Abstract
Osteoclasts are unique multinucleated cells that can resorb bone. Bone mass is determined by a tightly regulated balance between osteoclasts and osteoblasts, which generate bones. Thus, the excessive formation of osteoclasts leads to the pathological bone resorption observed in postmenopausal osteoporosis, rheumatoid arthritis, Paget’s disease, and bone tumor metastases. During osteoclast differentiation, NF-κB is activated by TRAF6-mediated signals from RANK expressed on the surface of osteoclast progenitor cells upon RANKL stimulation, activating NFATc1, a master transcription factor in osteoclastogenesis. However, in contrast regular NF-κB activation, sufficient NFATc1 activation requires long-term activation of NF-κB, which can be induced uniquely by RANK but not by CD40, a receptor that also uses TRAF6 to activate NF-κB. Through analysis of various RANK mutants, we identified the 60-amino-acid HCR domain (mouse RANK) in the cytoplasmic tail of RANK. HCR is highly conserved among vertebrates and is crucial for long-term NF-κB activation. Interestingly, when HCR was attached to the cytoplasmic tail of CD40, the chimeric receptor promoted osteoclast formation, even though CD40 itself cannot. In this chapter, we explore the molecular mechanisms of HCR-mediated signals and the possible application of the HCR peptide as an anti-bone-resorptive drug.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Activation of the Transcription Factor NF-κB
Nuclear factor κ-light-chain enhancer of activated B cells (NF-κB) is a family of transcription factors that consists of five members, viz., p50, p52, RelA, RelB, and c-Rel, which form homo- and heterodimers to induce the expression of target genes that are crucial for inflammation, immunoregulation, and cell differentiation (Hayden and Ghosh 2008). NF-κB is usually transcriptionally inactive because it is sequestered in the cytoplasm, where it complexes with members of the IκB family, including IκBα, IκBβ, IκBε, p105 (a precursor to p50), and p100 (a precursor to p52), which can mask the nuclear localization signal (NLS) of NF-κB. Two distinct activation pathways for NF-κB have been reported (Fig. 13.1). In the canonical pathway, the p50/RelA heterodimer is sequestered in the cytoplasm upon binding to IκBα. Various stimuli, including cytokines and bacterial and viral products, activate IκB kinase β (IKKβ), which phosphorylates IκBα. This phosphorylation in turn induces Lys48-linked polyubiquitination of IκBα and its subsequent degradation, which allows nuclear translocation of p50/RelA (Hacker and Karin 2006; Liu and Chen 2011). In the non-canonical pathway, RelB is sequestered in the cytoplasm by binding to p100, whose N-terminal half becomes p52 after stimulation-dependent degradation of the C-terminal half of p100, which acts as an IκB protein to mask the NLS of RelB. The non-canonical pathway is activated by receptors that are required for the formation of lymphoid organs and the maturation of immune cells, such as the lymphotoxin (LT) β receptor, the receptor activator of NF-κB (RANK), and the CD40 and BAFF receptor. Activation of these receptors by their specific ligands leads to activation of IKKα, which phosphorylates the C-terminal half of p100 (Sun 2010). This phosphorylation induces the polyubiquitination-dependent degradation of the C-terminal half, which allows nuclear translocation of the p52/RelB heterodimer.
2 Physiological and Pathological Roles of Osteoclasts
Osteoclasts are multinucleated large cells that form sealed zones with the bone, followed by acidification of the zones by proton transport activity (Blair et al. 1989; Vaananen et al. 1990; Teitelbaum et al. 1995). The osteoclasts then secrete proteases such as cathepsin K, which has optimal enzymatic activity in acidic conditions, into the zone. Because cathepsin K degrades type I collagen and other noncollagenous proteins, osteoclasts can resorb bones. Therefore, osteoclasts have a crucial role in bone homeostasis in concert with osteoblasts, which mediate bone formation (Takayanagi 2007). The excessive formation of osteoclasts in humans leads to pathological bone resorption, such as that found in postmenopausal osteoporosis, rheumatoid arthritis, Paget’s disease, and bone tumor metastases (Rodan and Martin 2000; Takayanagi et al. 2000). Therefore, to develop drugs or therapeutic strategies to treat such diseases, a precise understanding of the signal transduction pathways that promote osteoclastogenesis is needed.
3 NF-κB Activation Induced by the RANK-TRAF6 Signal Pathway Is Crucial for Osteoclastogenesis
Mice lacking NF-κB subunits p50 and p52 display osteopetrosis and do not have osteoclast-like cells, which express tartrate-resistant acid phosphatase (TRAP), an enzyme that is highly expressed in osteoclasts (Franzoso et al. 1997; Iotsova et al. 1997). Moreover, when primary osteoblasts derived from calvaria of wild-type newborn mice were co-cultured with either wild-type or double-knockout (KO) splenocytes, mature osteoclasts were not formed with splenocytes of p50/p52 double-KO mice, whereas mature osteoclasts were formed with wild-type splenocytes. These results clearly indicate that NF-κB is essential for osteoclastogenesis.
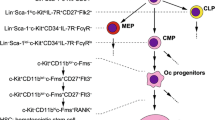
As already described, activation of NF-κB requires stimuli that activate either IKKα or IKKβ. What types of receptors and ligands are crucial for NF-κB activation during osteoclastogenesis? Studies of KO mice revealed that the expression of RANK (also known as the TRANCE receptor, a member of the TNF receptor superfamily (TNFRSF)) on the surface of osteoclast precursor cells, and the expression of its ligand RANK (RANKL, also known as ODF, OPGL, and TRANCE) on the surface of osteoblasts are essential for osteoclastogenesis (Dougall et al. 1999; Kong et al. 1999) (Fig. 13.2). The intracellular signaling pathways of TNFRSF are primarily mediated by members of the TNFR-associated factor (TRAF) family (Galibert et al. 1998; Wong et al. 1998; Darnay et al. 1999; Hsu et al. 1999). Seven members of the TRAF family have been identified to date (Inoue et al. 2000; Bouwmeester et al. 2004). We and others (Lomaga et al. 1999; Naito et al. 1999; Kobayashi et al. 2001; Kim et al. 2005) previously demonstrated that TRAF6-deficient mice display severe osteopetrosis resulting from lack of multinucleated functional osteoclasts. This defective osteoclast formation results from abrogated RANK signaling because RANK-induced activation of NF-κB and MAPKs is abrogated in osteoclast progenitor cells derived from TRAF6 deficient mice (Kobayashi et al. 2001). These results are consistent with previous findings that NF-κB and MAPKs are crucial in osteoclastogenesis (Matsumoto et al. 2000; Yamamoto et al. 2002; Takayanagi 2007).
4 HCR, a Unique Domain in RANK, Plays a Critical Role in Osteoclastogenesis
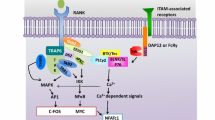
We originally identified TRAF6 as a protein binding to the cytoplasmic tail of CD40, another member of TNFRSF, by yeast two-hybrid cloning (Ishida et al. 1996). In addition, we demonstrated that TRAF6 is essential for the activation of NF-κB and MAPKs induction by signaling through CD40, which is expressed in osteoclast progenitor cells (Gohda et al. 2005). Interestingly, although RANK and CD40 activate NF-κB and MAPKs to a similar level in a TRAF6-dependent manner, stimulation of CD40 in osteoclast progenitor cells does not result in osteoclast formation (Gohda et al. 2005). These results led us to hypothesize that RANK, but not CD40, can trigger unique events essential for osteoclastogenesis in addition to NF-κB and MAPKs activation. Although we attempted to elucidate the unique activity of RANK, Koga et al. reported that RANK induces oscillations in Ca2+, which are required for the induction of NFATc1, a master transcription factor during osteoclastogenesis (Takayanagi et al. 2002; Koga et al. 2004; Asagiri et al. 2005). Oscillations in Ca2+ are mediated by phospholipase Cγ 2 (PLCγ2) (Mao et al. 2006) downstream of immunoreceptor tyrosine-based activation motif (ITAM)-harboring adaptors, such as the DNAX-activating protein (DAP)12 and the Fc-receptor common γ-subunit (FcRγ) (Koga et al. 2004), which associate with various immunoglobulin (Ig)-like receptors. DAP12 associates with triggering receptor expressed in myeloid cells 2 (TREM-2) and signal-regulatory protein β1 (SIRPβ1), whereas FcRγ associates with OSCAR and paired immunoglobulin-like receptor A (PIR-A) (Kim et al. 2002; Kubagawa et al. 1997; Colonna 2003; Dietrich et al. 2000; Tomasello et al. 2000; Kaifu et al. 2003; Takai et al. 1994). Because signals from these Ig-like receptor/ITAM adaptor complexes are crucial for NFATc1 induction but cannot induce osteoclastogenesis without the activation of NF-κB and AP-1, they are considered to be co-stimulatory signals (Koga et al. 2004; Takayanagi 2007) (Fig. 13.2). The activation of NF-κB and MAPKs begins during the first hour after RANKL stimulation (early phase of osteoclastogenic signals). NF-κB activation, but not MAPKs activation, is sustained for up to 24 or 48 h after stimulation (late phase of osteoclastogenic signals) (Taguchi et al. 2009). The co-stimulatory signals also begin to induce PLCγ2 activation in the early phase, which is sustained in the late phase, whereas Ca2+ oscillations and NFATc1 activation begin approximately 12 h after stimulation and reach a maximum during the late phase. Interestingly, we found that CD40 is not capable of activating PLCγ2 and NF-κB in the late phase, although it can activate NF-κB and AP-1 in the early phase (Taguchi et al. 2009).
To elucidate the molecular mechanism by which RANK, but not CD40, activates the late-phase RANK signals, which are essential for osteoclastogenesis, we compared the primary structure of the cytoplasmic tails of RANK and CD40. The cytoplasmic tail of RANK is 391 amino acids long (mouse RANK) and contains three TRAF6-binding sites, while that of CD40 is 74 amino acids long (mouse CD40) and has one TRAF6-binding site. We then tested the hypothesis that three TRAF6-binding sites are required for late-phase RANK signals and that one TRAF6-binding site is insufficient. Our series of studies using mutants of the RANK cytoplasmic region revealed that a single TRAF6-binding site is sufficient to promote osteoclastogenesis (Gohda et al. 2005). Therefore, the inability of CD40 to induce late-phase signals is not because it has fewer TRAF6-binding sites. These results led us to hypothesize that RANK, but not CD40, may harbor a specific domain that cooperates with the TRAF6-binding site to induce the late phase of osteoclastogenic signals by sustaining activation of NF-κB and PLCγ2 up to 48 h after stimulation.
To identify the specific domain in the cytoplasmic tail of RANK, we introduced various deletions into the cytoplasmic tail followed by analyzing the osteoclastogenic activity of each mutant (Taguchi et al. 2009). We identified a 60-amino-acid sequence in the RANK cytoplasmic tail that is highly conserved among vertebrates and whose deletion abrogates activation of the late-phase signals, thereby blocking osteoclast differentiation, without affecting the activation of the early-phase signals. We called this region “highly conserved domain in RANK” (HCR) (Figs. 13.3, 13.4).
Primary structure of HCR (“highly conserved domain in RANK”) in RANK. Upper: The amino acid sequences of HCR in RANK derived from mouse, human, dog, chicken, and puffer fish were aligned with CLUSTALW (version 1.83). Lower: Schematic diagram of RANK. Numbers indicate the amino acid position from the N-terminus in the mouse sequence. Amino acids that were identical among the four species, except for puffer fish, are shown in red; those that were identical among all five species are shown in purple. HCR, N-, C-, GY-peptides, and the region corresponding to RRI-peptides are indicated
Because the conservation of HCR is extremely high, we examined whether HCR could act as a functional domain to induce late-phase osteoclastogenic signals (Taguchi et al. 2009). To test this hypothesis, we generated chimeric receptors in which HCR was added to the cytoplasmic domain of human CD40, which by itself cannot induce osteoclast formation, to generate a chimeric receptor, hCD40/HCR. Surprisingly, stimulation of h40/HCR resulted in osteoclastogenesis, even though its efficiency was lower than that of RANK signals, indicating that HCR itself can render CD40 capable of inducing osteoclastogenesis. Collectively, HCR is a novel functional protein motif that induces long-term osteoclastogenic signals in concert with the TRAF6-binding site, leading to induction of Ca2+ oscillations and NFATc1 activation.
What is the molecular mechanism by which HCR induces the sustained signals? To answer this question, we determined whether the RANK cytoplasmic tail could bind to any proteins reported to be involved in osteoclastogenesis. Transient overexpression of each candidate protein and the RANK cytoplasmic tail in 293T cells followed by immunoprecipitation revealed that Gab2 and PLCγ2 specifically associated with the RANK cytoplasmic tail (Taguchi et al. 2009). Interestingly, only Gab2 bound RANK in an HCR-dependent manner, whereas PLCγ2 bound RANK in the absence of HCR. However, when we analyzed the stimulation-dependent association, an association of Gab2 with RANK was not detected in the early phase, although its association was clearly detected in the late phase. Recruitment of PLCγ2 to RANK was clearly observed in the early phase and their significant interaction was sustained up to the late phase. Interestingly, the PLCγ2/RANK association in the early phase was HCR independent while that in the late phase was HCR dependent. In addition, the stimulation-dependent association of Gab2 with PLCγ2 was observed in both the early and the late phases, although their association during the early phase was significantly weaker than in the late phase. Collectively, PLCγ2 is likely to interact directly with RANK in an HCR-independent manner, and Gab2 indirectly associates with RANK via PLCγ2 in the early phase (Fig. 13.3). In contrast, in the late phase, Gab2 is likely to bind RANK through HCR, and PLCγ2 indirectly associates with RANK via Gab2 (Taguchi et al. 2009) (Fig. 13.3). Kim et al. reported that Vav3 binds HCR, which could be crucial for osteoclast maturation (Kim et al. 2009). Given that TRAF6 binds to Gab2, HCR is crucial for the maintenance of the late phase of osteoclastogenic signals by sustaining activation of NF-κB and PLCγ2-mediated Ca2+ signaling in concert with TRAF6.
5 Peptides Derived from the HCR of the RANK Cytoplasmic Tail Are Anti-Osteoclastogenic
A human-type monoclonal antibody that targets RANKL (Denosumab) is used to treat cancerous bone lesions with multiple myeloma and bone metastasis (Hageman et al. 2013; Fizazi et al. 2009). Denosumab also inhibits pathogenic bone resorption in osteoporosis, rheumatoid arthritis, and Paget’s disease (Cummings et al. 2009; Cohen et al. 2008; Schwarz et al. 2012). Moreover, because of the long half-life of this antibody, its subcutaneous administration every 6 months is sufficient to inhibit bone resorption. However, it is an expensive injectable product with adverse effects, including hypocalcemia and osteonecrosis of the jaw. Therefore, inexpensive orally administered agents that show inhibitory effects on bone resorption need to be developed.
It is difficult to propose any of the intracellular signaling proteins involved in RANK signaling as a suitable target for developing drugs for pathogenic bone resorption because these proteins are commonly used in other signaling pathways and may lead to adverse effects. For example, NF-κB is crucial for osteoclastogenesis but is ubiquitous and required for various physiological processes, including inflammatory and immune responses (Hayden and Ghosh 2008). Thus, targeting NF-κB may not be the best strategy. In contrast, HCR may be a good target for developing anti-bone-resorptive drugs. The HCR peptide may work as a specific inhibitor of osteoclastogenesis with minimal adverse effects, because our search of the GenBank database did not identify any proteins homologous to the primary structure of HCR. We demonstrated that ectopic expression of the complete HCR peptide can inhibit RANK-induced osteoclast differentiation by blocking differentiation of TRAP+ mononuclear cells (Taguchi et al. 2009, 2012), which led us consider using the HCR peptide as an anti-osteoclastogenic drug. Therefore, we further analyzed the molecular mechanisms of the HCR peptide-mediated inhibition of osteoclastogenesis and also narrowed down the inhibitory domain in HCR to identify shorter inhibitory peptides. From a therapeutic perspective, peptide length is important because smaller peptides tend to enter into cells when added to cell-penetrating peptides, such as the TAT-peptide and the oligo-arginine peptide (Futaki et al. 2001; Brooks et al. 2005; Kosuge et al. 2008).
Based on the conservation of amino acid sequence, HCR peptides can be divided into two subdomains, the less conserved N-terminal region (N-peptide, aa 487–507) and the highly conserved C-terminal region (C-peptide, aa 508–548) (Fig. 13.4). Within the C-region, GQVMNF (aa 525–530) and IVVY (aa 535–538) were identical among various species, including puffer fish. Thus, we constructed a retroviral vector expressing peptides corresponding to aa 525–538 (GY-peptide), which covers both the GQVMNF and IVVY regions, in addition to the N-region and the C-region. Ectopic expression of the GY-, N- or C-peptides resulted in a 40 %, 60 %, or 75 % reduction in osteoclast formation, respectively (Taguchi et al. 2012) (Fig. 13.5). These results indicate that the C-peptide is the most potent among the HCR derivative peptides. Surprisingly, the N-peptide also significantly inhibits osteoclastogenesis, even though the N-region is much less conserved than the C-region (Figs. 13.4, 13.5). It is also interesting that the GY-peptide significantly inhibits osteoclastogenesis even though it is only 14 residues long (Fig. 13.5).
HCR derivative peptides block osteoclastogenesis. a Expression of HCR derivative peptides. Bone marrow-derived macrophages were infected with retroviruses expressing TAP (tandem affinity purification tag: FLAG-Strept-Strept-FLAG-)-HCR-, TAP-N-, TAP-C-, or TAP-GY-peptides. Cell lysates were subjected to immunoblot analysis using anti-FLAG and anti-α-tubulin antibodies. b Inhibition of osteoclastogenesis by various HCR derivative peptides. Cells were stimulated with RANKL for 3 days, fixed with formaldehyde, and stained with tartrate-resistant acid phosphatase (TRAP). Representative images of osteoclastogenesis at 25 ng/ml RANKL stimulation in the presence of various HCR derivative peptides (left). TRAP+ multinucleated cells containing more than five nuclei were classified as osteoclasts and were counted (right)
To elucidate the molecular mechanisms by which the HCR derivative peptides inhibit osteoclastogenesis, correlations between the expression levels of the HCR derivative peptides and the extent of differentiation were analyzed by TRAP staining and mono/multinucleated phenotypes. For cells expressing the N- or C-peptides, more than 80 % of the cells highly expressing the HCR derivative peptides did not express TRAP, whereas most of the cells expressing few or no HCR derivative peptides were positive for TRAP and had multiple nuclei (Taguchi et al. 2012) (Fig. 13.6, upper and middle). These results strongly suggest that the N- and C-peptides inhibit pre-fusion osteoclastogenic signals in a cell-autonomous manner. In contrast, most of the cells highly expressing the GY-peptides expressed TRAP, and most of the cells expressing little or no TAP-GY peptide were also positive for TRAP staining. More importantly, more than half of the TRAP+ cells that highly expressed the GY-peptide were mononuclear whereas most of the TRAP+ cells that expressed little or no TAP-GY peptide in the same culture well were multinuclear (Taguchi et al. 2012) (Fig. 13.6, lower). These results strongly suggest that the expression of the GY-peptide does not inhibit pre-fusion events but does inhibit cell–cell fusion in a cell-autonomous manner. These results are consistent with the finding by Kim et al., who reported that the RRI peptide, which covers a sequence similar to the GY-peptide, inhibits cell–cell fusion (Kim et al. 2009).
Two distinct modes of inhibition of osteoclastogenesis by HCR derivative peptides. Bone marrow-derived macrophages were stimulated with 25 ng/ml RANKL for 3 days, then fixed with formaldehyde and stained with TRAP. Cells were further immunostained using anti-FLAG and Alexa Fluor 488 goat anti-mouse IgG to visualize the HCR derivative peptides. Yellow arrows indicate cells expressing high levels of TAP-HCR derivative peptides; blue arrows indicate cells with low or undetectable levels of TAP-HCR derivative peptides
RANK has been reported to be an important molecule not only in osteoclastogenesis but also in lymph node development (Dougall et al. 1999), fever regulation (Hanada et al. 2009), thymus organogenesis (Akiyama et al. 2008), mammary gland development (Fata et al. 2000), and activation of dendritic cells (DC) (Josien et al. 2000), suggesting that therapeutic methods targeting the RANK–RANKL interaction, such as RANK-Fc or anti-RANKL antibody (Denosumab), may have various side effects. It is possible that the HCR derivative peptides might affect only osteoclastogenesis and not other functions of RANK signaling, which is partially supported by a study showing that the RRI peptide did not inhibit the production of cytokines from DCs upon RANKL stimulation (Kim et al. 2009). Further investigation of HCR peptide-mediated inhibition of osteoclastogenesis is required to develop therapeutic drugs aimed at inhibiting osteoclastogenic signals with the goal of treating pathological bone resorption with minimum adverse effects.
6 Conclusions
During osteoclast differentiation, NF-κB is activated by a signal from RANK expressed on the surface of osteoclast progenitor cells upon RANKL stimulation. Activation of NFATc1, a master transcription factor in osteoclastogenesis, requires long-term activation of NF-κB and the PLCγ2-mediated Ca2+ signal to activate calcineurin, a phosphatase that dephosphorylates NFATc1 for its nuclear translocation. HCR, a unique region in the cytoplasmic tail of RANK, is highly conserved among vertebrates and is crucial for the long-term activation of NF-κB and the PLCγ2 by forming a signal complex composed of TRAF6, Gab2, and PLCγ2. Because expression of HCR or HCR derivatives inhibits osteoclastogenesis, HCR is likely to be a molecular target to develop anti-bone-resorptive drugs that block pathogenic bone resorption, as observed in osteoporosis, rheumatoid arthritis, and Paget’s disease.
References
Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger JM, Matsumoto M, Nitta T, Takahama Y, Inoue J (2008) The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity 29(3):423–437. doi:10.1016/j.immuni.2008.06.015
Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E, Takayanagi H (2005) Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med 202(9):1261–1269. doi:10.1084/jem.20051150
Blair HC, Teitelbaum SL, Ghiselli R, Gluck S (1989) Osteoclastic bone resorption by a polarized vacuolar proton pump. Science 245(4920):855–857
Bouwmeester T, Bauch A, Ruffner H, Angrand PO, Bergamini G, Croughton K, Cruciat C, Eberhard D, Gagneur J, Ghidelli S, Hopf C, Huhse B, Mangano R, Michon AM, Schirle M, Schlegl J, Schwab M, Stein MA, Bauer A, Casari G, Drewes G, Gavin AC, Jackson DB, Joberty G, Neubauer G, Rick J, Kuster B, Superti-Furga G (2004) A physical and functional map of the human TNF-α/NF-κB signal transduction pathway. Nat Cell Biol 6(2):97–105. doi:10.1038/ncb1086
Brooks H, Lebleu B, Vives E (2005) Tat peptide-mediated cellular delivery: back to basics. Adv Drug Deliv Rev 57(4):559–577. doi:10.1016/j.addr.2004.12.001
Cohen SB, Dore RK, Lane NE, Ory PA, Peterfy CG, Sharp JT, van der Heijde D, Zhou L, Tsuji W, Newmark R (2008) Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum 58(5):1299–1309. doi:10.1002/art.23417
Colonna M (2003) TREMs in the immune system and beyond. Nat Rev Immunol 3(6):445–453. doi:10.1038/nri1106
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361(8):756–765. doi:10.1056/NEJMoa0809493
Darnay BG, Ni J, Moore PA, Aggarwal BB (1999) Activation of NF-κB by RANK requires tumor necrosis factor receptor-associated factor (TRAF) 6 and NF-κB-inducing kinase. Identification of a novel TRAF6 interaction motif. J Biol Chem 274(12):7724–7731
Dietrich J, Cella M, Seiffert M, Buhring HJ, Colonna M (2000) Cutting edge: signal-regulatory protein β1 is a DAP12-associated activating receptor expressed in myeloid cells. J Immunol 164 (1):9–12. doi:10.4049/jimmunol.164.1.9
Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J (1999) RANK is essential for osteoclast and lymph node development. Genes Dev 13(18):2412–2424
Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, Boyle WJ, Khokha R, Penninger JM (2000) The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103(1):41–50. doi:10.1016/S0092-8674(00)00103-3
Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, Gao G, Wu L, Sohn W, Jun S (2009) Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol 27(10):1564–1571. doi:10.1200/JCO.2008.19.2146
Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF, Siebenlist U (1997) Requirement for NF-κB in osteoclast and B-cell development. Genes Dev 11(24):3482–3496
Futaki S, Suzuki T, Ohashi W, Yagami T, Tanaka S, Ueda K, Sugiura Y (2001) Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem 276(8):5836–5840. doi:10.1074/jbc.M007540200
Galibert L, Tometsko ME, Anderson DM, Cosman D, Dougall WC (1998) The involvement of multiple tumor necrosis factor receptor (TNFR)-associated factors in the signaling mechanisms of receptor activator of NF-κB, a member of the TNFR superfamily. J Biol Chem 273(51):34120–34127
Gohda J, Akiyama T, Koga T, Takayanagi H, Tanaka S, Inoue J (2005) RANK-mediated amplification of TRAF6 signaling leads to NFATc1 induction during osteoclastogenesis. EMBO J 24(4):790–799. doi:10.1038/sj.emboj.7600564
Hacker H, Karin M (2006) Regulation and function of IKK and IKK-related kinases. Sci STKE 2006(357):re13. doi:10.1126/stke.3572006re13
Hageman K, Patel KC, Mace K, Cooper MR (2013) The role of denosumab for prevention of skeletal-related complications in multiple myeloma. Ann Pharmacother 47(7–8):1069–1074. doi:10.1345/aph.1R776
Hanada R, Leibbrandt A, Hanada T, Kitaoka S, Furuyashiki T, Fujihara H, Trichereau J, Paolino M, Qadri F, Plehm R, Klaere S, Komnenovic V, Mimata H, Yoshimatsu H, Takahashi N, von Haeseler A, Bader M, Kilic SS, Ueta Y, Pifl C, Narumiya S, Penninger JM (2009) Central control of fever and female body temperature by RANKL/RANK. Nature (Lond) 462(7272):505–509. doi:10.1038/nature08596
Hayden MS, Ghosh S (2008) Shared principles in NF-κB signaling. Cell 132(3):344–362. doi:10.1016/j.cell.2008.01.020
Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang L, Xia XZ, Elliott R, Chiu L, Black T, Scully S, Capparelli C, Morony S, Shimamoto G, Bass MB, Boyle WJ (1999) Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci U S A 96(7):3540–3545
Inoue J, Ishida T, Tsukamoto N, Kobayashi N, Naito A, Azuma S, Yamamoto T (2000) Tumor necrosis factor receptor-associated factor (TRAF) family: adapter proteins that mediate cytokine signaling. Exp Cell Res 254(1):14–24. doi:10.1016/j.cell.2008.01.020
Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R (1997) Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat Med 3(11):1285–1289
Ishida T, Mizushima S, Azuma S, Kobayashi N, Tojo T, Suzuki K, Aizawa S, Watanabe T, Mosialos G, Kieff E, Yamamoto T, Inoue J (1996) Identification of TRAF6, a novel tumor necrosis factor receptor-associated factor protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J Biol Chem 271(46):28745–28748
Josien R, Li HL, Ingulli E, Sarma S, Wong BR, Vologodskaia M, Steinman RM, Choi Y (2000) TRANCE, a tumor necrosis factor family member, enhances the longevity and adjuvant properties of dendritic cells in vivo. J Exp Med 191(3):495–502
Kaifu T, Nakahara J, Inui M, Mishima K, Momiyama T, Kaji M, Sugahara A, Koito H, Ujike-Asai A, Nakamura A, Kanazawa K, Tan-Takeuchi K, Iwasaki K, Yokoyama WM, Kudo A, Fujiwara M, Asou H, Takai T (2003) Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J Clin Invest 111(3):323–332. doi:10.1172/JCI16923
Kim N, Takami M, Rho J, Josien R, Choi Y (2002) A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J Exp Med 195(2):201–209
Kim N, Kadono Y, Takami M, Lee J, Lee SH, Okada F, Kim JH, Kobayashi T, Odgren PR, Nakano H, Yeh WC, Lee SK, Lorenzo JA, Choi Y (2005) Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J Exp Med 202(5):589–595. doi:10.1084/jem.20050978
Kim H, Choi HK, Shin JH, Kim KH, Huh JY, Lee SA, Ko CY, Kim HS, Shin HI, Lee HJ, Jeong D, Kim N, Choi Y, Lee SY (2009) Selective inhibition of RANK blocks osteoclast maturation and function and prevents bone loss in mice. J Clin Invest 119(4):813–825. doi:10.1172/JCI36809
Kobayashi N, Kadono Y, Naito A, Matsumoto K, Yamamoto T, Tanaka S, Inoue J (2001) Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J 20(6):1271–1280. doi:10.1093/emboj/20.6.1271
Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, Taniguchi T, Takayanagi H, Takai T (2004) Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature (Lond) 428(6984):758–763. doi:10.1038/nature02444
Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature (Lond) 397(6717):315–323. doi:10.1038/16852
Kosuge M, Takeuchi T, Nakase I, Jones AT, Futaki S (2008) Cellular internalization and distribution of arginine-rich peptides as a function of extracellular peptide concentration, serum, and plasma membrane associated proteoglycans. Bioconjug Chem 19(3):656–664. doi:10.1021/bc700289w
Kubagawa H, Burrows PD, Cooper MD (1997) A novel pair of immunoglobulin-like receptors expressed by B cells and myeloid cells. Proc Natl Acad Sci U S A 94(10):5261–5266
Liu S, Chen ZJ (2011) Expanding role of ubiquitination in NF-κB signaling. Cell Res 21(1):6–21. doi:10.1038/cr.2010.170
Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, van der Heiden A, Itie A, Wakeham A, Khoo W, Sasaki T, Cao Z, Penninger JM, Paige CJ, Lacey DL, Dunstan CR, Boyle WJ, Goeddel DV, Mak TW (1999) TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev 13(8):1015–1024
Mao D, Epple H, Uthgenannt B, Novack DV, Faccio R (2006) PLCγ2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2. J Clin Invest 116(11):2869–2879. doi:10.1172/JCI28775
Matsumoto M, Sudo T, Saito T, Osada H, Tsujimoto M (2000) Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-κB ligand (RANKL). J Biol Chem 275(40):31155–31161. doi:10.1074/jbc.M001229200
Naito A, Azuma S, Tanaka S, Miyazaki T, Takaki S, Takatsu K, Nakao K, Nakamura K, Katsuki M, Yamamoto T, Inoue J (1999) Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells 4(6):353–362. doi:10.1046/j.1365-2443.1999.00265.x
Rodan GA, Martin TJ (2000) Therapeutic approaches to bone diseases. Science 289(5484):1508–1514. doi:10.1126/science.289.5484.1508
Schwarz P, Rasmussen AQ, Kvist TM, Andersen UB, Jorgensen NR (2012) Paget’s disease of the bone after treatment with Denosumab: a case report. Bone (NY) 50(5):1023–1025
Sun SC (2010) Controlling the fate of NIK: a central stage in noncanonical NF-κB signaling. Sci Signal 3(123):pe18. doi:10.1126/scisignal.3123pe18
Taguchi Y, Gohda J, Koga T, Takayanagi H, Inoue J (2009) A unique domain in RANK is required for Gab2 and PLCγ2 binding to establish osteoclastogenic signals. Genes Cells 14(11):1331–1345. doi:10.1111/j.1365-2443.2009.01351.x
Taguchi Y, Kiga Y, Gohda J, Inoue J (2012) Identification and characterization of anti-osteoclastogenic peptides derived from the cytoplasmic tail of receptor activator of nuclear factor κB. J Bone Miner Metab 30(5):543–553. doi:10.1007/s00774-012-0353-5
Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV (1994) FcRγ chain deletion results in pleiotrophic effector cell defects. Cell 76(3):519–529. doi:10.1016/0092-8674(94)90115-5
Takayanagi H (2007) Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 7(4):292–304. doi:10.1038/nri2062
Takayanagi H, Iizuka H, Juji T, Nakagawa T, Yamamoto A, Miyazaki T, Koshihara Y, Oda H, Nakamura K, Tanaka S (2000) Involvement of receptor activator of nuclear factor κB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum 43(2):259–269. doi:10.1002/1529-0131(200002)43:2<259::AID-ANR4>3.0.CO;2-W
Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3(6):889–901. doi:10.1016/S1534-5807(02)00369-6
Teitelbaum SL, Abu-Amer Y, Ross FP (1995) Molecular mechanisms of bone resorption. J Cell Biochem 59(1):1–10. doi:10.1002/jcb.240590102
Tomasello E, Cant C, Buhring HJ, Vely F, Andre P, Seiffert M, Ullrich A, Vivier E (2000) Association of signal-regulatory proteins β with KARAP/DAP-12. Eur J Immunol 30(8):2147–2156. doi:10.1002/1521-4141(2000)30:8<2147::AID-IMMU2147>3.0.CO;2-1
Vaananen HK, Karhukorpi EK, Sundquist K, Wallmark B, Roininen I, Hentunen T, Tuukkanen J, Lakkakorpi P (1990) Evidence for the presence of a proton pump of the vacuolar H(+)-ATPase type in the ruffled borders of osteoclasts. J Cell Biol 111(3):1305–1311
Wong BR, Josien R, Lee SY, Vologodskaia M, Steinman RM, Choi Y (1998) The TRAF family of signal transducers mediates NF-κB activation by the TRANCE receptor. J Biol Chem 273(43):28355–28359
Yamamoto A, Miyazaki T, Kadono Y, Takayanagi H, Miura T, Nishina H, Katada T, Wakabayashi K, Oda H, Nakamura K, Tanaka S (2002) Possible involvement of IκB kinase 2 and MKK7 in osteoclastogenesis induced by receptor activator of nuclear factor κB ligand. J Bone Miner Res 17(4):612–621. doi:10.1359/jbmr.2002.17.4.612
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
Taguchi, Y., Gohda, J., Inoue, Ji. (2015). NF-κB Signaling in Osteoclastogenesis. In: Inoue, Ji., Takekawa, M. (eds) Protein Modifications in Pathogenic Dysregulation of Signaling. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55561-2_13
Download citation
DOI: https://doi.org/10.1007/978-4-431-55561-2_13
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55560-5
Online ISBN: 978-4-431-55561-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)