Abstract
Objective
The objective of this study was to analyze the microstructure and crystalline structures of ProRoot MTA, Biodentine, CEM Cement, and Retro MTA when exposed to phosphate-buffered saline, butyric acid, and blood.
Methods and materials
Mixed samples of ProRoot MTA, Biodentine, CEM Cement, and Retro MTA were exposed to either phosphate-buffered saline, butyric acid, or blood. Scanning electron microscope (SEM) and energy-dispersive X-ray spectroscopic (EDX) evaluations were conducted of specimens. X-ray diffraction (XRD) analysis was also performed for both hydrated and powder forms of evaluated calcium silicate cements.
Results
The peak of tricalcium silicate and dicalcium silicate detected in all hydrated cements was smaller than that seen in their unhydrated powders. The peak of calcium hydroxide (Ca(OH)2) in blood- and acid-exposed ProRoot MTA, CEM Cement, and Retro MTA specimens were smaller than that of specimens exposed to PBS. The peak of Ca(OH)2 seen in Biodentine™ specimens exposed to blood was similar to that of PBS-exposed specimens. On the other hand, those exposed to acid exhibited smaller peaks of Ca(OH)2.
Conclusion
Exposure to blood or acidic pH decreased Ca(OH)2 crystalline formation in ProRoot MTA, CEM Cement and Retro MTA. However, a decrease in Ca(OH)2 was only seen when Biodentine™ exposed to acid.
Clinical relevance
The formation of Ca(OH)2 which influences the biological properties of calcium silicate cements was impaired by blood and acid exposures in ProRoot MTA, CEM Cement, and Retro MTA; however, in the case of Biodentine, only exposure to acid had this detrimental effect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Calcium silicate cements (CSCs) have various indications of use in endodontics and their potential clinical applications have increased over the years. Mineral trioxide aggregate (MTA) was the first CSC introduced in endodontics. Although having a variety of favorable properties, this CSC has several disadvantages such as difficult handling properties and induction of tooth discoloration [1,2,3,4]. Recently, a variety of CSCs have been introduced to overcome these disadvantages.
Biodentine was introduced as a fast setting dentine replacement material [5] with high compressive strength [6, 7]. It is composed of tricalcium silicate, zirconium oxide, and calcium carbonate, which when mixed with its liquid containing calcium chloride and a water-soluble polymer, sets rapidly [8] and forms calcium hydroxide (Ca(OH)2) as a by-product of hydration [9].
Calcium-enriched mixture (CEM) Cement was introduced as a white CSC claiming to have no discoloration potential [10,11,12]. It is composed of CaO (51.75%), SO3 (9.53%), P2O5 (8.49%), SiO2 (6.32%), and minor components of Al2O3, Na2O, MgO, and Cl [13].
Retro MTA is another fast setting CSC and is said to have less discoloration potential [14]. It consists of calcium carbonate, silicon dioxide, aluminum oxide, and calcium zirconia [15].
The clinical applications of MTA have been suggested for all aforementioned CSCs [5, 16, 17]. In most clinical applications related to the field of endodontics such as root end filling, perforation sealing, vital pulp therapy, and regeneration, CSCs encounter blood and/or in infectious conditions, acidic pH. This contact occurs when these cements are undergoing hydration and maturation [1]. Therefore, knowledge of the effect of these environmental conditions on the chemical compounds and hydration process of these cements is of clinical significance. Some studies have shown that exposure to these conditions affect some properties in different cements [18,19,20] such as their setting, expansion and physical properties [21]. But data on the effect of different conditions on the properties of Retro MTA and CEM Cement are limited.
The aim of this study was to evaluate the effect of exposure to butyric acid, a metabolism by-products of anaerobic bacteria dominant in endodontic infections and the most common fatty acid found in infectious conditions with endodontic origin [22], and blood during setting on the chemical compounds and hydration process of ProRoot MTA, Biodentine, CEM Cement, and Retro MTA. The null hypothesis was that exposure to blood and acid will not affect these CSCs.
Methods and materials
Plexiglass molds with an internal diameter of 4 and 6 mm heights were fabricated by CNC laser cutting.
Four CSCs were evaluated (Table 1):
-
1.
ProRoot MTA (Dentsply Sirona, Ballaigues, Switzerland)
-
2.
Biodentine (Septodont, Saint Maur des Fosses, France)
-
3.
CEM Cement (Bionique, Tehran, Iran)
-
4.
Retro MTA (BioMTA, Seoul, Republic of Korea)
Three exposure conditions were evaluated:
-
a.
Phosphate-buffered saline (PBS) (pH = 7.4) (Merck, Darmstadt, Germany)
-
b.
Whole human blood (WHB)
-
c.
Butyric acid (BA)(pH = 5.4) (Merck, Darmstadt, Germany)
For preparation of ProRoot MTA and CEM Cement slurries, 1 g of their powder was placed in an empty, clean plastic capsule, and 0.33 mL of the respective liquid was added [23]. For Retro MTA, the powder content of one pack was placed in the same type of plastic capsule and three drops of its liquid were added as suggested by the manufacturer. Biodentine powder is delivered in a capsule, and its liquid is supplied in a single-dose container. Each capsule was mixed with five drops of its liquid. The encapsulated materials were then mechanically mixed for 30 s at 4500 rpm [18] using an amalgamator (Silamat; IvoclarVivadent AG, Liechtenstein).
Prior to slurry placement, each mold was filled with either PBS, WHB or BA according to the specific subgroup and excess liquid was aspirated after 20 s. The molds were then filled with the prepared cements using minimal pressure. The materials were then subjected to ultrasonic energy for 30 s at a power scale of 5 using a BUC-1 Spartan tip (Obtura Spartan, Fenton, MO, USA) attached to a Suprasson_P5 Booster (Satelec, Merignac, France) [18, 23]. After initial setting, specimens were placed in Eppendorf tubes containing the respective exposure liquid: PBS, WHB, or BA. Placement was in a way that the lower surface of each specimen would be in contact with the liquid. A moist cotton pellet was then placed over the specimens. They were incubated for 7 days in 37 °C and fully saturated conditions.
After the incubation period, the surface in contact with either PBS, WHB, or BA was coated with gold and analyzed with a scanning electron microscope (SEM) equipped with EDX (SEM-EDX 515, Phillips, Eindhoven, the Netherlands). Images with × 400 and × 2000 magnifications were considered adequate for the characterization of the microstructure. Element analysis was performed on selected segments with crystalline and amorphous structure.
Both hydrated (mixed) and unhydrated (powder) cements were mounted for XRD analysis. The hydrated cements were milled into a fine powder. An automated X-ray diffractometer (X’Pert PRO MPD, Philips) with Cu Kα radiation was set at 40 kVp and 30 mA. Data were collected from scan range of 15–55 with a step size of 0.02°. X’Pert High score plus software (Spectrum Viewer Basic 2.6.3, PANalytical, Nottingham, UK) was used for the analysis. The peaks of each sample were matched with those of the International Center for Diffraction Data (ICDD) database (International Center for Diffraction Data, Newton Square, PA, USA). By comparing the size of each peak representing a crystalline phase between groups, their relative amounts were estimated.
Results
The results of SEM and EDX evaluations of the CSCs exposed to the various media are shown in Figs. 1, 2, 3, and 4.
SEM images of ProRoot MTA specimens exposed to PBS and blood revealed the presence of globular crystalline structures which disappeared in those exposed to butyric acid. Instead, prismatic crystalline structures were seen on the surface of those exposed to butyric acid (Fig. 1). EDX evaluations revealed the addition of Fe in the composition of crystalline structures in specimens exposed to blood.
Biodentine specimens contained globular crystalline structures on their surface in all exposure conditions (Fig. 2). EDX evaluations revealed the presence of Fe in the composition of the crystalline structures of specimens exposed to blood while the amorphous phase in these specimens lacked both Fe and Si.
CEM Cement revealed hexagonal, cubical, and needle-like crystalline structures in specimens exposed to PBS, blood, and butyric acid, respectively (Fig. 3). EDX evaluations revealed the presence of P in both crystalline and amorphous structures in specimens exposed to PBS and blood whereas this element was not detectable in those exposed to butyric acid. BA was also detected in all specimens regardless of the exposure conditions.
Retro MTA exhibited prismatic, cubical, and needle-like crystalline structures in specimens exposed to PBS, blood, and butyric acid, respectively (Fig. 4). EDX evaluations revealed the presence BA in all specimens regardless of the exposure conditions.
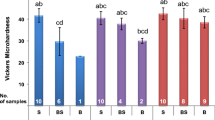
XRD evaluation of ProRoot MTA exposed to different media revealed the presence of tricalcium silicate and dicalcium silicate, alpha bismuth oxide, and Ca(OH)2 in all specimens. The peak of tricalcium silicate and dicalcium silicate was smaller than that of unhydrated powder. The peaks of Ca(OH)2 on blood- and acid-exposed specimens were smaller than that of specimens exposed to PBS. The unhydrated powder did not contain Ca(OH)2 (Fig. 5).
All Biodentine specimens exposed to different media contained tricalcium silicate and dicalcium silicate, calcium carbonate, zirconium oxide, and Ca(OH)2. The peak of tricalcium silicate and dicalcium silicate was smaller than that seen in the unhydrated powder. The peak of tricalcium silicate and dicalcium silicate and Ca(OH)2 seen in specimens exposed to blood were similar to that of PBS-exposed specimens. On the other hand, those exposed to acid exhibited smaller peaks of Ca(OH)2. The acid-exposed specimens were also associated with smaller peaks of tricalcium silicate and dicalcium silicate in comparison to the blood-exposed specimens (Fig. 5).
XRD evaluation of CEM Cement exposed to the various media revealed the presence of tricalcium silicate and dicalcium silicate, calcium carbonate, barium sulfate, and Ca(OH)2 in all specimens. The peak of tricalcium silicate and dicalcium silicate was smaller than that seen in unhydrated powder. The peak of Ca(OH)2 on specimens exposed to blood and acid was smaller than when exposed to PBS (Fig. 5).
XRD evaluation of Retro MTA exposed to the media revealed the presence of tricalcium silicate, zirconium oxide, and Ca(OH)2 in all specimens. The peak of tricalcium silicate was smaller than that seen in unhydrated powder. The peak of Ca(OH)2 seen on specimens exposed to blood and acid was smaller than those exposed to PBS (Fig. 5).
Discussion
In this study, three exposure conditions were evaluated: exposure to PBS (pH = 7.4), WHB, and BA (pH = 5.4). BA is a short-chain fatty acids produced by endodontic pathogens and the most common fatty acid found in endodontic infectious conditions [22]. It is usually used to reproduce acidic conditions in vitro [19, 24]. PBS is a simulated tissue fluid containing phosphate [25] that can be used for the purpose of mimicking normal in vivo conditions in laboratory studies [19, 26].
Scanning electron microscopy and EDX analysis together with X-ray diffraction analysis were used to assess the effect of exposure to several potential clinically relevant environmental conditions on the composition and hydration process of various CSCs.
XRD is useful for identifying the crystalline phases of specimens. The intensity of each peak representative of a crystalline phase in XRD is proportional to the phase concentration [27]. However, a disadvantage of this analysis for CSCs is the superimposition of the peaks and the presence of multiple compounds within the materials [27]. An example of this superimposition is observed at 52° 2θ for dicalcium silicate and tricalcium silicate crystalline structures as seen in Fig. 5. It should be noted that superimposition is also observed at 25.8° 2θ for hydroxyapatite and barium sulfate making them indistinguishable.
Reduction in the peak size of tricalcium silicate and dicalcium silicate peaks and detection of Ca(OH)2 in the hydrated forms of all the CSCs in comparison with their powder counterparts is a consequence of the hydration of these CSCs [28,29,30]. Carbonation of Ca(OH)2 should also be considered as it is inevitable in both clinical and experimental conditions but consistent in all specimens [31].
During the hydration process, calcium silicate hydrates (CSH) are also formed but due to their amorphous structure this phase was not detected with XRD analysis [27, 29, 30]. EDX analysis of the amorphous structures identified in SEM evaluations revealed the presence of Ca, Si, and O, which are indicative of CSH.
In ProRoot MTA, exposure to blood and acid reduced the peak size of Ca(OH)2 compared to specimens exposed to PBS. These results are consistent with previous studies [20, 26, 32]. Two hypotheses can be attributed to this decrease: (a) prohibition of hydration due to exposure to different environmental conditions or (b) dissolution of Ca(OH)2 formed as a consequence of hydration. Considering that the peak size of tricalcium silicate and dicalcium silicate was similar in all exposure conditions and smaller than that of the unhydrated powder, it may be concluded that the second hypothesis is more probable.
In the case of Biodentine, the peak size of Ca(OH)2 in specimens exposed to PBS and blood were similar, however, in specimens exposed to acid a smaller peak was seen. Interestingly, the peak size of tricalcium and dicalcium silicate was seen in those exposed to acid was quite similar to the unhydrated powder indicating an impairment in the hydration process. Previous studies have reported that several physical properties of Biodentine were affected by acidic environmental pH [32] but not affected by exposure to blood [33]. This may be explained by the presence of calcium chloride [34, 35] and calcium carbonate that both accelerate the setting process [30] and the presence of higher percentage of amorphous phase in its hydrated form [36], thus making this cement less susceptible to environmental conditions. Further research is required regarding this matter.
In the case of CEM Cement, the peak of Ca(OH)2 in specimens exposed to blood and acid was smaller than those exposed to PBS. However, their tricalcium and dicalcium silicate peaks were similar and smaller than those seen in the unhydrated powder. Hence, it may be concluded that when CEM Cement is exposed to either blood or acidic pH, hydration occurs and Ca(OH)2 is produced but is dissolved similar to what happens to ProRoot MTA. These conditions were also seen in Retro MTA.
Ettringite was not detectable in any of the specimens in the current study although trace amounts of Al were detected in EDX analysis of ProRoot MTA, CEM Cement, and Retro MTA. This may be due to either formation of small amounts of this phase [26] or limited detection due to dilution as a consequence of grinding the specimens [20]. The absence of ettringite peaks in the hydrated form of Biodentine was predictable as this cement lacks aluminate in its powder [30]. The reduction/absence of the aluminate phase is an added benefit with respect to the workability of the fresh cement paste [30], associated with biological improvements [37], e.g., avoiding the undesirable effects of aluminum (e.g., risks of Parkinson’s and Alzheimer’s disease) [38] and avoiding the leaching of this trace elements into the surrounding tissues [39, 40].
Conclusion
Blood or acidic pH exposure resulted in reduction in Ca(OH)2 in ProRoot MTA, Retro MTA, and CEM Cement. Blood did not affect the hydration of Biodentine, whereas, acidic pH interfered with its hydration.
References
Nekoofar MH, Davies TE, Stone D, Basturk FB, Dummer PM (2011) Microstructure and chemical analysis of blood-contaminated mineral trioxide aggregate. Int Endod J 44:1011–1018. https://doi.org/10.1111/j.1365-2591.2011.01909.x
Marconyak LJ Jr, Kirkpatrick TC, Roberts HW, Roberts MD, Aparicio A, Himel VT, Sabey KA (2016) A comparison of coronal tooth discoloration elicited by various endodontic reparative materials. J Endod 42(3):470–473. https://doi.org/10.1016/j.joen.2015.10.013
Kohli MR, Yamaguchi M, Setzer FC, Karabucak B (2015) Spectrophotometric analysis of coronal tooth discoloration induced by various bioceramic cements and other endodontic materials. J Endod 41(11):1862–1866. https://doi.org/10.1016/j.joen.2015.07.003
Nosrat A, Nekoofar MH, Bolhari B, Dummer PM (2012) Unintentional extrusion of mineral trioxide aggregate: a report of three cases. Int Endod J 45(12):1165–1176. https://doi.org/10.1111/j.1365-2591.2012.02082.x
Bakhtiar H, Nekoofar MH, Aminishakib P, Abedi F, Naghi Moosavi F, Esnaashari E, Azizi A, Esmailian S, Ellini MR, Mesgarzadeh V, Sezavar M, About I (2017) Human pulp responses to partial pulpotomy treatment with TheraCal as compared with Biodentine and ProRoot MTA: a clinical trial. J Endod 43:1786–1791. https://doi.org/10.1016/j.joen.2017.06.025
Butt N, Talwar S, Chaudhry S, Nawal RR, Yadav S, Bali A (2014) Comparison of physical and mechanical properties of mineral trioxide aggregate and Biodentine. Indian J Dent Res 25(6):692–697. https://doi.org/10.4103/0970-9290.152163
Kim JR, Nosrat A, Fouad AF (2015) Interfacial characteristics of Biodentine and MTA with dentine in simulated body fluid. J Dent 43(2):241–247. https://doi.org/10.1016/j.jdent.2014.11.004
Setbon HM, Devaux J, Iserentant A, Leloup G, Leprince JG (2014) Influence of composition on setting kinetics of new injectable and/or fast setting tricalcium silicate cements. Dent Mater 30(12):1291–1303. https://doi.org/10.1016/j.dental.2014.09.005
Camilleri J, Laurent P, About I (2014) Hydration of Biodentine, Theracal LC, and a prototype tricalcium silicate-based dentin replacement material after pulp capping in entire tooth cultures. J Endod 40(11):1846–1854. https://doi.org/10.1016/j.joen.2014.06.018
Esmaeili B, Alaghehmand H, Kordafshari T, Daryakenari G, Ehsani M, Bijani A (2016) Coronal discoloration induced by calcium-enriched mixture, mineral trioxide aggregate and calcium hydroxide: a spectrophotometric analysis. Iran Endod J 11(1):23–28. https://doi.org/10.7508/iej.2016.01.005
Shokouhinejad N, Nekoofar MH, Pirmoazen S, Shamshiri AR, Dummer PM (2016) Evaluation and comparison of occurrence of tooth discoloration after the application of various calcium silicate-based cements: an ex vivo study. J Endod 42(1):140–144. https://doi.org/10.1016/j.joen.2015.08.034
Rouhani A, Akbari M, Farhadi-Faz A (2016) Comparison of tooth discoloration induced by calcium-enriched mixture and mineral trioxide aggregate. Iran Endod J 11(3):175–178. https://doi.org/10.7508/iej.2016.03.005
Utneja S, Nawal RR, Talwar S, Verma M (2015) Current perspectives of bio-ceramic technology in endodontics: calcium enriched mixture cement—review of its composition, properties and applications. Restor Dent Endod 40(1):1–13. https://doi.org/10.5395/rde.2015.40.1.1
Kang SH, Shin YS, Lee HS, Kim SO, Shin Y, Jung IY, Song JS (2015) Color changes of teeth after treatment with various mineral trioxide aggregate-based materials: an ex vivo study. J Endod 41(5):737–741. https://doi.org/10.1016/j.joen.2015.01.019
Ha WN, Bentz DP, Kahler B, Walsh LJ (2015) D90: the strongest contributor to setting time in mineral trioxide aggregate and Portland cement. J Endod 41(7):1146–1150. https://doi.org/10.1016/j.joen.2015.02.033
Malkondu O, Karapinar Kazandag M, Kazazoglu E (2014) A review on biodentine, a contemporary dentine replacement and repair material. Biomed Res Int 2014:160951. https://doi.org/10.1155/2014/160951 10
Bakhtiar H, Mirzaei H, Bagheri MR, Fani N, Mashhadiabbas F, Baghaban Eslaminejad M, Sharifi D, Nekoofar MH, Dummer P (2017) Histologic tissue response to furcation perforation repair using mineral trioxide aggregate or dental pulp stem cells loaded onto treated dentin matrix or tricalcium phosphate. Clin Oral Investig 21:1579–1588. https://doi.org/10.1007/s00784-016-1967-0
Nekoofar MH, Stone DF, Dummer PM (2010) The effect of blood contamination on the compressive strength and surface microstructure of mineral trioxide aggregate. Int Endod J 43(9):782–791. https://doi.org/10.1111/j.1365-2591.2010.01745.x
Bolhari B, Nekoofar MH, Sharifian M, Ghabrai S, Meraji N, Dummer PM (2014) Acid and microhardness of mineral trioxide aggregate and mineral trioxide aggregate-like materials. J Endod 40(3):432–435. https://doi.org/10.1016/j.joen.2013.10.014
Nekoofar MH, Davies TE, Stone D, Basturk FB, Dummer PM (2011) Microstructure and chemical analysis of blood-contaminated mineral trioxide aggregate. Int Endod J 44(11):1011–1018. https://doi.org/10.1111/j.1365-2591.2011.01909.x
Sheykhrezae MS, Meraji N, Ghanbari F, Nekoofar MH, Bolhari B, Dummer PMH (2017) Effect of blood contamination on the compressive strength of three calcium silicate-based cements. Aust Endod J. https://doi.org/10.1111/aej.12227
Provenzano JC, Rocas IN, Tavares LF, Neves BC, Siqueira JF Jr (2015) Short-chain fatty acids in infected root canals of teeth with apical periodontitis before and after treatment. J Endod 41(6):831–835. https://doi.org/10.1016/j.joen.2015.02.006
Nekoofar M, Aseeley Z, Dummer P (2010) The effect of various mixing techniques on the surface microhardness of mineral trioxide aggregate. Int Endod J 43(4):312–320
Namazikhah MS, Nekoofar MH, Sheykhrezae MS, Salariyeh S, Hayes SJ, Bryant ST, Mohammadi MM, Dummer PM (2008) The effect of pH on surface hardness and microstructure of mineral trioxide aggregate. Int Endod J 41:108–116. https://doi.org/10.1111/j.1365-2591.2007.01325.x
Marques MR, Loebenberg R, Almukainzi M (2011) Simulated biological fluids with possible application in dissolution testing. Dissolut Technol 18(3):15–28. https://doi.org/10.14227/DT180311P15
Lee YL, Lee BS, Lin FH, Yun Lin A, Lan WH, Lin CP (2004) Effects of physiological environments on the hydration behavior of mineral trioxide aggregate. Biomaterials 25(5):787–793
Grazziotin-Soares R, Nekoofar MH, Davies TE, Bafail A, Alhaddar E, Hubler R, Busato AL, Dummer PM (2014) Effect of bismuth oxide on white mineral trioxide aggregate: chemical characterization and physical properties. Int Endod J 47:520–533. https://doi.org/10.1111/iej.12181
Camilleri J (2010) Hydration characteristics of calcium silicate cements with alternative radiopacifiers used as root-end filling materials. J Endod 36(3):502–508. https://doi.org/10.1016/j.joen.2009.10.018
Basturk FB, Nekoofar MH, Gunday M, Dummer PMH (2017) X-ray diffraction analysis of MTA mixed and placed with various techniques. Clin Oral Investig. https://doi.org/10.1007/s00784-017-2241-9
Camilleri J, Sorrentino F, Damidot D (2013) Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dent Mater 29(5):580–593. https://doi.org/10.1016/j.dental.2013.03.007
Lee YL, Wang WH, Lin FH, Lin CP (2017) Hydration behaviors of calcium silicate-based biomaterials. J Formos Med Assoc 116(6):424–431. https://doi.org/10.1016/j.jfma.2016.07.009
Kayahan MB, Nekoofar MH, McCann A, Sunay H, Kaptan RF, Meraji N, Dummer PM (2013) Effect of acid etching procedures on the compressive strength of 4 calcium silicate-based endodontic cements. J Endod 39:1646–1648. https://doi.org/10.1016/j.joen.2013.09.008
Bolhari B, Ashofteh Yazdi K, Sharifi F, Pirmoazen S (2015) Comparative scanning electron microscopic study of the marginal adaptation of four root-end filling materials in presence and absence of blood. J Dent (Tehran) 12(3):226–234
Grech L, Mallia B, Camilleri J (2013) Characterization of set intermediate restorative material, biodentine, bioaggregate and a prototype calcium silicate cement for use as root-end filling materials. Int Endod J 46(7):632–641. https://doi.org/10.1111/iej.12039
Formosa LM, Mallia B, Camilleri J (2012) The effect of curing conditions on the physical properties of tricalcium silicate cement for use as a dental biomaterial. Int Endod J 45(4):326–336. https://doi.org/10.1111/j.1365-2591.2011.01980.x
Grazziotin-Soares R, Nekoofar MH, Davies T, Hubler R, Meraji N, Dummer PMH (2017) Crystalline phases involved in the hydration of calcium silicate-based cements: semi-quantitative Rietveld X-ray diffraction analysis. Aust Endod J. https://doi.org/10.1111/aej.12226
De-Deus G, Canabarro A, Alves G, Linhares A, Senne MI, Granjeiro JM (2009) Optimal cytocompatibility of a bioceramic nanoparticulate cement in primary human mesenchymal cells. J Endod 35(10):1387–1390. https://doi.org/10.1016/j.joen.2009.06.022
Forbes WF, Gentleman JF (1998) Risk factors, causality, and policy initiatives: the case of aluminum and mental impairment. Exp Gerontol 33(1–2):141–154
Camilleri J, Kralj P, Veber M, Sinagra E (2012) Characterization and analyses of acid-extractable and leached trace elements in dental cements. Int Endod J 45(8):737–743. https://doi.org/10.1111/j.1365-2591.2012.02027.x
Basturk FB, Nekoofar MH, Gunday M, Dummer PM (2014) Effect of various mixing and placement techniques on the flexural strength and porosity of mineral trioxide aggregate. J Endod 40:441–445. https://doi.org/10.1016/j.joen.2013.08.010
Funding
The work was supported by Tehran University of Medical Sciences, Tehran, Iran (grant no. 27183).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Electronic supplementary material
ESM 1
(PDF 158 kb)
Rights and permissions
About this article
Cite this article
Ashofteh Yazdi, K., Ghabraei, S., Bolhari, B. et al. Microstructure and chemical analysis of four calcium silicate-based cements in different environmental conditions. Clin Oral Invest 23, 43–52 (2019). https://doi.org/10.1007/s00784-018-2394-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2394-1