Abstract
Parathyroid hormone (PTH) is widely accepted as an anabolic agent when administered intermittently. Here, we explored the influence of intermittent PTH(1-34) on the expression of local factors by human periodontal ligament (PDL) cells that modify osteoclast biology. This approach aimed at a further elucidation of the role of the hormone and of PDL cells in the regulation of periodontal tissue homeostasis and of repair processes. In a co-culture model of mature PDL cells and RAW 264.7 cells, intermittent PTH(1-34) induced an increased gene expression for tartrate-resistant acid phosphatase (+84%), cathepsin K (+56%), and vitronectin-receptor (+56%); and an enhanced resorptive activity of differentiated osteoclasts (+154%). These findings were correlated with a reduction of the osteoprotegerin (OPG)/receptor activator of nuclear factor kappaB ligand (RANKL) ratio in the presence of PTH(1-34; −44%). Similar results were obtained when RAW cells were cultured with the conditioned medium of PTH(1-34)-stimulated PDL cells. In contrast, when less mature PDL cells were co-cultured with RAW cells, PTH(1-34) induced an inhibition of osteoclastic differentiation (TRAP, −35%; cathepsin K, −28%; vitronectin-receptor, −35%), a reduction of the resorbed substrate area (−77%) and an increase of the OPG/RANKL ratio (+11%). The conditioned medium of PTH(1-34)-pretreated less mature PDL cells led to a down-regulation of the number and activity of multinucleated cells. These data indicate that intermittent PTH(1-34) modifies the expression of membrane-bound and secreted factors by PDL cells which then in turn alter osteoclast biology. The PDL cell response to PTH(1-34) is specific in terms of cell maturation and the mechanism involved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The RANK/RANKL/OPG-system (receptor activator of nuclear factor kappaB (RANK), RANK-ligand (RANKL), osteoprotegerin (OPG)) has been identified as a key regulatory component of alveolar bone loss associated with inflammatory periodontal disease [1, 2]. RANKL was found to be prominent in periodontitis sites and to be positively correlated with IL-6 expression in the gingival crevicular fluid [3]. Other groups found RANKL expression in periodontal ligament (PDL) cells to be stimulated by periodontal pathogenic microorganisms such as Porphyromonas gingivalis or Prevotella intermedia [4, 5]. In another study, smokers suffering from periodontitis displayed significantly lower serum concentrations of OPG and altered OPG/RANKL ratios compared to non-smoking subjects, and the authors concluded that bone loss in smoking-related periodontitis patients may be partially explained by suppression of OPG production [6]. Besides periodontal disease, orthodontic treatment displays a circumstance where changes of the RANKL/OPG-ratio are required to facilitate tooth movement [7] but at the same time represent a co-factor for the development of pathological tooth root resorption [8]. This knowledge led to the therapeutic approach to modify local expression levels of RANKL and OPG by local gene transfer. Local RANKL gene transfer to the periodontal tissue of rats significantly enhanced RANKL expression and osteoclastogenesis in periodontal tissues without any systemic effects. The tooth movement rate was significantly increased in the RANKL gene transfer side. Opposite effects were observed for local OPG gene transfer [9, 10]. Recently, PDL cells were shown to express several osteotropic cytokines [11] including both OPG and RANKL [12–14] suggesting a regulatory role for these cells in periodontal tissue homeostasis and repair processes [15]. In support to this, PDL cells exhibit phenotypic characteristics typical of osteoblasts [16, 17] and respond to hormonal stimulation in an osteoblast-like manner [18, 19]. Amongst those hormones, parathyroid hormone (PTH) is of particular interest since contemporary regenerative treatment approaches for bony tissues focus on its anabolic properties in an intermittent treatment regimen [20, 21]. This raises the question of whether pulsatile PTH might prove beneficial in the attempt to modify PDL cell characteristics and the expression of membrane-bound and secreted molecules such as OPG and RANKL which then in turn might modulate the PDL cell microenvironment. This approach holds out the perspective to influence the biology of osteoclasts and to interfere with the regulation of hard tissue resorption which is part of both hard tissue remodeling under physiological conditions and destruction under pathological circumstances such as inflammation. A better understanding of the role of PDL cells in the regulatory cascade of these events might provide a basis for the development of promising tools to prevent or at least limit the extent to which hard tissue resorption occurs in the course of inflammatory periodontal disease or orthodontic tooth movement or, once tissue loss has occurred, for the development of treatment strategies to re-establish the periodontal architecture. In previous research, we demonstrated that PDL cells express receptors for PTH and respond to PTH(1-34) with distinct changes in proliferation, survival, and differentiation [13, 22, 23] reinforcing the above considerations.
The present study was designed to determine the physiological relevance of the PTH-induced changes in the expression of OPG and membrane-bound RANKL by human PDL cells for the differentiation and function of osteoclasts in a co-culture model with osteoclastic precursors of the monocyte/macrophage lineage (RAW 264.7cells). Furthermore, we aimed at examining the effect of intermittent PTH(1-34) on the release of molecules by PDL cells in the local microenvironment that influence osteoclast biology. We hypothesized that PTH-induced changes in the expression and release of such factors would interfere with the differentiation of osteoclastic cells and modify their resorptive capabilities when cultured on calcium phosphate-coated slides. The response of PDL cells to such intermittent PTH(1-34) treatment regimen and the mechanisms involved were expected to depend on the maturation state of the PDL cells.
Materials and methods
PDL cell explantation
Human PDL cells were scraped from the middle third of the roots of premolars of six different human donors, aged between 12 and 14 years, showing no clinical signs of periodontitis. The teeth had been extracted for orthodontic reasons, with informed parental consent and following an approved protocol of the ethics committee of the University of Bonn (reference number 029/08). Cells were cultured in DMEM containing 10% fetal bovine serum and 0.5% antibiotics (diluted from a stock solution containing 5,000 U/ml penicillin and 5,000 U/ml streptomycin; Biochrom AG, Germany) and cultured at 37°C in an atmosphere of 100% humidity and 5% CO2.
Monocyte/macrophage cell culture
In order to establish a model for osteoclast-like cells, mononuclear cells of the monocytic and macrophage lineage (RAW 264.7 cells, CLS cell line services, Eppelheim, Germany) were cultured in the presence of 20 ng/ml M-CSF (BioCat, Heidelberg, Germany) and 30 ng/ml RANKL (Axxora, Lörrach, Germany) and shown to form multinuclear osteoclastic cells after 5 days in culture staining positively for tartrate-resistant acid phosphatase (TRAP, according to Barka and Anderson 1962 (9)).
Osteoclast formation assay
In a second step, the medium was supplemented with various concentrations of human RANKL and osteoprotegerin to demonstrate the impact of these factors on the formation of multinucleated resorptive cells and to show that those factors of human origin induce a response in murine RAW cells and, thereby, justify the cell culture model. The number of forming TRAP-positive osteoclasts was assessed histomorphometrically using images (×10) at the light microscopical level of two areas per well.
Resorption assay
In an analogous approach, RAW cells were cultured on calcium phosphate-coated slides (BD Biosciences, Heidelberg, Germany). After termination of culture, all remaining cells were lysed using 1 N NaOH and counter stained according to the von Kossa protocol [24]. Two images were captured per well at ×10 magnification and the resorbed area was quantified as a function of total area.
Co-culture of PDL cells and monocytes/macrophages and PTH(1-34) administration
To test the hypothesis that intermittent PTH(1-34) will modify the mRNA expression of RANKL bound to the PDL cell membrane and of OPG and, consequently, affect the differentiation of multinucleated, osteoclastic cells, fourth passage PDL cells (30,000/well) were co-cultured with RAW 264.7 cells (30,000/well) for 9 days and stimulated with 10−12 M PTH(1-34) (Sigma Aldrich, Germany) for 0, 1, and 24 h within a 48-h incubation cycle. For the remaining time, experimental media were replaced by tissue culture media without PTH(1-34). These cycles were carried out three times resulting in an experimental period of 6 days. Ethanol-treated cultures for each treatment group served as vehicle controls. No external RANKL was added to the medium.
To examine whether the response of PDL cells to intermittent PTH(1-34) depends on cellular maturation, the co-culture experiments were carried out using pre-confluent (~70%) and confluent PDL cells. The validity of the approach to use different stages of confluence as a model for cellular maturation was demonstrated by characterization of pre-confluent and confluent PDL cells for the expression of mesenchymal cell markers by means of microarray (SABiosciences, USA) and real-time PCR. For the microarray procedure, cDNA templates were mixed with a ready-to-use master mix provided with the kit and aliquoted into each well of the plates containing pre-dispensed gene-specific primer sets. Following real-time PCR, the relative gene expression was determined using the ∆∆Ct method.
At harvest, RNA was isolated using the RNeasy mini kit (Qiagen), reversely transcribed using the Amersham-Pharmacia-Biotech RT kit (Amersham Biosciences, Piscataway, USA) and the expression of specific marker genes typical of osteoclastic differentiation, namely TRAP, vitronectin-receptor, and cathepsin K, was quantified by means of real-time PCR as described below. The exact function of TRAP remains to be elucidated but it has been suggested to be secreted from the ruffled border of osteoclasts to dephosphorylate osteopontin and allow for osteoclast migration and further resorption to occur. TRAP−/− mice show mild osteopetrosis along with reduced osteoclast activity [25]. Cathepsin K is a cysteine protease that is predominantly expressed by osteoclasts and involved in the regulation of bone resorption by its ability to catabolize collagen, gelatin, and elastin [26]. Vitronectin-receptor is a cell-surface associated integrin that serves to anchor the cell to the extracellular matrix which is a prerequisite for osteoclasts to resorb bone [27]. The specificity of the primers for RAW cells was demonstrated by proving that the primers did not yield a product in PDL cells but only in RAW cells. The same procedure was carried out for OPG and RANKL with the primers demonstrated to be specific for human PDL cells but not murine RAW cells. To investigate the effect of the PTH(1-34) treatment on the resorptive activity of osteoclasts, the same co-culture experiments were carried out on calcium phosphate-coated slides as a substrate and resorption area was quantified as described above.
Real-time polymerase chain reaction
Optimal oligonucleotide primers were purchased from Qiagen, Hilden, Germany. Real-time PCR was performed on a light-cycler (Roche, Mannheim, Germany) using the light-cycler software 3.5.3. PCRs were carried out in a total volume of 20 μl in PCR master mix containing 10 μl SYBR Green, 2 μl of ×10 QuantiTect primer assay, 2 μl of the reverse transcription product filled up to 20 μl with RNase-free H2O. The amplifications were performed in duplicate for each sample and the optimal annealing temperature for all primers was 55°C for 40 cycles. The primer sequences used were as follows: cathepsin K sense 5’ CTC-CCT-CTC-GAT-CCT-ACA-GTA-ATG-A 3′, antisense 5′ TCA-GAG-TCA-ATG-CCT-CCG-TTC 3′ [28]; OPG sense 5′ AGA-GAA-AGC-GAT-GGT-GGA-TG 3′, antisense 5′ CGG-TGG-CAT-TAA-TAG-TGA-GAT-G 3′ [29]; RANKL sense 5′ CAC-TAT-TAA-TGC-CAC-CGA-C-3′, antisense 5′-GGG-TAT-GAG-AAC-TTG-GGA-TT 3′ [30]. Vitronectin-receptor (NM 008402) and TRAP (NM 007388) mRNA expression was quantified using a QuantiTect primer assay (Qiagen, Germany) yielding product sizes of 86 and 111 bp, respectively.
To normalize the content of cDNA samples, the comparative threshold (Ct) cycle method, consisting in the normalization of the number of target gene copies versus an endogenous reference gene such as GAPDH, was used. For comparison, the ∆∆Ct method was used. Through this method, a single sample, represented in our experiments by cells without any treatment, was designed as a calibrator and used for comparison of gene expression level of any unknown samples.
Intermittent PTH(1-34) exposure of PDL cells and collection of conditioned media
To address the question of whether additional factors produced by PDL cells and capable of affecting osteoclast biology might be altered by intermittent PTH(1-34) administration, fourth passage PDL cells were exposed to PTH(1-34) as described above. At harvest, the supernatant of each experimental group was collected, pooled, and assayed for the content of interleukin-1ß, tumor necrosis factor α, osteoprotegerin, receptor activator of nuclear factor kappaB ligand and PTH itself by means of ELISA according to the manufacturer's instructions (Immundiagnostik AG, Bensheim; Germany). The detection limits of the ELISAs were 2.0 pg/ml for interleukin-1ß, 10 pg/ml for TNF-α, 0.14 pmol/l for OPG, 1.56 pg/ml for RANKL, and 7.1 pg/ml for PTH. Likewise as in the co-culture experiments, the impact of the cellular maturation state for the cellular response to hormonal stimulation was examined using pre-confluent and confluent cultures as a model. To exclude the possibility that different levels of the target proteins in the conditioned medium simply result from different cell numbers in pre-confluent and confluent cells, protein data was analyzed as a function of cell number.
Exposure of RAW cells to the conditioned medium
To address the question of whether the conditioned media collected from the PTH(1-34)-challenged PDL cells would affect the differentiation of osteoclastic precursor cells towards multinucleated cells, RAW cells were plated in 24-well plates at a seeding density of 20,000 cells/well and cultured for 9 days in the presence of 50% regular RAW cell culture medium (containing 20 ng/ml M-CSF and 30 ng/ml RANKL as described above) and 50% conditioned medium from either pre-confluent or confluent PDL cells that had been exposed to PTH(1-34). At harvest, TRAP-staining was followed by histomorphometric quantification of the percentage of reactive cells over total cell number.
Analogous to the determination of osteoclast formation, the physiological relevance of a PTH(1-34)-induced release of local factors in the conditioned medium of PDL cells was determined with respect to the resorptive activity of RAW cells on calcium phosphate-coated slides as a substrate (BD Biosciences, Heidelberg, Germany). Fifty thousand cells per well were cultured for 5 days prior to a switch of the media towards the mixture of regular RAW cell culture medium and conditioned medium for another 8 days. The harvest and quantitative analysis of the resorbed area were conducted as described above.
Statistical analysis
For any given experiment, each data point represents the mean ± SEEM of six independent cultures. Variance and statistical significance of data were analyzed using Bonferroni's modification of Student's t test. P values <0.05 were considered to be significant. Each experiment was carried out twice and analyzed separately, and both sets of experiments yielded comparable results. Only one set of results from the two sets of experiments is presented.
Results
First, the results showed that RAW cells differentiated into multinucleated cells that stained positively for TRAP identifying them as osteoclasts when cultured in the presence of an osteoclast-inducing medium (Fig. 1a). When RAW cells were co-cultured with human PDL cells without the addition of any osteoclast-inducing factors to the medium, TRAP-positive multinucleated cells developed as well (Fig. 2b). The mRNA expression of the osteoclast-specific marker genes TRAP, cathepsin K, and vitronectin-receptor correlated with the culture period and increased with time (Fig. 1c). As a next step, RAW cells were cultured in the presence of various concentrations of RANKL and osteoprotegerin to prove the impact of these factors on the differentiation process of osteoclastic precursor cells. As expected, there was an increasing number of TRAP-positive cells with increasing concentrations of RANKL (Fig. 1d), while the opposite effect was observed for osteoprotegerin added to the medium (Fig. 1e).
a Positive staining for tartrate-resistent acid phosphatase in a monoculture of RAW 264.7 cells after 9 days in culture; magnification ×20. b Co-culturing of human PDL cells and RAW cells for 9 days without the addition of any RANKL to the medium. Despite of any exogenous RANKL, TRAP-positive osteoclasts developed indicating that the membrane-bound RANKL of PDL cells is sufficient to induce osteoclastogenesis. Multinucleated, osteoclastic cells (open arrowheads), PDL cells (arrows); magnification ×10. c When RAW cells were cultured in the presence of medium that contained RANKL, osteoclastic differentiation was promoted as evidenced by a culture-time-dependent increase of the mRNA expression for TRAP, cathepsin K, and vitronectin. Transcriptional changes were determined by real-time PCR. For comparison, the endogenous reference gene GAPDH was also quantified. Results were calculated according to the ΔΔCt method. d Demonstrates the impact of RANKL and OPG on the differentiation of osteoclast-like cells from RAW 264.7 precursors. Histomorphometric assessment of 12 cultures per experimental group revealed an increasing number of TRAP-positive cells when increasing concentrations of RANKL ranging from 0 to 30 ng/ml were added to the cell culture medium for 9 days. Analogous, the role of osteoprotegerin in hampering osteoclastic differentiation is depicted. e RANKL exerted a concentration-dependent effect on the resorptive activity of osteoclast-like cells. Increasing concentrations of RANKL were associated with enhanced number of resorption lacunae on calcium phosphate-coated slides as evidenced by von Kossa staining and subsequent histomorphometric quantification. In contrast, OPG inhibited osteoclastic resorption in a concentration-dependent manner
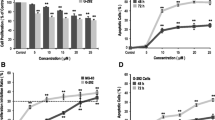
a Mesenchymal marker gene expression by confluent fourth passage PDL cell cultures normalized to the expression levels in pre-confluent cells. Pre-confluent (~70%) and confluent cells were characterized by the use of a microarray and differences were further quantified by real-time PCR using cDNA that was reversely transcribed from pooled RNA of six different donors. b Inhibitory effect of intermittent PTH(1-34) on the differentiation of osteoclastic cells in a co-culture model of pre-confluent PDL cells and RAW cells. An intermittent PTH(1-34) administration for 24 h/cycle induced a significant reduction of osteoclast-specific marker genes as determined by real-time PCR after 9 days of co-culture. Parallel to these data, the resorptive activity of the osteoclasts was inhibited by such intermittent PTH(1-34) treatment regimen as well. The co-culture of PDL cells and RAW cells for 9 days on calcium phosphate-coated slides resulted in a significant reduction of the resorbed area when cells PTH(1-34) was added intermittently to the medium (c). These observations correlated with changes of OPG and RANKL mRNA expression, which mostly remained at the sub-significant level, but displayed a trend towards an increase of the OPG/RANKL ratio (d). The experiments were performed twice, both sets of experiments yielded comparable results. Each value is the mean + SEM for six independent cultures. (asterisk) P < 0.05, experimental group vs. vehicle-treated control (vehicle); (black circle) P < 0.05, vehicle-treated control vs. specimen incubated without cells
PDL cell characterization at different stages of confluence
Confluent PDL cells exhibited an up to 26-fold higher expression of the marker genes typical of cells of mesenchymal origin, such as BMP-2 and -4 and their receptors BMPR-1a, -1b, and -2, alkaline phosphatase activity, osteocalcin, TGF-ß, PTH receptor, and collagen X (Fig. 2a).
Co-culture experiments
Co-culturing of pre-confluent PDL cells and RAW cells with a simultaneous intermittent PTH(1-34) exposure led to a decrease of mRNA expression for TRAP, cathepsin K, and vitronectin-receptor which displayed just a trend when PTH(1-34) was administered for 1 h/cycle but proved statistically significant for the 24 h/cycle treatment for all three marker genes (Fig. 2b). Paralleling these data, the resorptive activity of osteoclasts was down-regulated by intermittent PTH(1-34) in the same co-culture model as evidenced by a smaller area of substrate resorption in the PTH(1-34) treated groups (−62% to −77%; Fig. 2c). The OPG/RANKL mRNA expression ratio in PDL cells increased slightly with PTH(1-34) challenge of the co-culture (Fig. 2d).
When confluent PDL cells were co-cultured with RAW cells, the mRNA expression of the chosen osteoclastic marker genes increased significantly when the cultures were exposed to PTH(1-34) for 24 h/cycle (Fig. 3a). Likewise, the resorptive activity of the osteoclasts was enhanced in the presence of intermittent PTH(1-34) for 1 h/cycle (+60%) and this effect was even more pronounced when PTH(1-34) was administered for 24 h/cycle (+154%; Fig. 3b). The OPG mRNA expression was reduced by intermittent PTH(1-34) for 24 h/cycle as opposed to an increase of RANKL transcription by the same treatment regimen. Consequently, the OPG/RANKL showed a significant reduction (Fig. 3c).
a PTH(1-34) induction of osteoclastic differentiation in a co-culture model of confluent PDL cells and RAW cells. An intermittent PTH(1-34) administration for 24 h/cycle induced an increased mRNA expression for TRAP, cathepsin K, and vitronectin-receptor as determined by real-time PCR after 9 days of co-culture. Parallel to these data, the resorptive activity of the osteoclasts was enhanced 2.5-fold by such intermittent PTH(1-34) treatment regimen as well. The co-culture of PDL cells and RAW cells for 9 days on calcium phosphate-coated slides resulted in a significant increase of the resorbed area when cells PTH(1-34) was added intermittently to the medium (b). Intermittent PTH(1-34) for 24 per cycle reduced OPG mRNA expression significantly, whereas RANKL transcription was stimulated resulting in a reduction of the OPG/RANKL ratio (c). The experiments were performed twice, both sets of experiments yielded comparable results. Each value is the mean + SEM for six independent cultures. (asterisk) P < 0.05, experimental group vs. vehicle-treated control (vehicle); (number sign) P < 0.05, 24 h/cycle PTH(1-34) vs. 1 h/cycle PTH(1-34); (black circle) P < 0.05, vehicle-treated control vs. specimen incubated without cells
Experiments with conditioned medium obtained from PTH(1-34)-stimulated PDL cells
When RAW cells were cultured with conditioned medium of less mature, pre-confluent PDL cells that had been stimulated with PTH(1-34) intermittently, the number of differentiating TRAP-positive cells reduced significantly by up to 50%. In contrast, the conditioned medium of confluent PDL cells stimulated the differentiation of TRAP-positive cells significantly (Figs. 4a, b). This impression that was obtained by visual inspection of the staining results was validated by histomorphometric quantification and corroborated by statistical analysis (Fig. 4c).
Inhibitory effect of the conditioned medium of PTH(1-34)-stimulated pre-confluent human PDL cells on the differentiation of RAW 264.7 cells (TRAP staining; magnification ×10). The opposite was observed for the influence of the conditioned medium obtained from PTH(1-34)-challenged confluent PDL cells (b). Such medium supported osteoclastic differentiation (TRAP staining; magnification ×10). c The bar graph shows the results of the histomorphometric quantification of 12 cultures per experimental group. Data are from one of two separate experiments, which yielded comparable results. Each value is the mean + SEM for 12 independent cultures. (asterisk) P < 0.05, experimental group vs. vehicle-treated control (vehicle); (number sign) P < 0.05, experimental group vs. group exposed to the conditioned medium of PDL cells challenged with PTH(1-34) for 1 h/cycle
Paralleling these data, the conditioned medium obtained from PTH(1-34) exposed pre-confluent PDL cells reduced the resorbed substrate area significantly (Fig. 5a), while the conditioned medium collected from PTH(1-34)-treated, more mature PDL cells resulted in an enhanced loss of substrate area (Fig. 5b). The histomorphometrical quantification revealed that these changes were statistically significant (Fig. 5c).
Effects of the conditioned medium of PTH(1-34)-stimulated pre-confluent and confluent human PDL cells on the resorptive activity of osteoclast-like cells. Culturing RAW 264.7 cells in the presence of the conditioned medium of pre-confluent PDL cells inhibited osteoclastic resorption (a); magnification ×10. In contrast, the conditioned medium collected from PTH(1-34)-pretreated confluent PDL cells led to extended resorption areas on the substrate (b); magnification ×10. Histomorphometric quantification of resorption areas on the substrate yielded distinct differences between the influences of both conditioned media (c). Data are from one of two separate experiments, which yielded comparable results. Each value is the mean + SEM for 12 independent cultures. (asterisk) P < 0.05, experimental group vs. vehicle-treated control (vehicle); (number sign) P < 0.05, experimental group vs. group exposed to the conditioned medium of PDL cells challenged with PTH(1-34) for 1 h/cycle
Assaying of the conditioned media of PDL cells for factors that potentially affect osteoclast biology revealed that intermittent PTH(1-34) exposure of PDL cells elicited maturation-state dependent changes in osteoprotegerin production. In pre-confluent PDL cells, pulsatile PTH(1-34) treatment induced an increase of osteoprotegerin levels in the pooled conditioned media, whereas more mature cells responded with a reduction of osteoprotegerin production to such treatment regimen (Fig. 6). RANKL protein, bioactive PTH, interleukin-1ß, and tumor necrosis factor-alpha protein were not detectable in the conditioned medium with the assays applied; neither in the medium of pre-confluent PDL cells nor in the medium of confluent PDL cells (data not shown).
Time-dependent reduction in osteoprotegerin protein levels in the conditioned medium of confluent PDL cells exposed to intermittent PTH(1-34) challenge of confluent PDL cells. In contrast, there was an increase in osteoprotegerin protein production following intermittent PTH(1-34) challenge of pre-confluent cells. PDL cells were treated intermittently with 10−12 M PTH(1-34) for 0, 1, or 24 h during three cycles of 48 h each. Data are from one of two separate experiments, which yielded comparable results. Each value is the mean + SEM for six independent cultures. (asterisk) P < 0.05, experimental group vs. vehicle-treated control; (number sign) P < 0.05, experimental group vs. group exposed to PTH(1-34) for 1 h/cycle
Discussion
This study demonstrated that PTH(1-34) is capable of modifying the expression and release of factors by PDL cells which then interfere with the differentiation and activity of osteoclasts identifying PDL cells as competent regulators of bone remodeling. The PDL cell response was dependent on the cellular maturation state and associated with changes of OPG and RANKL mRNA expression and protein production. These mechanisms were shown in a co-culture model of PDL cells and RAW cells as well as in experiments using the conditioned medium of PTH(1-34)-pretreated PDL cells to culture RAW cells with.
Precursory to a further discussion of the results, two methodological considerations have to be addressed. As a model system for developing osteoclasts, RAW 264.7 cells were employed in this study. Upon treatment with RANKL, these cells stain positively for TRAP within 1 day and start to fuse after 4 to 5 days to generate multinucleated cells, a hallmark of fully differentiated osteoclasts [31]. RAW 264.7 cells serve as a model for osteoclasts in multiple contemporary studies dealing with osteoclast physiology or RANK/RANKL/OPG interaction [32–34]. Although RAW 264.7 cells are of murine origin which might be considered a certain drawback of the study design, they represent a well-established and reproducible model and the cells responded robustly to human OPG and RANKL (Fig. 1d, e) which legitimates the experimental approach of co-culturing human PDL cells with murine monocytes/macrophages. The characterization of osteoclasts by TRAP staining then again is accepted as a method to characterize these cells and also adopted in recent reviews on osteoclast biology [3]. Although used as a standard marker of osteoclasts, it has to be kept in mind that TRAP activity might also be present in other cells including macrophage polykaryons generated in murine bone marrow culture [35] as well as in human bone marrow macrophages in pathological states [36].
The experimental model we used to examine the role of cellular maturation, namely pre-confluent and confluent PDL cells, is based on our previous observation that the degree of confluence of PDL cells correlates well with the expression of marker genes typical of mesenchymal cells (data not shown) and also with osteoblastic differentiation markers as evidenced by lower levels of RUNX2, alkaline phosphatase specific activity, and osteocalcin expression in pre-confluent cells as opposed to higher levels in confluent cells [23]. These data support our experimental approach and justify considering pre-confluent cells to be less mature and confluent cells to be more mature. This model of cellular maturation is well established in the literature and was used in several studies examining PTH effects on osteoblasts [37–39].
In our co-culture model, TRAP-positive, multinucleated osteoclasts developed in the absence of any exogenous osteotropic factors indicating that molecules expressed by PDL cells might have influenced osteoclastic differentiation. In support to this, Uchiyama et al. recently presented a co-culture model of human PDL cells and CD14+ monocytes derived from human peripheral blood. Using this model, the authors demonstrated the ability of human PDL cells to induce osteoclastogenesis and proved an involvement of the RANK/RANKL/OPG-system in its regulation [40]. These results are in line with our observation that PDL cells constitutively express both OPG and RANKL mRNA and that an addition of either factor to the cell culture medium influenced RAW cell differentiation in a concentration-dependent manner. Therefore, the constitutive expression levels of OPG and RANKL by our PDL cells seemed to be sufficient to interfere with osteoclastogenesis. Intermittent PTH(1-34) affected the OPG/RANKL ratio in PDL cells significantly suggesting a potential mechanism by which the hormone modifies the PDL cell microenvironment and mediates its anabolic effect in periodontal repair processes. This conclusion is corroborated by Fukushima et al. who reported that human PDL cells supported mouse osteoclastic differentiation via RANK-RANKL signaling in co-cultures with mouse spleen cells in the presence of PTHrP [18]. Time course experiments for the PTH effect on OPG mRNA expression in the rat femur metaphysis found PTH to inhibit the transcription of OPG significantly within 1 h with a decline of the magnitude of the effect thereafter [41]. These findings suggest that the PTH effect on the OPG/RANKL ratio we observed in PDL cells might have been even more pronounced if we had analyzed it earlier than at the end of the third incubation cycle.
Recently, osteoclasts were demonstrated to express receptors for PTH and, consequently, it was proposed that PTH might exert a direct effect on osteoclasts [42]. To rule out this possibility in our experiments, we exposed RAW cells to intermittent PTH(1-34) for three cycles and did not observe any effect of such treatment regimen on the number and resorptive activity of those cells (data not shown).
The PTH(1-34)-induced effects on RAW cell differentiation and activity reflect differences in PDL cell maturation. When less mature PDL cells were co-cultured with RAW cells, an intermittent PTH(1-34) administration resulted in an inhibition of osteoclast differentiation and function, as opposed to a stimulation when more PDL cells were co-cultured. These results are in agreement with previous reports showing that cell density and the differentiation state of the cells are crucial to the cellular response to PTH. In these studies, osteoblasts at different states of confluence were employed as well as growth zone and resting zone chondrocytes [43, 44].
To address the question of whether factors other than PDL cell membrane-bound molecules but factors released by PDL cells in response to the PTH stimulus interfere with osteoclast biology, we performed the experiments with the conditioned medium from PTH(1-34)-treated PDL cells. Since the conditioned medium modified osteoclast function significantly as well, the present data indicate that PDL cells regulate the local microenvironment not only by membrane-bound molecules but also by secreting competent factors in response to the hormonal stimulation.
The regulatory effects of the conditioned media of PDL cells on RAW cell differentiation and activity were mainly associated with PTH(1-34)-induced changes in osteoprotegerin levels, suggesting that the osteoprotegerin secreted was active and able to counteract RANKL effects. Due to the key regulatory role of osteoprotegerin in the regulation of osteoclastic differentiation and activity [45–47], the observed changes regarding these two parameters might very well be attributed to the PTH(1-34)-induced modification of osteoprotegerin production by PDL cells. The significant increase in OPG protein observed in pre-confluent cells following PTH(1-34) exposure might result in favoring bone formation over bone resorption in vivo, whereas the PTH(1-34)-induced decrease of OPG in mature PDL cells suggests that less OPG protein would eventually be available at potential sites of osteoclast formation.
It is important to consider the way the conditioned medium of PTH(1-34)-treated PDL cells was collected and applied to the RAW cells in this investigation. We pooled the medium of each culture belonging to the same experimental group and then assayed for OPG and RANKL. This policy might have enhanced or blurred the measurement of the PTH(1-34) effect on OPG production. In fact, changes in OPG production in response to PTH(1-34) were a little less pronounced in the present investigation as compared to recent studies by our group where the supernatant of each culture had been assayed individually [13, 23]. Nevertheless, modifications in OPG expression remained distinct enough to alter osteoclastic differentiation and activity significantly. Despite of reports in the literature and our own observation that RANKL is expressed by PDL cells [4, 48, 49], the protein was not detectable in the conditioned medium by means of ELISA which might be related to the fact that any changes that may have occurred were below the threshold of detection of the immunoassay kit that was used. Based on this consideration and due to the fact that the amount of secreted RANKL is much less than the membrane-bound counterpart in the co-culture experiments, we added a moderate concentration of exogenous RANKL in the experiments with the conditioned medium but still the PTH(1-34)-induced PDL cell secretion of both OPG and RANKL in the conditioned medium were significant enough to modify osteoclast biology.
However, other factors in the conditioned medium related to the regulation of osteoclasts might have also been altered by PTH(1-34). With the absence of interleukin-1ß and tumor necrosis factor-α in our cultures, two potential candidate genes were excluded. Additionally, we assayed the conditioned medium of PDL cells for PTH to exclude a direct effect of the hormone on the osteoclasts and were unable to detect significant levels of bioactive PTH (data not shown). Therefore, the changes of osteoclastic differentiation and activity induced by the conditioned medium cannot be attributed to a direct effect of PTH.
New concepts of the pathogenesis of periodontitis have implicated inflammation triggered by host immune response to periodontal biofilm microorganisms in disease. Host response to bacteria involves activation of T and B cells in the inflammatory infiltrate which bear abundant RANKL that promotes osteoclastic bone resorption. Periodontal tissue destruction can be ameliorated by immunobiological interference with immune cell RANKL expression or function. The new disease concepts provide a foundation to build biological approaches to target RANKL production in periodontal lesions [50]. Our study picks up on this point and the data supports the promising policy, although we did not target RANKL production by immune competent cells but sought to modify the OPG/RANKL ratio by hormonal stimulation of cells that resemble constitutive components of the PDL.
Besides the implications in the regulation of the recruitment, differentiation, and resorptive activity of osteoclasts, our findings might also be of importance for the reparative capabilities of PDL cells. Together with previous reports of PTH-induced alterations of the phenotypic expression and survival of PDL cells [22, 23, 51], the present results add further support to the idea that PTH(1-34) might also modulate the local factor production by PDL cells which then in turn, based upon their osteoblastic character [17] and their ability to mineralize their extracellular matrix [52], might contribute to the regulation of periodontal repair processes.
References
Bostanci N, Ilgenli T, Emingil G et al (2007) Gingival crevicular fluid levels of RANKL and OPG in periodontal diseases: implications of their relative ratio. J Clin Periodontol 34:370–376
Nagasawa T, Kiji M, Yashiro R et al (2000) (2007) Roles of receptor activator of nuclear factor-kappaB ligand (RANKL) and osteoprotegerin in periodontal health and disease. Periodontol 43:65–84
Lu HK, Chen YL, Chang HC et al (2006) Identification of the osteoprotegerin/receptor activator of nuclear factor-kappa B ligand system in gingival crevicular fluid and tissue of patients with chronic periodontitis. J Periodontal Res 41:354–360
Belibasakis G, Bostanci N, Hashim A et al (2007) Regulation of RANKL and OPG gene expression in human gingival fibroblasts and periodontal ligament cells by Porphyromonas gingivalis: a putative role of the Arg-gingipains. Microb Pathog 43:46–53
Yamamoto T, Kita M, Oseko F et al (2006) Cytokine production in human periodontal ligament cells stimulated with Porphyromonas gingivalis. J Periodontal Res 41:554–559
Lappin DF, Sherrabeh S, Jenkins WM et al (2007) Effect of smoking on serum RANKL and OPG in sex, age and clinically matched supportive-therapy periodontitis patients. J Clin Periodontol 34:271–277
Nishijima Y, Yamaguchi M, Kojima T et al (2006) Levels of RANKL and OPG in gingival crevicular fluid during orthodontic tooth movement and effect of compression force on releases from periodontal ligament cells in vitro. Orthod Craniofac Res 9:63–70
Yamaguchi M, Aihara N, Kojima T et al (2006) RANKL increase in compressed periodontal ligament cells from root resorption. J Dent Res 85:751–756
Kanzaki H, Chiba M, Arai K et al (2006) Local RANKL gene transfer to the periodontal tissue accelerates orthodontic tooth movement. Gene Ther 13:678–685
Kanzaki H, Chiba M, Takahashi I et al (2004) Local OPG gene transfer to periodontal tissue inhibits orthodontic tooth movement. J Dent Res 83:920–925
Pinkerton MN, Wescott DC, Gaffey BJ et al (2008) Cultured human periodontal ligament cells constitutively express multiple osteotropic cytokines and growth factors, several of which are responsive to mechanical deformation. J Periodontal Res 43:343–351
Kanzaki H, Chiba M, Shimizu Y et al (2001) Dual regulation of osteoclast differentiation by periodontal ligament cells through RANKL stimulation and OPG inhibition. J Dent Res 80:887–891
Lossdorfer S, Gotz W, Jager A (2005) PTH(1-34) affects osteoprotegerin production in human PDL cells in vitro. J Dent Res 84:634–638
Hasegawa T, Yoshimura Y, Kikuiri T et al (2002) Expression of receptor activator of NF-kappa B ligand and osteoprotegerin in culture of human periodontal ligament cells. J Periodontal Res 37:405–411
Isaka J, Ohazama A, Kobayashi M et al (2001) Participation of periodontal ligament cells with regeneration of alveolar bone. J Periodontol 72:314–323
Chou AM, Sae-Lim V, Lim T et al (2002) Culturing and characterization of human periodontal ligament fibroblasts-a preliminary study. Mater Sci Eng 20:77–83
Basdra EK, Komposch G (1997) Osteoblast-like properties of human periodontal l igament cells: an in vitro analysis. Eur J Orthod 19:615–621
Fukushima H, Jimi E, Kajiya H et al (2005) Parathyroid-hormone-related protein induces expression of receptor activator of NF-{kappa}B ligand in human periodontal ligament cells via a cAMP/protein kinase A-independent pathway. J Dent Res 84:329–334
Zhang D, Yang YQ, Li XT et al (2004) The expression of osteoprotegerin and the receptor activator of nuclear factor kappa B ligand in human periodontal ligament cells cultured with and without 1alpha, 25-dihydroxyvitamin D3. Arch Oral Biol 49:71–76
Yang ZJ, Cheng V, Barnes S et al (1997) Pulsatile parathyroid hormone (PTH) treatment increases bone formation in vitro in fetal rat calvarial cell (FRCC) culture system. J Bone Miner Res 12(Suppl 1):S317
Sone T, Fukunaga M, Ono S et al (1995) A small dose of human parathyroid hormone(1-34) increased bone mass in the lumbar vertebrae in patients with senile osteoporosis. Miner Electrolyte Metab 21:232–235
Lossdorfer S, Gotz W, Jager A (2006) Parathyroid hormone modifies human periodontal ligament cell proliferation and survival in vitro. J Periodontal Res 41:519–526
Lossdorfer S, Stier S, Gotz W et al (2006) Maturation-state dependent response of human periodontal ligament cells to an intermittent parathyroid hormone exposure in vitro. J Periodontal Res 41:62–72
Bonewald LF, Harris SE, Rosser J et al (2003) Von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. Calcif Tissue Int 72:537–547
Hayman AR, Jones SJ, Boyde A et al (1996) Mice lacking tartrate-resistant acid phosphatase (Acp 5) have disrupted endochondral ossification and mild osteopetrosis. Development 122:3151–3162
Inaoka T, Bilbe G, Ishibashi O et al (1995) Molecular cloning of human cDNA for cathepsin K: novel cysteine proteinase predominantly expressed in bone. Biochem Biophys Res Commun 206:89–96
Felding-Habermann B, Cheresh DA (1993) Vitronectin and its receptors. Curr Opin Cell Biol 5:864–868
Andrade I Jr, Taddei SR, Garlet GP et al (2009) CCR5 down-regulates osteoclast function in orthodontic tooth movement. J Dent Res 88:1037–1041
Lossdorfer S, Schwartz Z, Wang L et al (2004) Microrough implant surface topographies increase osteogenesis by reducing osteoclast formation and activity. J Biomed Mater Res A 70:361–369
Kwan Tat S, Amiable N, Pelletier JP et al (2009) Modulation of OPG, RANK and RANKL by human chondrocytes and their implication during osteoarthritis. Rheumatology (Oxford) 48:1482–1490
Czupalla C, Mansukoski H, Pursche T et al (2005) Comparative study of protein and mRNA expression during osteoclastogenesis. Proteomics 5:3868–3875
Li X, Qin L, Bergenstock M et al (2007) Parathyroid hormone stimulates osteoblastic expression of MCP-1 to recruit and increase the fusion of pre/osteoclasts. J Biol Chem 282:33098–33106
Mozar A, Haren N, Chausseraud M et al (2008) High extracellular inorganic phosphate oncentration inhibits RANK-RANKL signaling in osteoclast-like cells. J Cell Physiol 215:47–54
Hsu H, Lacey D, Dunstan C et al (1999) Tumor necrosis factor receptor family member RANKL mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA 96:3540–3545
Suda T, Takahashi N, Martin T (1992) Modulation of osteoclast differentiation. Endocr Rev 13:66–80
Bianco P, Costantini M, Dearden L et al (1987) Expression of tartrate-resistant acid phosphatase in bone marrow macrophages. Basic Appl Histochem 31:433–440
Chen HL, Demiralp B, Schneider A et al (2002) Parathyroid hormone and parathyroid hormone-related protein exert both pro- and anti-apoptotic effects in mesenchymal cells. J Biol Chem 277:19374–19381
Jackson R, Kumarasuriyar A, Nurcombe V et al (2006) Long-term loading inhibits ERK1/2 phosphorylation and increases FGFR3 expression in MC3T3-E1 osteoblast cells. J Cell Physiol 209:894–904
Wang Y, Liu Y, Rowe D (2007) Effects of transient PTH on early proliferation, apoptosis, and subsequent differentiation of osteoblasts in primary osteoblast cultures. Am J Physiol Endocrinol Metab 292:E594–E603
Uchiyama M, Nakamichi Y, Nakamura M et al (2009) Dental pulp and periodontal ligament cells support osteoclastic differentiation. J Dent Res 88:609–614
Onyia JE, Miles RR, Yang X et al (2000) In vivo demonstration that human parathyroid hormone 1–38 inhibits the expression of osteoprotegerin in bone with the kinetics of an immediate early gene. J Bone Miner Res 15:863–871
Dempster D, Hughes-Begos C, Plavetic-Chee K et al (2005) Normal human osteoclasts formed from peripheral blood monocytes express PTH type 1 receptors and are stimulated by PTH in the absence of osteoblasts. J Cell Biochem 95:139–148
Isogai Y, Akatsu T, Ishizuya T et al (1996) Parathyroid hormone regulates osteoblast differentiation positively or negatively depending on the differentiation stages. J Bone Miner Res 11:1384–1393
Schwartz Z, Semba S, Graves D et al (1997) Rapid and long-term effects of PTH(1-34) on growth plate chondrocytes are mediated through two different pathways in a cell-maturation-dependent manner. Bone 21:249–259
Leibbrandt A, Penninger JM (2009) RANKL/RANK as key factors for osteoclast development and bone loss in arthropathies. Adv Exp Med Biol 649:100–113
Wright HL, McCarthy HS, Middleton J et al (2009) RANK, RANKL and osteoprotegerin in bone biology and disease. Curr Rev Musculoskelet Med 2:56–64
Kobayashi Y, Udagawa N, Takahashi N (2009) Action of RANKL and OPG for osteoclastogenesis. Crit Rev Eukaryot Gene Expr 19:61–72
Tsuji K, Uno K, Zhang GX et al (2004) Periodontal ligament cells under intermittent tensile stress regulate mRNA expression of osteoprotegerin and tissue inhibitor of matrix metalloprotease-1 and -2. J Bone Miner Metab 22:94–103
Kim T, Handa A, Iida J et al (2007) RANKL expression in rat periodontal ligament subjected to a continuous orthodontic force. Arch Oral Biol 52:244–250
Taubman MA, Kawai T, Han X (2007) The new concept of periodontal disease pathogenesis requires new and novel therapeutic strategies. J Clin Periodontol 34:367–369
Lossdorfer S, Gotz W, Rath-Deschner B et al (2006) Parathyroid hormone(1-34) mediates proliferative and apoptotic signaling in human periodontal ligament cells in vitro via protein kinase C-dependent and protein kinase A-dependent pathways. Cell Tissue Res 325:469–479
Nohutcu RM, McCauley LK, Koh AJ et al (1997) Expression of extracellular matrix proteins in human periodontal ligament cells during mineralization in vitro. J Periodontol 68:320–327
Acknowledgements
The authors thank K. Reifenrath for expert technical assistance and C. Maelicke for her help in preparing the manuscript. This research was supported by a research grant from the Deutsche Forschungsgemeinschaft (DFG, KFO 208; LO-1181/2-1). The authors do not have any conflict of interest.
Conflict of interest
The authors do not have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lossdörfer, S., Götz, W. & Jäger, A. PTH(1-34)-induced changes in RANKL and OPG expression by human PDL cells modify osteoclast biology in a co-culture model with RAW 264.7 cells. Clin Oral Invest 15, 941–952 (2011). https://doi.org/10.1007/s00784-010-0456-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-010-0456-0