonzalez M, Garcia-Bernal D, Onate-Sanchez RE, Forner L, Llena C, Lozano A, Castelo-Baz P, Moraleda JM, Rodriguez-Lozano FJ (2017) Comparative analysis of the biological effects of the endodontic bioactive cements MTA-angelus, MTA repair HP and NeoMTA plus on human dental pulp stem cells. Int Endod J 50(Suppl 2):e63–e72. https://doi.org/10.1111/iej.12859
Silva E, Carvalho NK, Guberman M, Prado M, Senna PM, Souza EM, De-Deus G (2017) Push-out bond strength of fast-setting mineral trioxide aggregate and Pozzolan-based cements: ENDOCEM MTA and ENDOCEM Zr. J Endod 43(5):801–804. https://doi.org/10.1016/j.joen.2016.12.007
Silva EJ, Carvalho NK, Zanon M, Senna PM, DE-Deus, G, Zuolo ML, Zaia AA (2016) Push-out bond strength of MTA HP, a new high-plasticity calcium silicate-based cement. Braz Oral Res 30(1):S1806-83242016000100269. https://doi.org/10.1590/1807-3107BOR-2016.vol30.0084
Duarte MA, Alves de Aguiar K, Zeferino MA, Vivan RR, Ordinola-Zapata R, Tanomaru-Filho M, Weckwerth PH, Kuga MC (2012) Evaluation of the propylene glycol association on some physical and chemical properties of mineral trioxide aggregate. Int Endod J 45(6):565–570
Gomes Cornelio AL, Salles LP, Campos da Paz M, Cirelli JA, Guerreiro-Tanomaru JM, Tanomaru Filho M (2011) Cytotoxicity of Portland cement with different radiopacifying agents: a cell death study. J Endod 37(2):203–210. https://doi.org/10.1016/j.joen.2010.11.017
Rodriguez-Lozano FJ, Garcia-Bernal D, Onate-Sanchez RE, Ortolani-Seltenerich PS, Forner L, Moraleda JM (2017) Evaluation of cytocompatibility of calcium silicate-based endodontic sealers and their effects on the biological responses of mesenchymal dental stem cells. Int Endod J 50(1):67–76. https://doi.org/10.1111/iej.12596
Vera-Sánchez M, Aznar-Cervantes S, Jover E, García-Bernal D, Oñate-Sánchez RE, Hernández-Romero D, Moraleda JM, Collado-González M, Rodríguez-Lozano FJ, Cenis JL (2016) Silk-fibroin and graphene oxide composites promote human periodontal ligament stem cell spontaneous differentiation into osteo/cementoblast-like cells. Stem Cells Dev 25(22):1742–1754. https://doi.org/10.1089/scd.2016.0028
Akbulut MB, Uyar Arpaci P, Unverdi Eldeniz A (2016) Effects of novel root repair materials on attachment and morphological behaviour of periodontal ligament fibroblasts: scanning electron microscopy observation. Microsc Res Tech 79(12):1214–1221. https://doi.org/10.1002/jemt.22780
Küçükkaya S, Görduysus M, Zeybek ND, Müftüoğlu SF (2016) In vitro cytotoxicity of calcium silicate-based endodontic cement as root-end filling materials. Scientifica (Cairo) 2016(9203932):1–5. https://doi.org/10.1155/2016/9203932
Collado-Gonzalez M, Garcia-Bernal D, Onate-Sanchez RE, Ortolani-Seltenerich PS, Lozano A, Forner L, Llena C, Rodriguez-Lozano FJ (2017) Biocompatibility of three new calcium silicate-based endodontic sealers on human periodontal ligament stem cells. Int Endod J 50(9):875–884. https://doi.org/10.1111/iej.12703
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317. https://doi.org/10.1080/14653240600855905
Wang Y, Zhou Y, Jin L, Pang X, Lu Y, Wang Z, Yu Y, Yu J (2018) Mineral trioxide aggregate enhances the osteogenic capacity of periodontal ligament stem cells via NF-kappaB and MAPK signaling pathways. J Cell Physiol 233(3):2386–2397. https://doi.org/10.1002/jcp.26110
Bueno C, Ramirez C, Rodríguez-Lozano FJ, Tabarés-Seisdedos R, Rodenas M, Moraleda JM, Jones JR, Martinez S (2013) Human adult periodontal ligament-derived cells integrate and differentiate after implantation into the adult mammalian brain. Cell Transplant 22(11):2017–2028. https://doi.org/10.3727/096368912X657305
Namazikhah MS, Nekoofar MH, Sheykhrezae MS, Salariyeh S, Hayes SJ, Bryant ST, Mohammadi MM, Dummer PM (2008) The effect of pH on surface hardness and microstructure of mineral trioxide aggregate. Int Endod J 41(2):108–116. https://doi.org/10.1111/j.1365-2591.2007.01325.x
Rajasekharan S, Vercruysse C, Martens L, Verbeeck R (2018) Effect of exposed surface area, volume and environmental pH on the calcium ion release of three commercially available tricalcium silicate based dental cements. Materials (Basel) 11(1):123. https://doi.org/10.3390/ma11010123
Yamamoto S, Han L, Noiri Y, Okiji T (2016) Evaluation of the Ca ion release, pH and surface apatite formation of a prototype tricalcium silicate cement. Int Endod J 50:e73–e82. https://doi.org/10.1111/iej.12737
Chen S, Shi L, Luo J, Engqvist H (2018) A novel fast-setting mineral trioxide aggregate: its formulation, chemical-physical properties and cytocompatibility. ACS Appl Mater Interfaces 10:20334–20341. https://doi.org/10.1021/acsami.8b04946
Zamparini F, Siboni F, Prati C, Taddei P, Gandolfi MG (2018) Properties of calcium silicate-monobasic calcium phosphate materials for endodontics containing tantalum pentoxide and zirconium oxide. Clin Oral Investig 23:445–457. https://doi.org/10.1007/s00784-018-2453-7
Chen I, Karabucak B, Wang C, Wang HG, Koyama E, Kohli MR, Nah HD, Kim S (2015) Healing after root-end microsurgery by using mineral trioxide aggregate and a new calcium silicate-based bioceramic material as root-end filling materials in dogs. J Endod 41(3):389–399. https://doi.org/10.1016/j.joen.2014.11.005
Ahmed HM, Luddin N, Kannan TP, Mokhtar KI, Ahmad A (2014) Cell attachment properties of Portland cement-based endodontic materials: biological and methodological considerations. J Endod 40(10):1517–1523. https://doi.org/10.1016/j.joen.2014.06.013
Ashofteh Yazdi K, Ghabraei S, Bolhari B, Kafili M, Meraji N, Nekoofar MH, Dummer PMH (2018) Microstructure and chemical analysis of four calcium silicate-based cements in different environmental conditions. Clin Oral Investig 23:43–52. https://doi.org/10.1007/s00784-018-2394-1
Ahmed HMA, Luddin N, Kannan TP, Mokhtar KI, Ahmad A (2017) White mineral trioxide aggregate mixed with calcium chloride dihydrate: chemical analysis and biological properties. Restor Dent Endod 42(3):176–187. https://doi.org/10.5395/rde.2017.42.3.176
Camilleri J, Formosa L, Damidot D (2013) The setting characteristics of MTA plus in different environmental conditions. Int Endod J 46(9):831–840. https://doi.org/10.1111/iej.12068
Guimaraes BM, Prati C, Duarte MAH, Bramante CM, Gandolfi MG (2018) Physicochemical properties of calcium silicate-based formulations MTA Repair HP and MTA Vitalcem. J Appl Oral Sci 26:e2017115. https://doi.org/10.1590/1678-7757-2017-0115
Cintra LTA, Benetti F, de Azevedo Queiroz IO, de Araujo Lopes JM, Penha de Oliveira SH, Sivieri Araujo G, Gomes-Filho JE (2017) Cytotoxicity, biocompatibility, and biomineralization of the new high-plasticity MTA material. J Endod 43(5):774–778. https://doi.org/10.1016/j.joen.2016.12.018
Funding
This work was supported by the Spanish Network of Cell Therapy (TerCel), RETICS subprograms of the I+D+I 2013–2016 Spanish National Plan, and projects “RD12/0019/0001” and “RD16/0011/0001” funded by the Instituto de Salud Carlos III to JMM and co-funded by the European Regional Development Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study protocol was approved by the Clinical Research Ethics Committee of the University of Murcia (procedure number: 1528/2017). Likewise, permission was obtained from the Health Department authorities to use the information contained in the CDHs, previously anonymized by one of the investigators belonging to the medical staff of the Health Department in order to protect patient confidentiality. All the information was processed in abidance with the confidentiality regulations defined under Act 15/1999 referred to personal data protection.
Informed consent
Informed consent was obtained from the parents of all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Collado-González, M., López-García, S., García-Bernal, D. et al. Biological effects of acid-eroded MTA Repair HP and ProRoot MTA on human periodontal ligament stem cells. Clin Oral Invest 23, 3915–3924 (2019). https://doi.org/10.1007/s00784-019-02822-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-02822-2