Abstract
Intracerebral hemorrhage (ICH) initiates a neuroinflammatory cascade that contributes to substantial neuronal damage and neurological deterioration. Taurine, an abundant amino acid in the nervous system, is reported to reduce inflammatory injury in various central nervous system diseases, but its role and the possible underlying mechanisms in the pathology following ICH remains unclear. This study was designed to evaluate the effect of taurine supplementation on neurological deficits, acute inflammatory responses and white matter injury in a model of ICH in rats. Adult male Sprague–Dawley (SD) rats subjected to collagenase-induced ICH injury were injected intravenously with different concentrations of taurine or vehicle 10 min after ICH and subsequently daily for 3 days. Behavioral studies, brain water content, and assessments of hemorrhagic lesion volume were quantified at day 1 and day 3 post-ICH. Neuronal damage, peri-hematomal inflammatory responses, and white matter injury were determined at 24 h, meanwhile, the content of hydrogen sulfide (H2S) along with the expression of cystathionine-β-synthase (CBS) and P2X7 receptor (P2X7R) in peri-hematomal tissues was analyzed to investigate the possible anti-inflammatory mechanism of taurine. Treatment with a high dosage of taurine (50 mg/kg) significantly attenuated functional deficits and reduced brain edema and hemorrhagic lesion volume after ICH. Taurine administration also resulted in significant amelioration of neuronal damage and white matter injury. These changes were associated with marked reductions in neutrophil infiltration, glial activation, and expression levels of inflammatory mediators. Moreover, the anti-inflammatory effect of taurine was accompanied by increased H2S content, enhanced CBS expression, and less expression of P2X7R. Our study demonstrated that the high dosage of taurine supplementation effectively mitigated the severity of pathological inflammation and white matter injury after ICH, and the mechanism may be related to upregulation of H2S content and reduced P2X7R expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spontaneous intracerebral hemorrhage (ICH) results from the rupture of blood vessels in the brain. It accounts for 8–15% of all strokes in Western societies and 20–30% among Asian populations (Keep et al. 2012). Since no satisfactory surgical or pharmacologic treatments have been found for clinical practice, most patients either die or are left with significant neurological deficit during insults (Sangha and Gonzales 2011). Mounting evidence suggests that neuroinflammation is a hallmark of hemorrhagic stroke pathology, which is characterized by glial activation, neutrophil and macrophage recruitment, upregulation of cytokines, adhesion molecules, and chemokines (Hwang et al. 2011; Chen et al. 2015). Excessive inflammatory responses are associated with diffuse brain edema, white matter injury, and consequent neurologic impairments (Wang 2010; Zuo et al. 2017). Therefore, strategies aimed at regulating the inflammatory response has become a focus of efforts to develop therapeutic approaches for ICH.
Taurine (2-aminoethane sulfonic acid) is a sulfur-containing free amino acid that is abundantly found in mammals (Jacobsen and Smith 1968). It can be synthesized in vivo by cysteine in the presence of cysteine dioxygenase and also obtained from dietary sources, such as eggs, meat, and seafood (Wu 1982; Yamori et al. 2010). Taurine possesses a number of cytoprotective properties through its actions as an osmoregulator (Huxtable 1992), neuromodulator (Kuriyama 1980), calcium regulator (Foos and Wu 2002), antioxidant (Schaffer et al. 2009), and anti-inflammation factor (Miao et al. 2012). There is a growing body of preclinical data that demonstrates taurine’s anti-inflammatory effects in both neural and systemic inflammation including spinal cord injury (Nakajima et al. 2010), ischemic stroke (Sun et al. 2012), traumatic brain injury (Wang et al. 2016), hepatic ischemia reperfusion (Zhang et al. 2010), and lung injury (Guler et al. 2014). However, the effects of taurine on neuroinflammation after ICH and its possible underlying mechanisms remain unknown.
H2S is synthesized from the 2 sulfur-containing amino acids, l-cysteine and l-methionine, mainly by the enzyme cystathionine-β-synthetase (CBS) in the central nervous system (Kamoun 2004). As taurine functions in the methionine cycle and can be converted by cysteine, it is probably a substrate for the synthesis of H2S to increase CBS expression, which has been investigated in some cardiovascular studies (Sun et al. 2016; DiNicolantonio et al. 2017). Our previous studies demonstrated that maintaining appropriate H2S concentrations in the central nervous system may represent a potential therapeutic strategy for ICH-induced inflammatory injury via downregulating P2X7R expression (Zhao et al. 2017). Therefore, we hypothesized that taurine supplementation may boost H2S production and attenuate ICH-induced inflammatory injury.
In this study, we examined the impact of taurine on cerebral edema, neutrophil infiltration, glial activation, inflammatory factors release, white matter injury, and neurological function in a rat model of ICH. We also investigate the interaction of taurine with the content of H2S along with the expression of cystathionine-β-synthase (CBS) and P2X7 receptor (P2X7R) in peri-hematomal tissues after experimental ICH.
Materials and methods
Animals
Twelve-week-old male SD rats weighing 275 ± 25 g were provided by the Animal Experimental Center of Third Military Medical University. All experimental procedures were approved by the Ethics Committee of Southwest Hospital and performed in accordance with the guidelines in the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and they followed the ARRIVE guidelines.
Animal treatments and experimental groups
A total of 140 rats were used. Animals were randomly divided into the following groups: sham (n = 32); vehicle (n = 42); ICH + low dosage taurine (10 mg/kg/day, n = 12, Sigma, St. Louis, MO, USA); ICH + middle dosage taurine (25 mg/kg/day, n = 12); ICH + high dosage taurine (50 mg/kg/day, n = 42). Taurine was dissolved in 0.9% saline and administered intravenously 10 min after ICH in a volume of 2 ml/kg and subsequently once daily for 3 days. Behavioral studies, brain water content, and assessments of hemorrhagic lesion volume were quantified at day 1 and day 3 post-ICH. Neuronal apoptosis, white matter injury, a series of inflammatory mediators including interleukin-1β (IL-1β), interleukin (IL-6), tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-2 (MIP-2), inducible nitric oxide synthase (iNOS), and the levels of H2S content along with the expression of CBS and P2X7R were assayed 24 h after ICH.
Intracerebral hemorrhage model in rats
Experimental ICH was induced by stereotactic-guided injection of collagenase type VII (0.2 units in 1 μL of saline) into the basal ganglia area as previously described (Zhao et al. 2017). The rats were fastened on a stereotaxic apparatus under sodium pentobarbital anesthesia (100 mg/kg intraperitoneally, Sigma-Aldrich, St. Louis, MO, USA), the skull exposed and the bregma revealed. A 1-mm cranial burr hole was drilled in the skull (coordinates: 1.5 mm posterior to the bregma, 3.0 mm lateral to the midline), and a microinfusion pump (Harvard Apparatus, Holliston, MA, USA) was inserted with collagenase infused into the right basal ganglia (5.5-mm deep from the dura mater) at a rate of 0.5 μL/min. The needle was left in place for an additional 10 min after injection to prevent reflux and then slowly withdrawn over 5 min. After the surgery, the skull hole was sealed with bone wax and the incision was closed with sutures. Rats were maintained at a 37.0 ± 0.5 °C body temperature using a heated pad throughout the surgery and recovery period. Sham-operated rats received an equal volume of saline in the same manner.
Modified Garcia test
A 12-point score system named modified Garcia test was conducted in a blinded fashion as previously reported (Zhao et al. 2016). Neurological deficits at 24 and 72 h after ICH were evaluated. Briefly, this test consisted of four individual tests covering side stroke, vibrissae touch, limb symmetry, and lateral turning. A score of 0 (worst performance) to 3 (best performance) was given for each sub-test and a total Garcia score was calculated as the sum of all subtests.
Corner turn test
Corner turn test was also conducted at 24 and 72 h after ICH to assess the neurological deficits as we previously reported (Zhao et al. 2016). Briefly, rats were allowed to walk into a 30-degree corner. On exiting the corner, the rat could turn either to the left or the right, and this choice was recorded. Trials were repeated ten times with 30 s interval, and the percentage of right turns was calculated.
Fluoro-Jade C (FJC) staining
FJC is a polyanionic fluorescein derivative that binds with high sensitivity and specificity to degenerating neurons. Fluoro-Jade C staining (Millipore, Temecula, CA, USA) of brain sections was performed as previously described with slight modifications (Zhao et al. 2017). Briefly, selected sections were rehydrated in graded ethanol solutions (80, 70, and 50%, 5 min each) and distilled water, incubated in 0.06% KMnO4 for 10 min, rinsed in distilled water for 2 min, stained with a 0.0001% solution of FJC in 0.1% acetic for 10 min, and rinsed in distilled water for 5 min. Sections were observed and photographed under a confocal microscope (Zeiss AxioCam, Germany), and FJC-positive neurons were counted by Image J (National Institutes of Health, Bethesda, MD, USA).
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining
The TUNEL assay was performed using a commercial kit that labels DNA strand breaks with fluorescein isothiocyanate (FITC; In Situ Cell Death Detection Kit, Roche Molecular Biochemicals, Mannheim, Germany). Briefly, selected sections were pretreated with 20 mg/mL proteinase-K in 10 mM Tris–HCl at 37 °C for 15 min. These sections were then rinsed in phosphate-buffered saline and incubated in 0.3% hydrogen peroxide dissolved in a hydrous methanol for 10 min. The sections were then incubated in 0.1% sodium citrate and 0.1% Triton X-100 solution for 2 min at 4 °C. After several washes with phosphate-buffered saline, sections were incubated with 50 μL of TUNEL reaction mixture containing terminal deoxynucleotidyl transferase (TdT) and fluorescein-dUTP for 60 min at 37 °C under humidified conditions, and neuronal nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Each section was observed and photographed under a confocal microscope. Negative controls were obtained by omitting the TdT enzyme.
Measurements of hemorrhage volume and hemorrhagic lesion volume
The hemorrhage volume of ICH brains was quantified using a hemoglobin assay as previously described (Wang et al. 2017). Briefly, rats were transcardially perfused and their ipsilateral hemispheres were collected following ICH. Distilled water (3 mL) was added to each hemisphere, followed by homogenization for 60 s, sonication on ice for 1 min and centrifugation at 13,000g for 30 min. Drabkin’s reagent (400 μL, Sigma-Aldrich, St Louis, MO, USA) was added to a 100-μL aliquot of supernatant (which contained the hemoglobin) and allowed to stand for 15 min at room temperature. The hemoglobin absorbance was measured at 540 nm by a spectrophotometer and quantified using a standard curve. The results are presented as microliters of blood to represent the hemoglobin content in the ipsilateral hemispheres.
Hemorrhagic lesion volume of ICH brains was determined by hematoxylin and eosin (H&E) staining of coronal sections (1 mm apart) and calculated using Image-Pro Plus 6.0 software (Media Cybernetics, USA) to span the entire hematoma.
Brain water content
Animals were euthanized under deep anesthesia and decapitated for brain water content determination as previously described (Yang et al. 2015). Briefly, the brain specimen was quickly divided into ipsilateral cortex (Ipsi-CX) and basal ganglia (Ipsi-BG) and contralateral cortex (Cont-CX) and basal ganglia (Cont-BG). All specimens were weighed as wet weight, then dried in an oven at 105 °C for 72 h and weighed again as dry weight. Brain water content was calculated as percentage of (wet weight − dry weight)/wet weight × 100%.
Immunofluorescence staining
Rats were perfused transcardially with saline, followed by 4% paraformaldehyde under deep anesthesia. Frozen sections (20-μm thickness) were obtained using a cryostat and then washed with a 0.01 M phosphate buffer solution after 30 min of heating at 37 °C. The sections were incubated for 30 min in 5% bovine serum albumin and incubated at 4 °C overnight with the following primary antibodies: rabbit polyclonal anti-myeloperoxidase (MPO) antibody (1:50, Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal anti-ionized calcium binding adapter molecule 1 (Iba1) antibody (1:500, Abcam, Cambridge, MA, USA), goat polyclonal anti-glial fibrillary acidic protein (GFAP) antibody (1:500, Abcam, Cambridge, MA, USA), mouse monoclonal anti-neural/glial antigen 2 (NG2) antibody (1:400, Invitrogen, Carlsbad, CA, USA), and rabbit polyclonal anti-myelin basic protein (MBP) antibody (1:400; Millipore Bioscience Research Reagents). The specimens were incubated with appropriate secondary antibodies for 3 h at 37 °C. Immunofluorescence was examined under a confocal microscope. The number of MPO, Iba1, GFAP, and NG2-positive cells were counted by Image J and the axon density (MBP positive) was counted using Image-Pro Plus software (Media Cybernetics, Rockville, MD, USA).
Transmission electron microscopy
At 24 h post-ICH induction, anesthetized rats were transcardially perfused with 4% formaldehyde in phosphate-buffered saline. The peri-hematomal brain tissues were removed and postfixed with 2% glutaraldehyde and 2% formaldehyde for 15 min. Then, tissues were minced into pieces of 1 × 1 × 1 mm and stored overnight in 2% glutaraldehyde and 2% formaldehyde at 4 °C. After dehydration, samples were impregnated with epoxy resin and sectioned. The sections were double stained with lead citrate and uranyl acetate, and images were obtained using an H-7100 transmission electron microscope (Hitachi, Tokyo, Japan).
Enzyme-linked immunosorbent assay (ELISA)
The peri-hematomal brain tissues were collected and homogenized. The homogenates were centrifuged at 4 °C at 12,000g for 15 min, and supernatants were collected carefully and evaluated in duplicate using IL-1β, IL-6, TNF-α, MCP-1, and MIP-2 assay kits (R&D Systems, Minneapolis, MN, USA), in accordance with the manufacturer’s guidelines. Tissue cytokine concentrations are expressed in picograms per milligram of protein.
Methylene blue assay
As described in our previous study (Zhao et al. 2017), H2S contents were measured using a methylene blue assay to determine the potential for H2S production of taurine.
Quantitative polymerase chain reaction (qPCR)
Total RNA was extracted from peri-hematomal brain tissues using Trizol reagent (Invitrogen, Camarillo, CA, USA). Isolated RNA was reverse-transcribed into cDNA using a cDNA synthesis kit (Vazyme, Jiangsu, China), in accordance with the manufacturer’s protocols. qPCR was performed using synthetic primers and SYBR Green (Thermo, Rockford, IL, USA) with an IQ5 Detection System. After incubating at 50 °C for 2 min and 95 °C for 10 min, the samples were subjected to 40 cycles of 95 °C for 15 s and 60 °C for 1 min. β-Actin was used as an endogenous control gene. The sequences of the primers specific for the P2X7R was as follows:
P2X7R, 5′-CTACTCTTCGGTGGGGGCTT-3′ (forward primer),
P2X7R, 5′-CTCTGGATCCGGGTGACTTT-3′ (reverse primer),
β-actin, 5′-TGCCCATCTATGAGGGTTACG-3′ (forward primer),
β-actin, 5′-TAGAAGCATTTGCGGTGCACG-3′ (reverse primer).
Western blot analysis
Total protein was extracted from brain tissues surrounding the hemorrhagic region at 24 h after ICH and subjected to western blot analysis, as described in our previous studies (Zhao et al. 2016). The following primary antibodies were used: rabbit polyclonal anti-iNOS antibody (1:500, Abcam, Cambridge, MA, USA), rabbit polyclonal anti-CBS antibody (1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and rabbit polyclonal anti-P2X7R antibody (1:500, Alomone Labs, Jerusalem, Israel); β-actin (1:1000, Beyotime, Shanghai, China) was used as a loading control. The proteins were detected on nitrocellulose membranes with enhanced chemiluminescence reagents (GE Healthcare, Beijing, China), and the blot bands were quantified by densitometry with Image J software (National Institutes of Health, Bethesda, MD, USA). The results are expressed as a relative density ratio, which was normalized to the mean value of the sham group.
Statistical analysis
Data were expressed as mean ± SEM. Neurobehavioral data were analyzed using Kruskal–Wallis one-way analysis of variance on ranks, followed by the Student–Newman–Keuls method. All other data were analyzed using one-way analysis of variance, followed by Tukey post hoc test. p value < 0.05 was considered to be statistically significant. All statistical analyses were performed using SigmaPlot 10.0 for Windows (Systat Software Inc., San Jose, CA, USA).
Results
Taurine attenuated neurological deficits post-ICH
To examine whether taurine supplementation results in improved neurologic function, the scores of modified Garcia test and corner turn test were assessed on days 1 and 3 after ICH. Compared with the sham group, ICH rats treated with vehicle exhibited significant neurobehavioral deficits on both modified Garcia test and corner turn test at 24 and 72 h after ICH (p < 0.05, Fig. 1a, b). Treatment with taurine 10 mg/kg did not improve neurobehavioral deficits at 24 and 72 h after ICH (p > 0.05 vs. vehicle), but 25 and 50 mg/kg treatments significantly ameliorated neurobehavioral outcomes 72 h after ICH, whereas only 50 mg/kg treatment was effective at 24 h after ICH when compared with the vehicle group (p < 0.05, Fig. 1a, b).
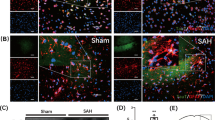
The effects of taurine supplementation on neurological deficits and degenerating neurons after ICH. a Modified Garcia score and b corner turn score in the sham, vehicle, and taurine (different dosages) groups at 24 and 72 h after ICH. c Representative Fluoro-Jade C staining images and d quantitative analyses of Fluoro-Jade C-positive neurons surrounding the hematoma in the sham, vehicle, and taurine (50 mg/kg) groups at 24 h after ICH. e Representative images of TUNEL-stained (green) and DAPI-stained (blue) brain sections in the peri-hematomal area in the sham, vehicle, and taurine (50 mg/kg) groups at 24 h after ICH. f Quantitative analyses of TUNEL-positive cells surrounding the hematoma in the sham, vehicle, and taurine (50 mg/kg) groups at 24 h after ICH. n = 6 for modified Garcia test and corner turn test; n = 5 for Fluoro-Jade C and TUNEL staining; Scale bar = 50 μm; *p < 0.05 vs. sham; #p < 0.05 vs. vehicle
We employed FJC staining to evaluate the neuronal damage around the hematoma. The results indicated that ICH induced a significant increase in FJC-positive neurons, and treatment with taurine 50 mg/kg decreased about 28% of neuronal damage in the peri-hematomal area when compared with the vehicle group at 24 h after ICH (p < 0.05, Fig. 1c, d). In addition, compared with the sham group, the vehicle group exhibited an increased number of TUNEL-positive cells in the peri-hematomal area at 24 h after ICH (p < 0.05, Fig. 1e, f). Remarkably, taurine 50 mg/kg treatment induced a significant reduction of about 25% in the number of TUNEL-positive cells in the peri-hematomal area compared with the vehicle group (p < 0.05, Fig. 1e, f).
Taurine reduced hemorrhagic lesion volume and brain water content after ICH
Using hemoglobin assay analysis, we demonstrated that taurine at a dosage of 50 mg/kg did not significantly affect hemorrhage volume compared with vehicle treatment at 24 h after ICH (p > 0.05, Fig. 2a). This suggests that taurine had no effect on collagenase-induced bleeding.
The effects of taurine supplementation on hemorrhagic lesion volume and brain edema after ICH. a Representative hemorrhage volume measured at 24 h after ICH by spectrophotometric hemoglobin assay in rats treated with either vehicle or taurine (50 mg/kg). b Representative hematoxylin and eosin-stained sections and quantification of the hemorrhagic lesion volume in rats treated with either vehicle or taurine (50 mg/kg) at 72 h after ICH. Brain water content assessment at c 24 h and d 72 h after ICH in the sham, vehicle, and taurine (different dosages) groups. n = 5 for the quantification of hemorrhage volume and hemorrhagic lesion volume; n = 6 for brain water content assessment. Scale bar = 1 mm; *p < 0.05 vs. sham; #p < 0.05 vs. vehicle
Brain lesions were identified by lack of color on sections stained with H&E. Our results demonstrated that the brain lesion was evidently mitigated by taurine 50 mg/kg treatment (39.53 ± 11.24) compared with that in the vehicle-treated group (70.05 ± 18.42) on day 3 after ICH (p < 0.05, Fig. 2b), indicating that taurine could promote tissue reconstruction.
Because swelling contributes to brain damage and cell death after severe stroke, we also measured the percentage of water content in different brain sections to evaluate brain edema. At 24 h after ICH, brain water content in the ipsilateral cortex and ipsilateral and contralateral basal ganglia were significantly increased compared with the sham group, and only in the ipsilateral and contralateral basal ganglia brain water content was increased at 72 h post-ICH (p < 0.05, Fig. 2c, d). Compared with the vehicle group, taurine 50 mg/kg treatment significantly reduced brain water content in the ipsilateral basal ganglia at both 24 and 72 h post-ICH (taurine 50 mg/kg, 80.68 ± 0.34% vs. vehicle, 82.21 ± 0.13%, taurine 50 mg/kg, 81.73 ± 0.29% vs. vehicle, 83.56 ± 0.16%, respectively, p < 0.05), but not in the ipsilateral cortex and contralateral basal ganglia at 24 h post-ICH or in the contralateral basal ganglia at 72 h post-ICH (p > 0.05, Fig. 2c, d). However, treatment with taurine 10 and 25 mg/kg could not reduce the percentage of water content in the ipsilateral basal ganglia at both 24 and 72 h post-ICH compared with the vehicle group (p > 0.05). Though 25 mg/kg treatment had a mitigating effect on brain water content in the ipsilateral basal ganglia at 72 h post-ICH, it did not reach statistical significance compared with the vehicle group (p > 0.05, Fig. 2c, d).
Taurine reduced microglial accumulation, neutrophil infiltration, and astrocytic activity after ICH
Iba-1 immunofluorescence labeling was used to detect the effect of taurine treatment on microglial accumulation after ICH. The number of microglia in the peri-hematomal region on day 1 after ICH was evidently increased compared with the sham group (p < 0.05), but rats treated with taurine were induced about 40% less Iba1-positive cells than those in the vehicle group (p < 0.05, Fig. 3a).
The effects of taurine supplementation on microglial accumulation, neutrophil infiltration, and astrocytic activity after ICH. a Representative photographs of immunofluorescence staining and quantitative analyses of Iba1-positive cells surrounding the hematoma in the sham, vehicle, and taurine (50 mg/kg) groups at 24 h after ICH. b Representative photographs of immunofluorescence staining and quantitative analyses of MPO-positive cells surrounding the hematoma in the sham, vehicle, and taurine (50 mg/kg) groups at 24 h after ICH. c Representative photographs of immunofluorescence staining and quantitative analyses of GFAP-positive cells surrounding the hematoma in the sham, vehicle, and taurine (50 mg/kg) groups at 24 h after ICH. n = 5 for immunofluorescence staining analysis; n = 6 for brain water content assessment; Scale bar = 25 μm; *p < 0.05 vs. sham; #p < 0.05 vs. vehicle
Neutrophil infiltration occurs after activation of microglia and contributes to early hemorrhagic brain injury after ICH. MPO-positive neutrophils were evidently increased in the peri-hematomal area on day 1 after ICH. Taurine treatment (50 mg/kg) reduced about 30% of their number compared with that in vehicle-treated rats (p < 0.05, Fig. 3b).
GFAP immunofluorescence labeling was used to examine the effect of taurine treatment on ICH-induced astrocyte reactivity. Reactive astrocytes in the peri-hematomal area had more GFAP-immunoreactive processes than did resting astrocytes on the striatum in the sham group (p < 0.05). Additionally, the immunoreactivity was more intense, and the processes were longer and thicker. Taurine treatment (50 mg/kg) reduced about 33% of the fluorescence intensity of GFAP in the peri-hematomal area at 24 h post-ICH compared with the vehicle group (p < 0.05, Fig. 3c).
Taurine inhibited inflammatory mediator release after ICH
We then assessed whether taurine influenced the expression of inflammatory mediators following ICH. ELISA results demonstrated that ICH induced a significant increase in IL-1β, IL-6, TNF-α, MCP-1, and MIP-2 protein expression in peri-hematomal brain tissues, but treatment with taurine (50 mg/kg) reduced over 25% of the IL-1β, IL-6, TNF-α, MCP-1, and MIP-2 levels at 24 h post-ICH (p < 0.05 vs. vehicle, Fig. 4a–e). Similarly, ICH-induced upregulation of iNOS expression was significantly attenuated by taurine (50 mg/kg) compared with that in the vehicle group (p < 0.05, Fig. 4f), which was identified by western blotting.
The effects of taurine supplementation on inflammatory mediator release after ICH. Bar graphs of a IL-1β, b IL-6, c TNF-α, d MCP-1, and e MIP-2 protein concentrations, as assessed by ELISA in the peri-hematomal brain tissues at 24 h after ICH in the sham, vehicle, taurine (50 mg/kg) groups. f Representative immunoblot and quantification of iNOS protein in the peri-hematomal brain tissues at 24 h after ICH in the sham, vehicle, taurine (50 mg/kg) groups. n = 5 for the ELISA and western blotting analysis; n = 6 for brain water content assessment; *p < 0.05 vs. sham; #p < 0.05 vs. vehicle
Taurine decreased NG2 expression, myelin loss, and disorganization after ICH
NG2 is an oligodendrocyte precursor cell marker. NG2 expression correlated with axonal degeneration and oligodendrocyte death (Egashira et al. 2014). Our immunofluorescence results demonstrated that ICH induced a significant increase in the number of NG2-positive cells in the peri-hematomal area at 24 h after ICH, and treatment with taurine (50 mg/kg) reduced almost 50% of this response compared with the vehicle group (p < 0.05, Fig. 5a).
The effects of taurine supplementation on white matter injury induced by ICH. a Representative photographs of immunofluorescence staining and quantitative analyses of of NG2-positive cells surrounding the hematoma in the sham, vehicle, and taurine (50 mg/kg) groups at 24 h after ICH. b Representative photographs of immunofluorescence staining and quantitative analyses of of MBP-positive myelinating cluster surrounding the hematoma in the sham, vehicle, and taurine (50 mg/kg) groups at 24 h after ICH. c Representative ultrastructural changes of myelinated nerve fibers in internal capsule investigated by transmission electron microscopy and quantitative analyses of axon density in the sham, vehicle, and taurine (50 mg/kg) groups at 24 h after ICH. The red arrow indicates the layers of myelinated fibers that became loose and disorganized at 24 h after ICH. n = 5 for immunofluorescence staining and transmission electron microscopy analysis; Scale bar = 100 μm; *p < 0.05 vs. sham; #p < 0.05 vs. vehicle
MBP is the major protein of the myelin sheath, and MBP breakdown is also a characteristic of demyelination after ICH (Li et al. 2016; Zou et al. 2017). We used MBP immunofluorescence labeling to examine the effect of taurine treatment on ICH-induced demyelination. The number of MBP-positive myelinating cluster in ICH groups was significantly less than that in the sham group (p < 0.05), and, notably, taurine (50 mg/kg) significantly protected MBP breakdown at 24 h after ICH compared with the vehicle group (p < 0.05, Fig. 5b).
The ultrastructure features of myelinated nerve fibers in the internal capsule were detected by transmission electron microscope after ICH. In the sham group, the myelinated fibers showed a normal axoplasm with well-preserved cellular structure. The multiple layers of myelin sheath also showed normal appearance: regularly and tightly organized. 24 h after ICH, the myelin sheath became loose and swollen, and the layers of the myelin sheath were disorganized exhibiting an onion-like appearance. However, the pathology of white matter in ICH rats treated with taurine (50 mg/kg) could be improved to some extent compared with that in the vehicle group (Fig. 5c). In addition, we observed that taurine could increase the axon density over 20% at 24 h post-ICH, which was similar to MBP immunofluorescence detection (p < 0.05, Fig. 5c).
Taurine elevated H2S levels and CBS expression, and decreased P2X7R expression after ICH
To elucidate the mechanisms underlying the effects of taurine on neuroinflammation after ICH, we further examined the H2S levels, CBS, and P2X7R expression in the peri-hematomal brain tissues. By methylene blue analysis, we showed that the H2S content was evidently decreased at 24 h after ICH, and ICH rats treated with taurine (50 mg/kg) had about 35% elevated H2S contents compared with the vehicle group (p < 0.05, Fig. 6a).
The effects of taurine supplementation on H2S levels, and the expression of CBS and P2X7R after ICH. a H2S content assessed by methylene blue assay in peri-hematomal brain tissues at 24 h after ICH in the sham, vehicle, and taurine (50 mg/kg) groups. b Representative immunoblot and quantification of CBS protein in the peri-hematomal brain tissues at 24 h after ICH in the sham, vehicle, and taurine (50 mg/kg) groups. c Quantitative analysis of P2X7R mRNA levels by qPCR in the peri-hematomal brain tissues at 24 h after ICH in the sham, vehicle, and taurine (50 mg/kg) groups. d Representative bands and quantitative analysis of P2X7R protein levels by western blotting in the peri-hematomal brain tissues at 24 h after ICH in the sham, vehicle, and taurine (50 mg/kg) groups. n = 5 for the qPCR and western blotting analysis; *p < 0.05 vs. sham; #p < 0.05 vs. vehicle
We then examined the key H2S-synthesizing enzyme CBS by western blotting. The result indicated that ICH induced significant downregulation of CBS protein level, and administration of taurine (50 mg/kg) significantly upregulated CBS expression at 24 h after ICH compared with the vehicle group (p < 0.05, Fig. 6b).
Finally, we tested the effect of taurine on P2X7R expression which mediates the anti-inflammatory effect of H2S after ICH, as previously reported. Both qPCR and western blotting results demonstrated that the elevation of P2X7R expression could be significantly inhibited by taurine (50 mg/kg) supplementation compared with the vehicle group at 24 h after ICH (p < 0.05, Fig. 6c, d).
Discussion
Taurine is one of the most abundant free amino acids in mammalian tissue including the brain. The intracellular/extracellular concentration ratio has been estimated at 400 in neurons, and there is a functional equilibrium among active uptake, passive release, and biosynthesis from cysteine (Jacobson et al. 1985). The acute increases in the extracellular taurine could reflect the severity of brain injury, as higher humoral taurine levels were found in patients suffering from more serious subarachnoid hemorrhage (Barges-Coll et al. 2013), traumatic brain injury (Seki et al. 2005), and Alzheimer’s disease (Chouraki et al. 2017). Meanwhile, the depletion of intracellular taurine may result in the disruption of intracellular homeostasis or enantiostasis, leading to neuronal damage (Huxtable 1992). Taurine may exert its cytoprotective effect through both extracellular and intracellular mechanisms. The extracellular action involves the inhibition of calcium influx, which is attributed to the suppression of taurine on glutamate-mediated depolarization through opening of the chloride channel (Saransaari and Oja 2000). The intracellular action of taurine may be related to its protection of the mitochondrial function by preventing mitochondrial dysfunction from calcium overload (El Idrissi 2008). Other functions of taurine, such as anti-oxidation, anti-inflammation, and osmoregulation, are also contributed to its cytoprotective action. Lack of taurine in the body can cause many functional disorders. Hayes and colleagues (Hayes et al. 1975) reported that cats fed a taurine-deficient diet developed central retinal degeneration. It has also been reported that taurine deficiency is associated with cardiovascular disease (Zulli 2011).
Previous studies have shown that taurine is neuroprotective and might be a useful therapeutic agent for traumatic brain injury, ischemic stroke, and spinal cord injury. To our knowledge, this is the first study to test the efficacy of taurine supplementation in a clinically relevant ICH model, in which the inflammatory responses rapidly occur and contribute to early brain injury. We found that taurine intravenously administered at a dose of 50 mg/kg reduced glial responses, neutrophil infiltration, and expression of inflammatory molecules (e.g., IL-1β, IL-6, TNF-α, MCP-1, MIP-2, and iNOS), which are in line with previous studies showing that taurine suppresses inflammatory responses in animal models of CNS diseases (Nakajima et al. 2010; Sun et al. 2012; Su et al. 2014; Ward et al. 2011). It is known that peri-hematomal edema increases by approximately 75% in the first 24 h after ICH and is associated with adverse outcomes (Hwang et al. 2011). In this study, we showed that taurine treatment reduces brain lesion volume and brain swelling early after ICH, likely by reducing cellular inflammatory responses from microglia and astrocytes and infiltrating neutrophils and macrophages. Inflammatory responses can also activate receptor-dependent apoptotic pathways and contribute to neuronal damage after ICH. In our study, taurine reduced neuronal damage and diminished neurological deficits at 24 h post-ICH, which correlated with a reduction of inflammatory responses. Thus, it seems very likely that taurine may limit brain injury following ICH, in part by blocking the inflammatory responses.
Currently, white matter damage induced by inflammatory mediators, edema, and mechanical force is an important consideration in ICH (Zuo et al. 2017). The demyelination and axonal injury around the hematoma promote neuronal damage and apoptosis, and inundation of inflammatory cells (Ziai 2013). In this study, we evaluated the effect of taurine supplementation on white matter fibers in rats after ICH. Previous studies have suggested that axonal degeneration induces a unique sequence of newly generated NG2-positive cells, so the NG2 expression is considered as a marker of axonal degeneration and oligodendrocyte death (Nielsen et al. 2006; Egashira et al. 2014). In the present study, we observed that taurine treatment led to less NG2 expression in the peri-hematomal brain tissues after ICH compared with the vehicle group, which indicated that taurine had a protective effect on axons from degenerating. MBP is essential for myelin sheath formation. In animal model of multiple sclerosis, the cleavage of MBP to smaller peptides sensitized T cells, causing further damage to the myelin and consequently demyelination (Weissert 2013). Therefore, MBP breakdown is a characteristic of demyelination. In the present study, MBP expression in the internal capsule was downregulated after ICH, but taurine effectively reversed the MBP breakdown. The pathological features of the myelin sheath in transmission electron microscope were the direct evidence of demyelination. In the present study, we found that the myelin sheaths of nerve fibers became swollen and loose at 24 h post-ICH, which was similar to a previous study described (Zhuo et al. 2016). However, taurine not only attenuated the pathological structure, but also increased the axon density after ICH.
Our previous study demonstrated that endogenous H2S synthesis, which plays a pivotal role in P2X7R/NLRP3 inflammasome-associated neuroinflammatory response in the pathogenesis of secondary brain injury, was impaired after ICH (Zhao et al. 2017). For the exploration of the role of taurine in the anti-inflammatory mechanism, we assumed that a correlation may exist between taurine and H2S due to the similar cysteine and methionine cycle they shared. In the present study, we found taurine not only restored the content of H2S whose synthesis was impaired after ICH injury, but also upregulated the expression of CBS, the predominant cerebral H2S-generating enzyme, the results of which were similar to a study aimed at investigating the effect of taurine on lowering blood pressure (Sun et al. 2016). In addition, we showed that the expression of P2X7R which mediates the anti-inflammatory effect of H2S after ICH was also inhibited by taurine supplementation.
Our study has several potential weaknesses. First, in view of the fact that taurine is known to play an important role in reducing oxidative stress, endoplasmic reticulum (ER) stress, and mitochondrial dysfunction (Jong et al. 2012, 2017; Menzie et al. 2013), it is reasonable to speculate that taurine could exert its neuroprotective roles through other mechanisms aside from anti-inflammation following ICH, as was noted in the present study. Second, it has been reported that classically activated (M1) microglia and macrophages release destructive pro-inflammatory mediators (e.g., IL-1β, IL-6, and TNF-α); by contrast, alternatively activated (M2) microglia and macrophages clear cellular debris through phagocytosis and release numerous protective and trophic factors (e.g., IL-4, IL-10, and BDNF) (Gordon 2003). Therefore, although an induction of pro-inflammatory cytokines in ICH is reversed by taurine, whether taurine supplementation could promote the neuroprotective M2 phenotypic activation of microglia and increase the expression of anti-inflammatory cytokines should be clarified in a further study. Finally, we only evaluated the neuroprotective effects of taurine supplementation within 3 days after ICH in this study. The long-term outcome of taurine supplementation after ICH should be addressed.
Conclusion
Taken together, inflammation is an important contributor to secondary brain injury after ICH, which gives rise to the damage of white matter, growth of brain edema, and impairment of neurological functions. Taurine (50 mg/kg) supplementation has a potential therapy for experimental ICH by its anti-inflammatory effect and the novel mechanism may be related to upregulation of H2S content and reduced P2X7R expression. Further study is needed to elucidate the detailed anti-inflammatory mechanism of taurine against experimental ICH.
References
Barges-Coll J, Perez-Neri I, Avendano J, Mendez-Rosito D, Gomez-Amador JL, Rios C (2013) Plasma taurine as a predictor of poor outcome in patients with mild neurological deficits after aneurysmal subarachnoid hemorrhage. J Neurosurg 119(4):1021–1027. https://doi.org/10.3171/2013.4.JNS121558
Chen S, Yang Q, Chen G, Zhang JH (2015) An update on inflammation in the acute phase of intracerebral hemorrhage. Transl Stroke Res 6(1):4–8. https://doi.org/10.1007/s12975-014-0384-4
Chouraki V, Preis SR, Yang Q, Beiser A, Li S, Larson MG, Weinstein G, Wang TJ, Gerszten RE, Vasan RS, Seshadri S (2017) Association of amine biomarkers with incident dementia and Alzheimer’s disease in the Framingham Study. Alzheimers Dement. https://doi.org/10.1016/j.jalz.2017.04.009
DiNicolantonio JJ, OK JH, McCarty MF (2017) Boosting endogenous production of vasoprotective hydrogen sulfide via supplementation with taurine and N-acetylcysteine: a novel way to promote cardiovascular health. Open Heart 4(1):e000600. https://doi.org/10.1136/openhrt-2017-000600
Egashira Y, Hua Y, Keep RF, Xi G (2014) Acute white matter injury after experimental subarachnoid hemorrhage: potential role of lipocalin 2. Stroke 45(7):2141–2143. https://doi.org/10.1161/STROKEAHA.114.005307
El Idrissi A (2008) Taurine increases mitochondrial buffering of calcium: role in neuroprotection. Amino Acids 34(2):321–328. https://doi.org/10.1007/s00726-006-0396-9
Foos TM, Wu JY (2002) The role of taurine in the central nervous system and the modulation of intracellular calcium homeostasis. Neurochem Res 27(1–2):21–26
Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3(1):23–35. https://doi.org/10.1038/nri978
Guler L, Tavlasoglu M, Yucel O, Guler A, Sahin MA, Kurkluoglu M, Sirin Y, Eken A, Gamsizkan M, Dakak M, Gurkok S, Genc O (2014) Taurine attenuates lung ischemia-reperfusion injury after lung transplantation in rats. J Anesth 28(3):347–353. https://doi.org/10.1007/s00540-013-1741-2
Hayes KC, Carey RE, Schmidt SY (1975) Retinal degeneration associated with taurine deficiency in the cat. Science 188(4191):949–951
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72(1):101–163
Hwang BY, Appelboom G, Ayer A, Kellner CP, Kotchetkov IS, Gigante PR, Haque R, Kellner M, Connolly ES (2011) Advances in neuroprotective strategies: potential therapies for intracerebral hemorrhage. Cerebrovasc Dis 31(3):211–222. https://doi.org/10.1159/000321870
Jacobsen JG, Smith LH (1968) Biochemistry and physiology of taurine and taurine derivatives. Physiol Rev 48(2):424–511
Jacobson I, Sandberg M, Hamberger A (1985) Mass transfer in brain dialysis devices—a new method for the estimation of extracellular amino acids concentration. J Neurosci Methods 15(3):263–268
Jong CJ, Azuma J, Schaffer S (2012) Mechanism underlying the antioxidant activity of taurine: prevention of mitochondrial oxidant production. Amino Acids 42(6):2223–2232. https://doi.org/10.1007/s00726-011-0962-7
Jong CJ, Ito T, Prentice H, Wu JY, Schaffer SW (2017) Role of mitochondria and endoplasmic reticulum in taurine-deficiency-mediated apoptosis. Nutrients. https://doi.org/10.3390/nu9080795
Kamoun P (2004) Endogenous production of hydrogen sulfide in mammals. Amino Acids 26(3):243–254. https://doi.org/10.1007/s00726-004-0072-x
Keep RF, Hua Y, Xi G (2012) Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol 11(8):720–731. https://doi.org/10.1016/S1474-4422(12)70104-7
Kuriyama K (1980) Taurine as a neuromodulator. Fed Proc 39(9):2680–2684
Li R, Ma K, Zhao H, Feng Z, Yang Y, Ge H, Zhang X, Tang J, Yin Y, Liu X, Tan L, Feng H (2016) Cattle encephalon glycoside and ignotin reduced white matter injury and prevented post-hemorrhagic hydrocephalus in a rat model of intracerebral hemorrhage. Sci Rep 6:35923. https://doi.org/10.1038/srep35923
Menzie J, Prentice H, Wu JY (2013) Neuroprotective mechanisms of taurine against ischemic stroke. Brain Sci 3(2):877–907. https://doi.org/10.3390/brainsci3020877
Miao J, Zhang J, Zheng L, Yu X, Zhu W, Zou S (2012) Taurine attenuates Streptococcus uberis-induced mastitis in rats by increasing T regulatory cells. Amino Acids 42(6):2417–2428. https://doi.org/10.1007/s00726-011-1047-3
Nakajima Y, Osuka K, Seki Y, Gupta RC, Hara M, Takayasu M, Wakabayashi T (2010) Taurine reduces inflammatory responses after spinal cord injury. J Neurotrauma 27(2):403–410. https://doi.org/10.1089/neu.2009.1044
Nielsen HH, Ladeby R, Drojdahl N, Peterson AC, Finsen B (2006) Axonal degeneration stimulates the formation of NG2+ cells and oligodendrocytes in the mouse. Glia 54(2):105–115. https://doi.org/10.1002/glia.20357
Sangha N, Gonzales NR (2011) Treatment targets in intracerebral hemorrhage. Neurotherapeutics 8(3):374–387. https://doi.org/10.1007/s13311-011-0055-z
Saransaari P, Oja SS (2000) Taurine and neural cell damage. Amino Acids 19(3–4):509–526
Schaffer SW, Azuma J, Mozaffari M (2009) Role of antioxidant activity of taurine in diabetes. Can J Physiol Pharmacol 87(2):91–99. https://doi.org/10.1139/Y08-110
Seki Y, Kimura M, Mizutani N, Fujita M, Aimi Y, Suzuki Y (2005) Cerebrospinal fluid taurine after traumatic brain injury. Neurochem Res 30(1):123–128
Su Y, Fan W, Ma Z, Wen X, Wang W, Wu Q, Huang H (2014) Taurine improves functional and histological outcomes and reduces inflammation in traumatic brain injury. Neuroscience 266:56–65. https://doi.org/10.1016/j.neuroscience.2014.02.006
Sun M, Zhao Y, Gu Y, Xu C (2012) Anti-inflammatory mechanism of taurine against ischemic stroke is related to down-regulation of PARP and NF-kappaB. Amino Acids 42(5):1735–1747. https://doi.org/10.1007/s00726-011-0885-3
Sun Q, Wang B, Li Y, Sun F, Li P, Xia W, Zhou X, Li Q, Wang X, Chen J, Zeng X, Zhao Z, He H, Liu D, Zhu Z (2016) Taurine supplementation lowers blood pressure and improves vascular function in prehypertension: randomized, double-blind, placebo-controlled study. Hypertension 67(3):541–549. https://doi.org/10.1161/HYPERTENSIONAHA.115.06624
Wang J (2010) Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol 92(4):463–477. https://doi.org/10.1016/j.pneurobio.2010.08.001
Wang Q, Fan W, Cai Y, Wu Q, Mo L, Huang Z, Huang H (2016) Protective effects of taurine in traumatic brain injury via mitochondria and cerebral blood flow. Amino Acids 48(9):2169–2177. https://doi.org/10.1007/s00726-016-2244-x
Wang J, Wang G, Yi J, Xu Y, Duan S, Li T, Sun XG, Dong L (2017) The effect of monascin on hematoma clearance and edema after intracerebral hemorrhage in rats. Brain Res Bull 134:24–29. https://doi.org/10.1016/j.brainresbull.2017.06.018
Ward RJ, Lallemand F, de Witte P, Crichton RR, Piette J, Tipton K, Hemmings K, Pitard A, Page M, Della Corte L, Taylor D, Dexter D (2011) Anti-inflammatory actions of a taurine analogue, ethane beta-sultam, in phagocytic cells, in vivo and in vitro. Biochem Pharmacol 81(6):743–751. https://doi.org/10.1016/j.bcp.2010.12.030
Weissert R (2013) The immune pathogenesis of multiple sclerosis. J Neuroimmune Pharmacol 8(4):857–866. https://doi.org/10.1007/s11481-013-9467-3
Wu JY (1982) Purification and characterization of cysteic acid and cysteine sulfinic acid decarboxylase and l-glutamate decarboxylase from bovine brain. Proc Natl Acad Sci USA 79(14):4270–4274
Yamori Y, Taguchi T, Hamada A, Kunimasa K, Mori H, Mori M (2010) Taurine in health and diseases: consistent evidence from experimental and epidemiological studies. J Biomed Sci 17(Suppl 1):S6. https://doi.org/10.1186/1423-0127-17-S1-S6
Yang F, Wang Z, Zhang JH, Tang J, Liu X, Tan L, Huang QY, Feng H (2015) Receptor for advanced glycation end-product antagonist reduces blood-brain barrier damage after intracerebral hemorrhage. Stroke 46(5):1328–1336. https://doi.org/10.1161/STROKEAHA.114.008336
Zhang F, Mao Y, Qiao H, Jiang H, Zhao H, Chen X, Tong L, Sun X (2010) Protective effects of taurine against endotoxin-induced acute liver injury after hepatic ischemia reperfusion. Amino Acids 38(1):237–245. https://doi.org/10.1007/s00726-009-0233-z
Zhao H, Zhang X, Dai Z, Feng Y, Li Q, Zhang JH, Liu X, Chen Y, Feng H (2016) P2X7 receptor suppression preserves blood-brain barrier through inhibiting rhoa activation after experimental intracerebral hemorrhage in rats. Sci Rep 6:23286. https://doi.org/10.1038/srep23286
Zhao H, Pan P, Yang Y, Ge H, Chen W, Qu J, Shi J, Cui G, Liu X, Feng H, Chen Y (2017) Endogenous hydrogen sulphide attenuates NLRP3 inflammasome-mediated neuroinflammation by suppressing the P2X7 receptor after intracerebral haemorrhage in rats. J Neuroinflammation 14(1):163. https://doi.org/10.1186/s12974-017-0940-4
Zhuo F, Qiu G, Xu J, Yang M, Wang K, Liu H, Huang J, Lu W, Liu Q, Xu S, Huang S, Sun S (2016) Both endoplasmic reticulum and mitochondrial pathways are involved in oligodendrocyte apoptosis induced by capsular hemorrhage. Mol Cell Neurosci 72:64–71. https://doi.org/10.1016/j.mcn.2016.01.009
Ziai WC (2013) Hematology and inflammatory signaling of intracerebral hemorrhage. Stroke 44(6 Suppl 1):S74–S78. https://doi.org/10.1161/STROKEAHA.111.000662
Zou X, Wu Z, Zhu W, Chen L, Mao Y, Zhao F (2017) Effectiveness of minocycline in acute white matter injury after intracerebral hemorrhage. J Neurosurg 126(6):1855–1862. https://doi.org/10.3171/2016.5.JNS152670
Zulli A (2011) Taurine in cardiovascular disease. Curr Opin Clin Nutr Metab Care 14(1):57–60. https://doi.org/10.1097/MCO.0b013e328340d863
Zuo S, Pan P, Li Q, Chen Y, Feng H (2017) White matter injury and recovery after hypertensive intracerebral hemorrhage. Biomed Res Int 2017:6138424. https://doi.org/10.1155/2017/6138424
Funding
This work was supported by the National Basic Research Program of China (973 Program, Grant number 2014CB541600), the National Natural Science Foundation of China (Grant numbers 81501002), the Basic Science and Advanced Technology Research Project of Chongqing (Grant number cstc2016jcyjA0114), and the Major Innovation Project of Southwest Hospital (Grant number SWH2016ZDCX1011).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: S. W. Schaffer.
Rights and permissions
About this article
Cite this article
Zhao, H., Qu, J., Li, Q. et al. Taurine supplementation reduces neuroinflammation and protects against white matter injury after intracerebral hemorrhage in rats. Amino Acids 50, 439–451 (2018). https://doi.org/10.1007/s00726-017-2529-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-017-2529-8