Abstract
Introduction
In bone tissue, bone resorption by osteoclasts and bone formation by osteoblasts are repeated continuously. Osteoclasts are multinucleated cells that derive from monocyte-/macrophage-lineage cells and resorb bone. In contrast, osteoblasts mediate osteoclastogenesis by expressing receptor activator of nuclear factor-kappa B ligand (RANKL), which is expressed as a membrane-associated cytokine. Osteoprotegerin (OPG) is a soluble RANKL decoy receptor that is predominantly produced by osteoblasts and which prevents osteoclast formation and osteoclastic bone resorption by inhibiting the RANKL–RANKL receptor interaction.
Materials and Methods
In this review, we would like to summarize our experimental results on signal transduction that regulates the expression of RANKL and OPG.
Results
Using OPG gene-deficient mice, we have demonstrated that OPG and sclerostin produced by osteocytes play an important role in the maintenance of cortical and alveolar bone. In addition, it was shown that osteoclast-derived leukemia inhibitory factor (LIF) reduces the expression of sclerostin in osteocytes and promotes bone formation. WP9QY (W9) is a peptide that was designed to be structurally similar to one of the cysteine-rich TNF-receptortype-I domains. Addition of the W9 peptide to bone marrow culture simultaneously inhibited osteoclast differentiation and stimulated osteoblastic cell proliferation. An anti-sialic acid-binding immunoglobulin-like lectin 15 (Siglec-15) antibody inhibited multinucleated osteoclast formation induced by RANKL and macrophage colony-stimulating factor (M-CSF). Pit-forming activity of osteoclasts was also inhibited by the anti-Siglec-15 antibody. In addition, anti-Siglec-15 antibody treatment stimulated the appearance of osteoblasts in cultures of mouse bone marrow cells in the presence of RANKL and M-CSF.
Conclusions
Bone mass loss depends on the RANK–RANKL–OPG system, which is a major regulatory system of osteoclast differentiation induction, activation, and survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone is continuously destroyed by osteoclasts and reformed by osteoblasts to maintain bone volume and calcium homeostasis throughout the life span of vertebrates [1]. Osteoclasts are multinucleated cells that derive from monocyte/macrophage-lineage cells and resorb bone [2]. In contrast, osteoblasts mediate osteoclastogenesis [3] by producing macrophage colony-stimulating factor (M-CSF), which is essential for osteoclast differentiation [4]. Receptor activator of nuclear factor-kappa B (NF-κB) ligand (RANKL) is another cytokine that is essential for osteoclastogenesis, and it is expressed by osteoblasts as a membrane-associated cytokine [5]. Osteoclast precursors express RANK (a RANKL receptor), recognize RANKL expressed by osteoblasts via cell–cell interaction, and differentiate into osteoclasts in the presence of M-CSF [6]. Osteoprotegerin (OPG) is a soluble RANKL decoy receptor that is predominantly produced by osteoblasts [7, 8], which prevents osteoclast formation and osteoclastic bone resorption by inhibiting the RANKL–RANK interaction. In contrast, bone resorption-stimulating hormones and cytokines enhance RANKL expression in osteoblasts. Mature osteoclasts also express RANK and RANKL both support osteoclast survival and stimulate osteoclast bone-resorbing activity.
Inhibition of RANKL–RANK signaling in bone can increase bone mass by preventing osteoclastic bone resorption. RANKL- and RANK-deficient mice have been shown to exhibit severe osteopetrosis, accompanying lack of osteoclast differentiation [9, 10]. In contrast, OPG-deficient mice exhibit severe osteoporosis arising from enhanced adult-stage osteoclastogenesis [11, 12]. Accordingly, OPG and soluble RANK have been investigated as potential therapeutic targets, and an anti-human RANKL-antibody called denosumab has been employed in the clinical setting for the treatment of osteoporosis and cancer-related bone disorders [13].
Although bone formation is generally thought to be dynamically coupled to bone resorption, the mechanism(s) underlying this process have not been determined systematically in vivo or in vitro. Mice deficient in OPG have been shown to exhibit a high bone turnover rate [12, 14]. We reported that daily injection of OPG-deficient osteoporotic mice with bisphosphonate induced a sharp decrease in various bone formation-related parameters, indicative of suppressed osteoclastic bone resorption; however, the high serum RANKL concentration in these mice was unchanged [15]. The same study showed that although bone morphogenic protein 2 (BMP-2) implantation induced a high rate of bone turnover, it did not increase the rate of ectopic bone formation [16]. Together, these results suggested that osteoclastic bone resorption directly activates osteoblast function; however, serum RANKL levels appeared not to correlate with the coupling of these processes.
The classical mechanism of bone remodeling is that osteoclasts activate transforming growth factor-beta (TGF-β) in the bone matrix and activate osteoblasts. This TGF-β story is still being modified and evolving [17]. On the other hand, it has been reported that various molecules such as sphingosine-1-phosphate (S1P) [18], ephrinA2 [19], ephrinB2 [20], semaphorin 4D [21], platelet-derived growth factor (PDGF)-BB [22], Wnt10b [23], collagen triple-helix repeat-containing 1 (Cthrc1) [24], C3a [25], and cathepsin K [26] that are expressed and produced by osteoclasts are important in the bone coupling mechanism (Fig. 1). The importance of osteoblast-derived osteoclast differentiation suppressor semaphorin 3a [27] and OPG [14] in bone remodeling has been reported. In addition, the reverse signaling hypothesis that the osteoclast differentiation factor RANKL signal promotes osteoblastic bone formation has been proposed [28, 29] (Fig. 1). Analyzing the molecular mechanism of mechanical stress is very important in considering the mechanism of bone remodeling [30]. The relationship between the expression control mechanism of sclerostin produced by osteocytes and bone remodeling is also a future subject.
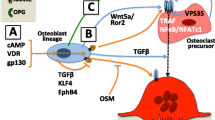
Reverse signaling from osteoclast to osteoblast activation. TGF-β released from the bone matrix acts directly on osteoclasts, promoting Wnt1 secretion, and acts on osteoblasts to promote bone formation. Osteoclasts produce various factors and act to promote osteogenesis in osteoblasts. Mature osteoclasts secrete exosomes that express RANK on the surface, bind to RANKL in osteoblasts (reverse signal), and activate the PI3K–Akt pathway, thereby increasing osteoblast activity. OPG produced by osteoblasts blocks excessive enhancement of bone resorption by binding to RANKL expressed by itself
In this review, we would like to summarize our experimental results on signal transduction that regulates the expression of RANKL and OPG.
Bone formation is coupled to resorption via suppression of sclerostin expression by osteoclasts
Sclerostin (encoded by the Sost gene), an antagonist of Wnt/β-catenin signaling, is secreted from osteocytes and inhibits bone formation [31, 32]. Sost-deficient mice exhibited increased bone mass [33]. The administration of an anti-sclerostin-neutralizing antibody has been shown to increase bone mass with increased bone formation [34]. The expression of sclerostin was reportedly suppressed by mechanical stimulation [35], parathyroid hormone [36], prostaglandin E2 (PGE2) [37], and IL-6 family members, such as oncostatin M (OSM) [38], leukemia inhibitory factor (LIF) [39], and cardiotrophin-1 (CT-1) [40]. OSM, LIF, and osteoclast-derived CT-1 promoted bone formation in vitro and in vivo [38,39,40,41]. Thus, it has been proposed that osteoclast-derived CT-1 as a coupling factor suppresses sclerostin expression in osteocytes to promote transitions from bone resorption to formation [42]. Furthermore, several studies in humans and mice demonstrated that the expression of sclerostin was decreased in conditions that enhanced bone resorption such as osteoporosis. TGF-β induced the expression of LIF in osteoclasts [43]. However, it remains to be clarified how bone resorption regulates the expression of sclerostin during bone remodeling. Here, we found that osteoclast-secreted factors, including LIF, suppress the expression of sclerostin, thereby promoting bone formation. Thus, osteoclast-derived LIF as well as CT-1 suppresses the expression of sclerostin to regulate bone remodeling.
Using OPG-deficient mice, in which bone formation is clearly coupled to bone resorption, we found that osteoclasts suppress the expression of sclerostin, a Wnt antagonist, thereby promoting bone formation [44]. Wnt/β-catenin signals were higher in OPG-deficient and RANKL-transgenic mice with a low level of sclerostin. Conditioned medium from osteoclast cultures suppressed sclerostin expression in UMR106 cells and osteocyte cultures [44]. In vitro experiments revealed that osteoclasts secreted LIF and inhibited sclerostin expression. Anti-RANKL antibodies, antiresorptive agents, suppressed LIF expression, and increased sclerostin expression, thereby reducing bone formation in OPG-deficient mice. Taken together, osteoclast-derived LIF regulates bone turnover through sclerostin expression (Fig. 2). Thus, LIF represents a target for improving the prolonged suppression of bone turnover by antiresorptive agents.
The W9 peptide directly stimulates osteoblast differentiation via RANKL signaling
We reported previously that OPG-deficient mouse-derived osteoblasts strongly support osteoclast formation when co-cultured with OPG-deficient bone-marrow hemopoietic cells, even in the absence of bone-resorbing factors [45]. In contrast, when OPG-deficient osteoblasts and hemopoietic cells were co-cultured, but direct contact between them was prevented, no osteoclasts were observed to form, even in the presence of 1α,25(OH)2D3 but not in the presence of sRANKL and M-CSF [45]. These findings suggested that OPG produced by osteoblasts is a physiologically important regulator of osteoclast differentiation. RANKL-deficient osteoblasts failed to induce osteoclast differentiation when co-cultured with wild-type (WT) bone-marrow hemopoietic cells, even in the presence of 1α,25(OH)2D3. Thus, it is likely that RANKL expressed by osteoblasts functions in a membrane-associated form during osteoclastogenesis [8].
W9 is a peptide that was designed to be structurally similar to one of the cysteine-rich TNF-receptor-type-I domains, and was demonstrated to bind to TNFα and block its activity [46]. W9 also binds RANKL and inhibits RANKL-induced osteoclast differentiation and function both in vitro and in vivo [47]. We examined the effects of treating mouse bone-marrow cell cultures, including osteoblastic stromal cells and osteoclast progenitors, with the W9 peptide in the presence of sRANKL and/or M-CSF. The results of the analysis showed that multinucleated osteoclasts formed in violet-stained tartrate-resistant acid phosphatase (TRAP)-positive cultures (Fig. 3a). Addition of W9 peptide to the bone-marrow culture simultaneously inhibited osteoclast differentiation, and stimulated purple-stained alkaline phosphatase (ALP)-positive osteoblastic cell proliferation, in a dose-dependent manner [28, 48]. Treatment of the cultures with a high concentration (200 μM) of W9 was found to stimulate the formation of typical osteoblastic-calcified nodules (Fig. 3) [28, 48].
Effects of W9 on the differentiation of osteoclasts and osteoblasts in mouse bone-marrow cultures and human peripheral blood mononuclear cell cultures. A Bone marrow cells were cultured in α-minimum essential medium supplemented with 10% fetal bovine serum, in the presence of sRANKL (100 ng/ml) and M-CSF (50 ng/ml), without or with W9 peptide (200 μM). After 7 days, the cells were fixed and then stained for TRAP and ALP as described. B Human peripheral blood mononuclear cell cultured in α-minimum essential medium supplemented with 10% fetal bovine serum, in the presence of sRANKL (100 ng/ml) and M-CSF (50 ng/ml), without or with W9 peptide (100 μM). After 14 days, the cells were fixed and then stained for TRAP
We used RANKL-deficient mouse-derived osteoblasts to evaluate whether osteoblastic differentiation is mediated by RANKL signaling in vitro. We found that RANKL-deficient osteoblasts exhibited weak ALP activity compared with WT osteoblasts, even in the presence of W9, parathyroid hormone, and/or BMP-2 [48]. In addition, RANKL-deficient osteoblasts displayed no supporting TRAP-positive osteoclast formation activity when co-cultured with WT bone-marrow hematopoietic cells in the presence of bone-resorbing factors [48]. Together, these results suggest that the RANKL–RANK signaling in osteoblasts may be essential for the dynamic regulation of bone formation and resorption.
In this experiment, we used primary cultured cells derived from newborn mouse calvaria. Notably, this type of culture contains mesenchymal cells other than osteoblasts. Therefore, it is not possible to exclude the possibility that W9 peptide may stimulate the differentiation of mesenchymal cells other than osteoblasts, resulting in the secretion of BMP-like soluble factors that may induce osteoblast differentiation via paracrine signaling. Therefore, further study is necessary to elucidate and verify the mechanism by which the W9 peptide stimulates osteoblast differentiation.
Osteoclasts and dendritic cells are derived from common progenitors, such as bone-marrow-derived macrophages. W9 strongly inhibited multinucleated osteoclast formation in human peripheral blood mononuclear cell cultures in the presence of RANKL and M-CSF (Fig. 3b). In contrast, W9 have no effect on dendritic cell differentiation in human peripheral blood mononuclear cell cultures in the presence of GM-CSF and IL-4. These results indicate that W9 inhibit human osteoclast formation but not dendritic cell differentiation.
Sialic acid-binding immunoglobulin-like lectin 15 plays important roles in the induction of both bone-resorbing activity of osteoclasts and osteoblast differentiation
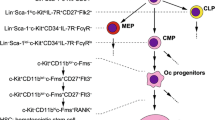
Analysis of RANKL-inducible genes revealed that NFATc1, a molecule belonging to the NFAT family, is a transcription factor whose expression is strongly induced by RANKL in osteoclasts [49, 50]. The NFAT family has been thought to be a transcription factor that plays an important role in activated T cells, but NFATc1 has been demonstrated to be a co-stimulatory signal essential for osteoclast differentiation at the biological level [51, 52]. Although calcium signaling is essential for osteoclast differentiation, RANK cannot directly activate calcium signaling. On the other hand, a study focusing on a molecule with a sequence called an immunoreceptor tyrosine-based activation motif (ITAM) that induces a calcium signal in immune system cells identified DNAX-activating protein 12 (DAP12) and Fc receptor common γ subunit (FcRγ). It was reported that osteoclast differentiation was impaired in double-deficient mice, resulting in severe osteopetrosis [53, 54]. This finding indicated that signals mediated by immunoglobulin-like receptors that associate with DAP12 and FcRγ are essential for osteoclast differentiation. That is, the existence of immunoglobulin-like receptors as new essential receptors in osteoclast differentiation was revealed through analysis of c-Fms, which is a receptor for M-CSF, and RANK, which is a receptor for RANKL (Figs. 4, 5).
Three signals that regulate osteoclast differentiation. To differentiate osteoclasts, in addition to the signals of RANKL and M-CSF expressed in osteoblasts, the signals of DAP12 and FcRγ with an ITAM motif are essential as co-stimulatory signals. Siglec-15, which binds to sialic acid, was identified as an immunoglobulin-like receptor that associates with DAP12. Siglec-15 expression is induced with osteoclast differentiation
Difference in action of bisphosphonate or RANKL neutralizing antibody and Siglec-15 neutralizing antibody on bone metabolism coupling mechanism. Bone resorption promoting factors and cytokines produced by cancer cells promote RANKL expression in osteoblasts and suppress OPG production. Siglec-15 neutralizing antibody increases bone mass without inhibiting osteogenic activity, unlike bisphosphonate administration
We and other groups reported sialic acid-binding immunoglobulin-like lectin 15 (Siglec-15) as a protein that regulates differentiation of osteoclasts [55,56,57,58,59]. The expression of Siglec-15 was increased with osteoclast formation in mouse bone-marrow cultures [55]. In Siglec-15-deficient mice, bone resorption marker was suppressed, but bone formation marker was unchanged or moderately increased, leading to increase in bone mass [58]. From the results of histology, it was indicated that osteoblast activity was significantly increased in anti-Siglec-15 Ab-treated mice [59]. These results suggested that bone formation is maintained when the function of Siglec-15 is suppressed.
Treatment of bone-marrow cell cultures with anti-Siglec-15 antibody (Siglec-15 Ab) inhibited TRAP-positive multinucleated cell formation induced by RANKL and M-CSF [55, 60]. However, anti-Siglec-15 Ab failed to suppress TRAP-positive mononuclear cell (mononuclear osteoclast) differentiation. In contrast, anti-Siglec-15 Ab treatment stimulated the appearance of ALP-positive osteoblasts in those cultures in the presence of RANKL and M-CSF. We then examined the effects of anti-Siglec-15 Ab on the appearance of osteoclast precursors, which expressed RANK and c-Fms but not TRAP, in mouse co-cultures of osteoblasts and bone-marrow cells. Anti-Siglec-15 Ab showed no effects on the appearance of osteoclast precursors in the co-culture. Osteoclasts prepared from mouse co-cultures were further cultured on dentin slices in the presence or absence of anti-Siglec-15 Ab. Pit-forming activity of osteoclasts was inhibited by anti-Siglec-15 Ab [60]. The actin rings in osteoclasts on dentin slices completely disappeared within 8 h in the presence of anti-Siglec-15 Ab. In contrast, treatment with alendronate for 1 h completely disrupted actin rings in mature osteoclasts. Treatment with anti-Siglec-15 Ab for 24 h decreased the number of multinucleated osteoclasts, but alendronate treatment did not. We next examined the effects of anti-Siglec-15 Ab on sclerostin expression in UMR106 rat osteosarcoma cells. Sclerostin is secreted from osteocytes and inhibits bone formation. We reported that conditioned medium from osteoclast cultures suppressed sclerostin expression in UMR106 cells. In vitro experiments revealed that osteoclasts secreted LIF, which in turn inhibited sclerostin expression. Both conditioned medium from osteoclasts treated with anti-Siglec 15Ab for 48 h and that not treated with anti-Siglec-15 Ab similarly inhibited sclerostin expression in UMR106 cells. Anti-Siglec-15 Ab did not inhibit LIF expression in osteoclasts (unpublished data). These results indicated the possibility that maintenance of LIF expression may be involved in promoting bone formation in osteoclasts treated with anti-Siglec-15 Ab. We showed previously that osteoblasts derived from Wnt5a-deficient mice had decreased ALP activity, and Wnt5a produced by osteoclasts acted on osteoblasts leading to the promotion of their differentiation. In this study, Wnt5a expression was increased in osteoclasts by treatment with anti-Siglec-15 Ab (unpublished data), suggesting that Wnt5a induced by anti-Siglec-15 Ab is involved in osteoblast differentiation. Our findings suggested that Siglec-15 plays important roles in the induction of both bone-resorbing activity of osteoclasts and osteoblast differentiation.
Experimental results have been reported on increasing bone density by administering anti-Siglec-15 Ab to normal mice [59]. In a preclinical pharmacological study using ovariectomized rats and cynomolgus monkeys, the effect of improving bone density, bone quality, and bone strength has been confirmed [61, 62]. Furthermore, as a characteristic of the efficacy of the anti-Siglec-15 Ab, an ideal efficacy profile similar to that of cathepsin K inhibitor, that is, suppression of bone resorption and maintenance of bone formation, was also confirmed in preclinical studies and a Phase 1 study [63].
Conclusions
Bone mass loss depends on the RANK–RANKL–OPG system, which is a major regulatory system of osteoclast differentiation induction, activation, and survival. In this complex system, we expect further developments in future studies on the importance of the bone coupling mechanism by osteoclasts, osteoblasts, and osteocytes.
References
Rodan GA, Martin TJ (1981) Role of osteoblasts in hormonal control of bone resorption—a hypothesis. Calcif Tissue Int 33:349–351. https://doi.org/10.1007/BF02409454
Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, Koga T, Martin TJ, Suda T (1990) Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acid Sci 87:7260–7264. https://doi.org/10.1073/pnas.87.18.7260
Takahashi N, Akatsu T, Udagawa N, Sasaki T, Yamaguchi A, Jane MM, Jone M, Suda T (1987) Osteoblastic cells are involved in osteoclast formation. Endocrinology 123:2600–2602. https://doi.org/10.1210/endo-123-5-2600
Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa S (1990) The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345:442–444. https://doi.org/10.1038/345442a0
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acid Sci 95:3597–3602. https://doi.org/10.1073/pnas.95.7.3597
Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ (1999) Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endoc Rev 20:345–357. https://doi.org/10.1210/edrv.20.3.0367
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS et al (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319. https://doi.org/10.1016/s0092-8674(00)80209-3
Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N, Sato Y, Goto M, Yamaguchi K, Kuriyama M, Kanno T, Murakami A, Tsuda E, Morinaga T, Higashio K (1998) Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 139:1329–1337. https://doi.org/10.1210/endo.139.3.5837
Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315–323. https://doi.org/10.1038/16852
Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J (1999) RANK is essential for osteoclast and lymph node development. Genes Dev 13:2412–2424. https://doi.org/10.1101/gad.13.18.2412
Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS (1998) Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 12:1260–1268. https://doi.org/10.1101/gad.12.9.1260
Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N, Kanno T, Sato Y, Nakagawa N, Yasuda H, Mochizuki S, Gomibuchi T, Yano K, Shim N, Washida N, Tsuda E, Morinaga T, Higashino K, Ozawa H (1998) Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commum 247:610–625. https://doi.org/10.1006/bbrc.1998.8697
Zebaze RM, Libanati C, McClung MR, Zanchetta JR, Kendler DL, Høiseth A, Wang A, Ghasem-Zadeh A, Seeman E (2016) Denosumab reduces cortical porosity of the proximal femoral shaft in postmenopausal women with osteoporosis. J Bone Miner Res 31:1827–1834. https://doi.org/10.1002/jbmr.2855
Koide M, Kobayashi Y, Ninomiya T, Nakamura M, Yasuda H, Arai Y, Okahashi N, Yoshinari N, Takahashi N, Udagawa N (2013) Osteoprotegerin-deficient male mice as a model for severe alveolar bone loss. Comparison with RANKL-overexpressing transgenic male mice. Endocrinology 154:773–782. https://doi.org/10.1210/en.2012-1928
Nakamura M, Udagawa N, Matsuura S, Mogi M, Nakamura H, Horiuchi H, Saito N, Hiraoka BY, Kobayashi Y, Takaoka K, Ozawa H, Miyazawa H, Takahashi N (2003) Osteoprotegerin regulates bone formation through a coupling mechanism with bone resorption. Endocrinology 144:5441–5449. https://doi.org/10.1210/en.2003-0717
Yamamoto Y, Udagawa N, Matsuura S, Nakamichi Y, Horiuchi H, Hosoya A, Nakamura M, Ozawa H, Takaoka K, Penninger JM, Noguchi T, Takahashi N (2006) Osteoblasts provide a suitable microenvironment for the action of receptor activator of nuclear factor-κB ligand. Endocrinology 147:3366–3374. https://doi.org/10.1210/en.2006-0216
Weivoda MM, Ruan M, Pederson L, Hachfeld C, Davey RA, Zajac JD, Westendorf JJ, Khosla S, Oursler MJ (2016) Osteoclast TGF-β receptor signaling induces Wnt1 secretion and couples bone resorption to bone formation. J Bone Miner Res 31:76–85. https://doi.org/10.1002/jbmr.2586
Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ (2008) Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci USA 105:20764–20769. https://doi.org/10.1073/pnas.0805133106
Irie N, Takada Y, Watanabe Y, Matsuzaki Y, Naruse C, Asano M, Iwakura Y, Suda T, Matsuo K (2009) Bidirectional signaling through ephrinA2-ephA2 enhances osteoclastogenesis and suppresses osteoblastogenesis. J Biol Chem 284:14637–14644. https://doi.org/10.1074/jbc.M807598200
Takyar FM, Tonna S, Ho PW, Crimeen-Irwin B, Baker EK, Martin TJ, Sims NA (2013) EphrinB2/ephB4 inhibition in the osteoblast lineage modifies the anabolic response to parathyroid hormone. J Bone Miner Res 28:912–925. https://doi.org/10.1002/jbmr.1820
Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH, Takayanagi H (2011) Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med 17:1473–1480. https://doi.org/10.1038/nm.2489
Xie H, Cui Z, Wang L, Xia Z, Hu Y et al (2014) PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med 20:1270–1278. https://doi.org/10.1038/nm.3668
Ota K, Quint P, Ruan M, Pederson L, Westendorf JJ, Khosla S, Oursler MJ (2013) TGF-β induces Wnt10b in osteoclasts from female mice to enhance coupling to osteoblasts. Endocrinology 154:3745–3752. https://doi.org/10.1210/en.2013-1272
Takeshita S, Fumoto T, Matsuoka K, Park KA, Aburatani H, Kato S, Ito M, Ikeda K (2013) Osteoclast-secreted CTHRC1 in the coupling of bone resorption to formation. J Clin Invest 123:3914–3924. https://doi.org/10.1172/JCI69493
Matsuoka K, Park KA, Ito M, Ikeda K, Takeshita S (2014) Osteoclast-derived complement component 3a stimulates osteoblast differentiation. J Bone Miner Res 29:1522–1530. https://doi.org/10.1002/jbmr.2187
Lotinun S, Kiviranta R, Matsubara AJA, Neff L, Lüth A, Koskivirta I, Kleuser B, Vacher J, Vuorio E, Horne WC, Baron R (2013) Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J Clin Invest 123:666–681. https://doi.org/10.1172/JCI64840
Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H (2012) Osteoprotection by semaphorin 3A. Nature 485:69–74. https://doi.org/10.1038/nature11000
Furuya Y, Inagaki A, Khan M, Mori K, Penninger JM, Nakamura M, Udagawa N, Aoki K, Ohya K, Uchida K, Yasuda H (2013) Stimulation of bone formation in cortical bone of mice treated with a preceptor activator of nuclear factor-κB ligand (RANKL)-binding peptide that possesses osteoclastogenesis inhibitory activity. J Biol Chem 288:5562–5571. https://doi.org/10.1074/jbc.M112.426080
Ikebuchi Y, Aoki S, Honma M, Hayashi M, Sugamori Y, Khan M, Kariya Y, Kato G, Tabata Y, Penninger JM, Udagawa N, Aoki K, Suzuki H (2018) Coupling of bone resorption and formation by RANKL reverse signalling. Nature 561:195–200. https://doi.org/10.1038/s41586-018-0482-7
Shoji-Matsunaga A, Ono T, Hayashi M, Takayanagi H, Moriyama K, Nakashima T (2017) Osteocyte regulation of orthodontic force-mediated tooth movement via RANKL expression. Sci Rep 7:8753. https://doi.org/10.1038/s41598-017-09326-7
Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D (2005) Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 280:19883–19887. https://doi.org/10.1074/jbc.M413274200
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19:179–192. https://doi.org/10.1038/nm.3074
Li X, Ominsky MS, Niu QT, Sun N, Daugherty B et al (2008) Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23:860–869
Li X, Ominsky MS, Warmington KS, Morony S, Gong J et al (2009) Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 24:578–588. https://doi.org/10.1359/jbmr.080216
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH (2008) Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 283:5866–5875. https://doi.org/10.1074/jbc.M705092200
Leupin O, Kramer I, Collette NM, Loots GG, Natt F, Kneissel M, Keller H (2007) Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J Bone Miner Res 22:1957–1967. https://doi.org/10.1359/jbmr.070804
Galea GL, Sunters A, Meakin LB, Zaman G, Sugiyama T, Lanyon LE, Price JS (2011) Sost down-regulation by mechanical strain in human osteoblastic cells involves PGE2 signaling via EP4. FEBS Lett 585:2450–2454. https://doi.org/10.1016/j.febslet.2011.06.019
Walker EC, McGregor NE, Poulton IJ, Solano M, Pompolo S, Fernandes TJ, Constable MJ, Nicholson GC, Zhang JG, Nicola NA, Gillespie MT, Martin TJ, Sims NA (2010) Oncostatin M promotes bone formation independently of resorption when signaling through leukemia inhibitory factor receptor in mice. J Clin Invest 120:582–592. https://doi.org/10.1172/JCI40568
Poulton IJ, McGregor NE, Pompolo S, Walker EC, Sims NA (2012) Contrasting roles of leukemia inhibitory factor in murine bone development and remodeling involve region-specific changes in vascularization. J Bone Miner Res 27:586–595. https://doi.org/10.1002/jbmr.1485
Walker EC, McGregor NE, Poulton IJ et al (2008) Cardiotrophin-1 is an osteoclast-derived stimulus of bone formation required for normal bone remodeling. J Bone Miner Res 23:2025–2032. https://doi.org/10.1359/jbmr.080706
Cornish J, Callon K, King A, Edgar S, Reid IR (1993) The effect of leukemia inhibitory factor on bone in vivo. Endocrinology 132:1359–1366. https://doi.org/10.1210/endo.132.3.8440191
Sims NA, Martin TJ (2015) Coupling signals between the osteoclast and osteoblast: how are messages transmitted between these temporary visitors to the bone surface? Front Endocrinol 6:41. https://doi.org/10.3389/fendo.2015.00041
Ota K, Quint P, Weivoda MM, Ruan M, Pederson L, Westendorf JJ, Khosla S, Oursler MJ (2013) Transforming growth factorβ1 induces CXCL16 and leukemia inhibitory factor expression in osteoclasts to modulate migration of osteoblast progenitors. Bone 57:68–75. https://doi.org/10.1016/j.bone.2013.07.023
Koide M, Kobayashi Y, Yamashita T, Uehara S, Nakamura M, Hiraoka BY, Ozaki Y, Iimura T, Yasuda H, Takahashi N, Udagawa N (2017) Bone Formation is coupled to resorption via suppression of sclerostin expression by osteoclasts. J Bone Miner Res 32:2074–2086. https://doi.org/10.1002/jbmr.3175
Udagawa N, Takahashi N, Yasuda H, Mizuno A, Itoh K, Ueno Y, Shinki T, Gillespie MT, Martin TJ, Higashio K, Suda T (2000) Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology 141:3478–3484. https://doi.org/10.1210/endo.141.9.7634
Takasaki W, Kajino Y, Kajino K, Murali R, Greene MI (1997) Structure-based design and characterization of exocyclic peptidomimetics that inhibit TNF alpha binding to its receptor. Nat Biotechnol 15:1266–1270. https://doi.org/10.1038/nbt1197-1266
Aoki K, Saito H, Itzstein C, Ishiguro M, Shibata T, Blanque R, Mian AH, Takahashi M, Suzuki Y, Yoshimatsu M, Yamaguchi A, Deprez P, Mollat P, Murali R, Ohya K, Horne WC, Baron R (2006) A TNF receptor loop peptide mimic blocks RANK ligand-induced signaling, bone resorption, and bone loss. J Clin Invest 116:1525–1534. https://doi.org/10.1172/JCI22513
Nakamura M, Nakamichi Y, Mizoguchi T, Koide M, Yamashita T, Ara T, Nakamura H, Penninger JM, Furuya Y, Yasuda H, Udagawa N (2017) The W9 peptide directly stimulates osteoblast differentiation via RANKL signaling. J Oral Biosci 59:146–151
Ishida N, Hayashi K, Hoshijima M, Ogawa T, Koga S, Miyatake Y, Kumegawa M, Kimura T, Takeya T (2002) Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J Biol Chem 277:41147–41156. https://doi.org/10.1074/jbc.M205063200
Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3:889–901. https://doi.org/10.1016/s1534-5807(02)00369-6
Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E, Takayanagi H (2005) Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med 202:1261–1269. https://doi.org/10.1084/jem.20051150
Aliprantis AO, Ueki Y, Sulyanto R, Park A, Sigrist KS, Sharma SM, Ostrowski MC, Olsen BR, Glimcher LH (2008) NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest 118:3775–3789. https://doi.org/10.1172/JCI35711
Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, Taniguchi T, Takayanagi H, Takai T (2004) Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428:758–763. https://doi.org/10.1038/nature02444
Kaifu T, Nakahara J, Inui M, Mishima K, Momiyama T, Kaji M, Sugahara A, Koito H, Ujike-Asai A, Nakamura A, Kanazawa K, Tan-Takeuchi K, Iwasaki K, Yokoyama W, Kudo A, Fujiwara M, Asou H, Takai T (2003) Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J Clin Invest 111:323–332. https://doi.org/10.1172/JCI16923
Hiruma Y, Hirai T, Tsuda E (2011) Siglec-15, a member of the sialic acid-binding lectin, is a novel regulator for osteoclast differentiation. Biochem Biophy Res Commun 409:424–429. https://doi.org/10.1016/j.bbrc.2011.05.015
Ishida-Kitagawa N, Tanaka K, Bao X, Kimura T, Miura T, Kitaoka Y, Hayashi K, Sato M, Maruoka M, Ogawa T, Miyoshi J, Takeya T (2012) Siglec-15 protein regulates formation of functional osteoclasts in concert with DNAX-activating protein of 12 kDa (DAP12). J Biol Chem 287:17493–17502. https://doi.org/10.1074/jbc.M111.324194
Kameda Y, Takahata M, Komatsu M, Mikuni S, Hatakeyama S, Shimizu T, Angata T, Kinjo M, Minami A, Iwasaki N (2013) Siglec-15 regulaters osteoclast differentiation by modulating RANKL-induced phosphatidylinositol 3-kinase/Akt and Erk pathways in association with signaling adaptor DAP12. J Bone Miner Res 28:2463–2475. https://doi.org/10.1002/jbmr.1989
Hiruma Y, Tsuda E, Maeda N, Okada A, Kabasawa N, Miyamoto M, Hattori H, Fukuda C (2013) Impaired osteoclast differentiation and function and mild osteopetrosis development in Siglec-15-deficient mice. Bone 53:87–93. https://doi.org/10.1016/j.bone.2012.11.036
Stuible M, Moraitis A, Fortin A, Saragosa S, Kalbakji A, Filion M, Tremblay GB (2014) Mechanism and function of monoclonal antibodies targeting siglec-15 for therapeutic inhibition of osteoclastic bone resorption. J Biol Chem 289:6498–6512. https://doi.org/10.1074/jbc.M113.494542
Udagawa N, Uehara S, Koide M, Arai A, Mizoguchi T, Nakamura M, Kobayashi Y, Takahashi N, Fukuda C, Tsuda E (2017) Anti-Siglec-15 antibody inhibits bone-resorbing activity of osteoclasts and stimulates osteoblast differentiation. J Bone Miner Res Suppl 32:349
Fukuda C, Okada A, Karibe T, Hinuma Y, Kumakura S, Tsuda E (2017) A novel bone formation-sparing anti-resorptive agent, DS-1501a, increased BMD and bone biomechanical properties of cortical bone in ovariectomized cynomolgus monkeys. J Bone Miner Res 32:112
Fukuda C, Tsuda E, Okada A, Amizuka N, Hasegawa T, Karibe T, Hinuma Y, Takagi N, Kumakura S (2017) Anti-Siglec-15 antibody reduced bone resorption while maintaining bone formation in ovariectomized (OVX) rats and monkeys. J Bone Miner Res 32:112
Dishy V, Kang D, Warren V, Maxwell W, Levinson B, Kochan J, He L, Baz-Hecht M, Fukuda C, Koga J, Tsuda E, Watanabe K (2017) A phase 1, subject and investigator blinded, sponsor unblended, placebocontrolled, radamized, 2 part, sequential, single ascending dose study to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of DS-1501a in healthy young subjects and healthy postmenopausal woman. J Bone Miner Res 32:107–108
Acknowledgements
This work was supported by JSPS KAKENHI (Grant nos 19K10395, 17K19776, 16H05508, 16K11818, 15K11377, and 15K15688).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Eisuke Tsuda and Chie Fukuda are employees of Daiichi Sankyo Co., Ltd. The other authors (Nobuyuki Udagawa, Masanori Koide, Midori Nakamura, Yuko Nakamichi, Teruhito Yamashita, Shunsuke Uehara, and Yasuhiro Koide) have financial interest and/or other relationship with Daiichi Sankyo Co., Ltd. Yuriko Furuya and Hisataka Yasuda are employees of Oriental Yeast Co., Ltd.
Ethics approval
All experiments performed in the Matsumoto Dental University were conducted in strict accordance with the Guidelines for Studies with Laboratory Animals of the Matsumoto Dental University Experimental Animal Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Udagawa, N., Koide, M., Nakamura, M. et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab 39, 19–26 (2021). https://doi.org/10.1007/s00774-020-01162-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-020-01162-6