Abstract
Taurine (Tau) is reported to have a key role in the regulation of the innate immune response and thus reduce tissue damage induced by bacterial infection. In this study, the effects of Tau on a rat model of mastitis induced by Streptococcus uberis (S. uberis) and the changes of T regulatory cells (Tregs) were assessed. Starting on gestation day 14 and continuing until parturition, 100 mg/kg of taurine (group TS) or an equal volume of physiological saline (group CS) was administered daily, per os. Seventy-two hours after parturition, rats were infused with approximately 100 cfu of S. uberis into each of two mammary glands. The results showed that the resultant inflammation, evidenced by swelling, secretory epithelial cell degeneration, increased adipose tissue and neutrophil (PMN) infiltration were evident in mammary tissue following injection with S. uberis. Pre-treatment with Tau attenuated these morphologic changes, the expression of interleukin (IL)-2, interferon (INF)-γ mRNA, myeloperoxidase (MPO) activity and N-acetyl-β-D-glucosaminidase (NAGase) in mammary tissue. The percentages of Foxp3 + CD25 + CD4 +/lymphocytes (Tregs) were dramatically increased after the S. uberis challenge. Significant differences (P < 0.05) were observed at 24, and 72 h post S. uberis - injection (PI) in CS. Pre-treatment further increased the percentage of Tregs and a significant difference between CS and TS (P < 0.05) was apparent at 24 h PI. Our data indicate that in rats, Tau can be used to regulate the immune response following infection by S. uberis and consequently prevent mammary tissue damage by increasing Tregs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mastitis is a debilitating disease that occurs in dairy herds worldwide. Its manifestations are either clinical with localized symptoms including depressed milk production and milk content abnormalities, or subclinical with marginal production loss and lowered milk quality. Both forms of disease produce significant economic loss to producers (Bradley 2002; Hillerton and Berry 2005). Streptococcus uberis (S. uberis) accounts for approximately 33% of the isolates obtained from clinical cases of bovine mastitis (Hillerton and Berry 2005). The relative contribution of S. uberis to clinical mastitis has increased since the introduction of hygienic milking practices (Hillerton et al. 1993). The reason that currently recommended hygiene procedures fail to control S. uberis infections lies in the unique interaction of this organism with the host’s defense mechanisms. Previous work has demonstrated that S. uberis can adhere to, internalize and persist in mammary epithelial cells, and especially encapsulated strains, which can escape the recognitions by the host immune system due to the presence of their outer capsule (Tamilselvam et al. 2006). Phagocytosis and killing of this pathogen by neutrophils (PMNs) has been shown to be ineffective in the prevention of infection, while phagocytosis of Escherichia coli and Staphylococcus aureus by bovine PMNs has been well documented (Hill et al. 1978; Barrio et al. 2003; Field et al. 2003). Thus, a better understanding of the immune response within the mammary gland following S. uberis infection is the first step in formulating effective control programs for this disease.
Although the immune response associated with S. uberis infection of the mammary gland is a very complex biological process differing from that associated with other pathogens, the systemic and innate immune systems are clearly over activated (Bannerman et al. 2004; Moyes et al. 2009). T regulatory cells (Tregs) are thought to be a functionally unique population of T cells, playing an important role in negative regulation of the immune response, thus maintaining immune homeostasis (Golovina and Vonderheide 2010). It has been reported that Tregs have an immunosuppressive property that in some diseases prevents over activation and self-injury by effector cells (Ricciardelli et al. 2008; Wei et al. 2008). These data have led to our interest in the role of Tregs in the development of S. uberis mastitis.
Current practices for the control of mastitis include the use of antibiotics in combination with preventive management techniques. These strategies have worked well against pathogens such as Staphylococcus aureus (Bradley 2002; Hillerton and Berry 2005). However, the extensive use of antibiotics in the treatment and control of mastitis has possible negative implications for human health through an increased risk of antibiotic resistant strains of bacteria that may then enter the food chain (Hillerton and Berry 2005). Clearly, new and innovative approaches for mastitis control are needed. In recent years, research has focused on regulating the natural defense mechanisms of the mammary gland during periods of heightened disease susceptibility (Diarra et al. 2003; Kawai et al. 2003). Our group has demonstrated that retinoid, CpG-ODN, and Bacillus Calmette Guerin polysaccharide nucleic acid (BCG-PSN) administration are effective tools in reducing the incidence of mastitis induced by different causative agents (Zhu et al. 2007b; Miao et al. 2009; Gu et al. 2010).
Taurine (2-aminoethane sulfonic acid) (Tau) is the most abundant free amino acid in most animal tissues and plays an important role in several essential biologic processes (Grimble 2006). A large number of reports suggest a key role for Tau and its derivatives in the innate immune response and its potential use in the prevention and treatment of various topical infections and chronic inflammatory diseases (Erdem et al. 2008; Nagl et al. 2000; Verdrengh and Tarkowski 2005). For example, it has been reported that Tau can protect the organ against endotoxin-induced injury (Erdem et al. 2008). Tau chloramine exerts an inhibitory effect on the development of bone and cartilage damage in infected joints when administered intra-articularly (Verdrengh and Tarkowski 2005). This may attribute to the functions that Tau possesses antioxidant properties and regulates the release of cytokines in animals and humans (Kontny et al. 2000; Grimble 1994; Huxtable 1996). It has been demonstrated in vitro that Tau chloramine inhibits the production of cytokines via fibroblast-like synoviocytes isolated from rheumatoid arthritis patients (Kontny et al. 2000). In a preliminary study, we demonstrated that Tau attenuates endotoxin-induced dysfunction in mouse mammary epithelial cells through reduction in the release of inflammatory mediators (cytokines, NO and so on) (data not published). There are no reports documenting the effect of Tau on mastitis. Herein, we report the effects of Tau on S. uberis-induced mastitis in rats and document the associated changes of Tregs.
Materials and methods
Animals
Seventy-two healthy pregnant SD rats (weighing 300–350 g) were purchased from the Experimental Animal Center of Southeast University (Nanjing, China). They were housed in individual cages and provided water and food ad libitum. Following acclimatization, the rats were randomly divided into two groups (n = 36). The experiments followed the guidelines of the regional Animal Ethics Committee.
Treatment
On gestation day 14, Tau (100 mg/kg) dissolved in sterile pyrogen-free physiological saline (treatment group, TS) or an equal volume of physiological saline (control group, CS) was administrated by gavage each morning until parturition. In order to avoid the stress of gavage, the administration of Tau was terminated at parturition. Seventy-two hours after parturition, 30 rats from each group were injected with approximately 100 cfu of S. uberis (S. uberis 0140 J, capsular strain, ATCC) in 100 μL into the left 4 (L4) and right 4 (R4) teats. Six rats from each group served as controls. The offspring were weaned 2 h prior to experimental inoculation. Following administration of ether anesthesia, the teat area of L4 and R4 was moistened with 75% ethanol, a 33-gauge needle fitted to a 1-mL syringe that was gently inserted into the mammary duct, and 100 μL of S. uberis were slowly injected. Just before the inoculation (control group defined as 0 h) and at 8, 16, 24, 48 and 72 h post S. uberis - injection (PI), six rats at each time point were euthanized. Blood samples were obtained via jugular venapuncture and the serum separated. Mammary tissues were aseptically collected and directly stored at −80°C freezer until analyzed. One milliliter of EDTA anticoagulated blood was collected at the 0, 24, and 72 h timepoints for analysis by flow cytometry.
Preparation of mammary tissue and serum
Mammary tissues were weighed and homogenized (Kinematica AG Switzerland) with sterile physiological saline (1:4, W/V) on ice and then centrifuged at 2,000×g for 40 min at 4°C. Fat was removed, and the supernatant was collected and centrifuged again at 2,000×g for 20 min at 4°C to remove any remaining fat. The resultant supernatant was collected and stored at −20°C for later analysis. Protein concentration was determined using the Bradford method. Serum was separated by centrifugation (2,000×g for 15 min) and stored at −20°C until analyzed.
Histologic examination
Tissue specimens were fixed in 10% formalin for 24 h. Standard dehydration and paraffin-wax embedding procedures were used to produce tissue blocks. Hematoxylin and eosin stained slides were prepared using standard methods. The presence of PMNs in mammary alveoli and histologic changes were estimated by light microscopic (Olympus BH2 Japan) analyses at a magnification of 250× . Four sections of rat mammary tissue were quantified for each animal. Ten fields were randomly selected per tissue section and assigned a score of 1, 2 or 3 according to the number of PMNs per microscopic field, where 1 = none or few PMNs present, 2 = moderate PMN infiltration, and 3 = marked PMN infiltration. Areas occupied by adipose tissue in the tissue samples were semi-quantified using a scoring system where 1 = less than 20% adipose tissue, 2 = 20% to 50% adipose tissue, and 3 = more than 50% adipose tissue. Bleeding and degeneration in mammary alveoli were scored as 1 = no or minimal bleeding and degeneration, 2 = mild bleeding and degeneration, and 3 = severe bleeding and degeneration (Trinidad et al. 1990). Evaluation of each section was carried out in a blinded fashion by two of the authors.
Detection of NAGase and MPO
The activities of N-acetyl-β-D-glucosaminidase (NAGase) and myeloperoxidase (MPO) were determined using commercial kits purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Mammary gland homogenate and serum enzyme activity were quantified, following the manufacturers’ protocols. Briefly, for NAGase, the optical density of paranitrophenol during the reaction (at 37°C) between 4-methylumbelliferyl-N-acetyl-β-glucosaminide substrate with the NAGase contained in the analyzed samples was measured spectrophotometrically in triplicate at a wavelength of 400 nm. One unit of NAGase activity represents the amount of paranitrophenol released from 1-L of mammary homogenate or serum in 15 min at 37°C.
For MPO, tissues were thawed immediately before the assay and homogenized in 10% (W/V) of 20 mM phosphate buffer (pH 7.4). The homogenate was centrifuged at 10,000×g for 10 min at 4°C. The pellet was re-suspended by sonication in 50 mM phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide and then further disrupted by three freeze–thaw cycles. Samples were then centrifuged at 10,000×g for 5 min at 4°C, and the supernatants were collected for assay. MPO activity was assessed by mixing the sample with 3, 3′, 5, 5′-tetramethyl benzidine (TMB) chromogen substrate solution and incubating for 180 s. The reaction was terminated by the addition of 0.18 M H2SO4. The absorbance at 460 nm was determined on the resulting mixture. Horseradish peroxidase was used as a standard, and the results were expressed as U/g protein or U/L.
RNA extraction
Total RNA was extracted from mammary tissue using TRIZOL reagent (Takara, Dalian, China) according to the manufacturer’s protocols. The concentration was quantified by measuring absorbance at 260 nm (Eppendorf Biophotometer). The ratios of absorption (260/280 nm) of all samples were between 1.8 and 2.0. Aliquots of the RNA samples were subjected to electrophoresis through a 1.4% agarose formaldehyde gel to verify their identity.
Real-time quantitative RT-PCR
Synthesis of first strand complementary DNA (cDNA) was performed with reverse transcriptase and Oligo(dT)18 primer (TaKaRa, Dalian, China), according to the manufacturer’s instructions. The final volume of 20 μl contained 10 units of avian myeloblastosis virus (AMV) reverse transcriptase, 1 mM dNTP mixture (TaKaRa, Dalian, China), 20 units of recombinant RNasin ribonuclease inhibitor (TaKaRa, Dalian, China), and 50 pmol of Oligo(dT)18 primer. After incubation (42°C, 60 min), the mixture was heated (95°C, 5 min). An aliquot of the cDNA samples was mixed with 25 μl SYBR® Green PCR Master Mix (TaKaRa, Dalian, China) in the presence of 10 pmol of each forward and reverse primer for INF-γ, IL-2 and IL-4 (Table 1) and then subjected to PCR under standard conditions (43 cycles). As an internal control, the same RT products were subjected to PCR in the presence of a second pair of primers specific to rat β-actin. All primer sequences were obtained from previous studies and synthesized by Invitrogen Biological Company (Shanghai, China) (Zhu et al. 2007a; Ohtsuka et al. 2005). Mixtures were incubated in an ABI Prism 7300 Sequence Detection System (Applied Biosystems) programmed to conduct one cycle (95°C for 10 min) and 43 cycles (95°C for 15 s and 62°C for 1 min). Results (fold changes) were expressed as 2−ΔΔCt with ΔΔCt = (Ct ij − Ct β-actin j) − (Ct i1 − Ct β-actin1), where Ct ij and Ct β-actin j are the Ct for gene i and for β-actin in a sample (named j), and where Ct i1 and Ct β-actin1 are the Ct in sample 1, expressed as the standard. In this study, 0 h for the CS group is designated as standard, thus leading to a relative expression of 1 = 20 at this time point.
Flow cytometric analysis
Peripheral blood leucocytes were isolated from anti-coagulated venous blood using lymphocyte separation medium (diatrizoate-Ficoll 1.083, TBD, Tianjin, China) according to the manufacturer’s instructions. Briefly, after a 1:1 dilution with phosphate-buffered saline, the mixture was placed on lymphocyte separation medium and centrifuged at 600×g for 30 min. The layer of cells remaining at the interface was collected and washed three times in phosphate buffered saline (PBS) at 400×g for 10 min and then re-suspended in PBS.
Phenotypic characteristics of the cells were determined using multicolor fluorescent staining. Peripheral blood leucocytes were stained with combinations of antibodies conjugated with the fluorescent dyes allophycocyanin (APC) and fluoresceine isothiocyanate (FITC) to the following cell surface antigens: cluster of differentiation (CD) 4 and CD25 (eBioscience) before staining the intracellular antigen forkhead box P3 (Foxp3). The expression of Foxp3 was determined via intracellular staining with monoclonal phycoerythrin (PE)-conjugated antibodies to Foxp3 (eBioscience). Fluorescence was measured using a FACSCalibur flow cytometer (BD Biosciences, USA) (Fig. 1), and data analysis was performed using WinMDI software.
Flow cytometry analysis of regulatory T cells in rat peripheral blood. A forward/side scatter plot demonstrates gating of the lymphocyte population (region R1) for further analysis (a). The dot plots reflect the CD4+ cells (region R2) within the lymphocytes gate (b), CD25+ FoxP3+ cells (regulatory T cells, upper right corner) within the CD4+ gate (c), CD4+ FoxP3+ cells (upper right corner) within the lymphocytes gate (d), CD25+ FoxP3+ cells (upper right corner) within the lymphocytes gate (e). CD cluster of differentiation, Foxp3 forkhead box P3, APC allophycocyanin, FITC fluoresceine isothiocyanate, PE phycoerythrin
Statistical analyses
All statistical procedures were computed using statistical software SPSS16.0. Data were expressed as means ± SE. Differences were evaluated by using two-way analysis of variance (ANOVA) followed by a least significant difference t test (LSD) (the levels of NAGase, MPO, IL-2, INF-γ, IL-4 and Tregs). The histologic data were expressed as median ± SD and analyzed with a non-parametric test (χ2 test). Differences were considered significant at P < 0.05.
Results
Histologic analyses
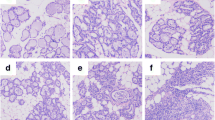
As shown in Fig. 2, no histologic changes were observed in either CS or TS mammary glands before infection (Fig. 2a, g), whereas inflammation was present in mammary tissue after infection with S. uberis. This response was characterized by PMN infiltration, increased bleeding, epithelial cell degeneration, and excess adipose tissue. PMN infiltration reached a maximum at 16 h PI (Fig. 2c, i). Bleeding and degeneration were most severe at 24 h PI (Fig. 2d, j), followed by a gradual decrease in both groups. More adipose tissue was evident at 48 h PI in CS (Fig. 2e), but the most serious changes in TS were observed at 16 h PI (Fig. 2i). Compared to CS, there was a significant reduction in PMN infiltration at 16, 24, 48, and 72 h PI. Adipose tissue reduction was seen at 48 h PI and bleeding and epithelial cell degeneration at 48 and 72 h PI. The values for PMN infiltration, adipose tissue, bleeding and epithelial degeneration are presented in Tables 2 and 3.
Histology of the mammary gland after injection with Streptococcus uberis (H.E. ×250) a–f illustrate histologic changes in the control mammary gland before and 8, 16, 24, 48, 72 h post infection with Streptococcus uberis, respectively. g–l Illustrate histologic changes in the mammary gland in the treatment group before and 8, 16, 24, 48, 72 h post infection with Streptococcus uberis, respectively. No pathological changes were observed in either control group or treatment group mammary glands before infection, whereas inflammation, as evidenced by bleeding, degeneration of secretory epithelium (ellipse), increased adipose tissue (coattails arrow) and infiltrating PMNs (arrow) were present in mammary tissue after infection with S. uberis
NAGase and MPO activity
The activity of NAGase in mammary tissue in CS was higher after S. uberis injection than it was pre-injection (0 h; 75.43 ± 12.11 U/g), and activity peaked at 24 h PI (197.86 ± 15.54 U/g; P < 0.05). NAGase in TS also peaked at 24 h PI (182.67 ± 31.95 U/g; P < 0.05). A significant difference (P < 0.05) between CS and TS was present at 48 h PI (Fig. 3a). Serum NAGase reached its nadir at 16 h PI in CS (45.67 ± 5.78 U/L) and at 24 h PI in TS (52.16 ± 3.9 U/L). There was no significant difference in serum NAGase between CS and TS (Fig. 3b).
Changes of N-acetyl-β-D-glucosaminidase (NAGase) and myeloperoxidase (MPO) activity in mammary tissue and serum. Rats were injected with Streptococcus uberis (100 cfu in 100 μL/gland). After 0, 8, 16, 24, 48 and 72 h, mammary tissues and sera were collected and the activities of NAGase and MPO were determined using commercial kits. Data are presented as the means ± SE (n = 6). Means without common letters differ significantly between different time points in the same group (P < 0.05). *(P < 0.05) significant difference between group TS and group CS at the same time point. a Changes of NAGase activity in mammary tissue. b Changes of NAGase activity in sera. c Changes of MPO activity in mammary tissue. d Changes of MPO activity in sera. CS control group, TS treatment group
The activity of MPO in mammary tissue peaked at 16 h PI with values of 21.09 ± 1.58 U/g (CS) and 14.89 ± 1.83 U/g (TS), respectively. Significant differences (P < 0.05) between CS and TS were present at 16, 48 and 72 h PI (Fig. 3c). Serum MPO decreased in both groups after injection with S. uberis, and the lowest values were reached at 48 h PI in CS and 8 h PI in TS. There were no significant differences in serum MPO between CS and TS (Fig. 3d).
Expression of IL-2, INF-γ, and IL-4 mRNA in mammary tissue
Gavage of Tau increased the expression of IL-2 mRNA in mammary tissue of mock S. uberis-challenged rats (P < 0.05). The values obtained at 0 h in CS and TS were 1.00 ± 0.16 and 1.53 ± 0.1, respectively. IL-2 mRNA expression in CS peaked at 24 h PI (4.14 ± 0.22; P < 0.05), and then decreased at all subsequent time points. Significant elevations (P < 0.05) were observed at 8 h (1.89 ± 0.23), 16 h (3.01 ± 0.55), and 48 h PI (2.05 ± 0.29). IL-2 mRNA expression in TS peaked at 16 h PI (2.94 ± 0.49; P < 0.05) and then decreased. Significant increases (P < 0.05) were observed at 8 h (2.57 ± 0.23), and 24 h PI (2.39 ± 0.31) compared to 0 h (1.53 ± 0.1) (Fig. 4a). Relative to CS, pre-treatment significantly decreased the expression of IL-2 mRNA in mammary tissue at 24 h (P < 0.05).
Changes of mRNA expression of interleukin (IL)-2, interferon (INF)-γ and IL-4 in mammary tissue. Rats were injected with Streptococcus uberis (100 cfu in 100 μL/gland). After 0, 8, 16, 24, 48 and 72 h, mammary tissues were collected and the expression of IL-2, INF-γ, and IL-4 mRNA was determined. Data are presented as the means ± SE (n = 6). Means without common letters differ significantly between different time points in the same group (P < 0.05). *(P < 0.05) significant difference between group TS and group CS at the same time point. a Changes of mRNA expression of IL-2 in mammary tissue. b Changes of mRNA expression of INF-γ in mammary tissue. c Changes of mRNA expression of IL-4 in mammary tissue. CS control group, TS treatment group
The levels of INF-γ mRNA expression in CS peaked at 24 h PI (4.32 ± 0.62; P < 0.05). At subsequent time points, there was a marked decrease in its expression. Significant elevations were observed at 16 h (3.45 ± 0.21), 48 h (3.51 ± 0.44) (P < 0.05), and 72 h PI (3.03 ± 0.63) (P < 0.05). The levels of INF-γ mRNA expression in TS peaked at 16 h PI (3.19 ± 0.42; P < 0.05) and then decreased. A significant increase (P < 0.05) was observed at 24 h PI (2.91 ± 0.31) compared to 0 h (1.21 ± 0.2). Significant differences (P < 0.05) between CS and TS were present at 24 h, 48 h and 72 h PI (Fig. 4b).
IL-4 mRNA expression in CS decreased with S. uberis infection. Significant reductions were observed (P < 0.05) at 16 h (0.47 ± 0.05) and 24 h (0.44 ± 0.14) PI. Pre-treatment with Tau inhibited this decrease. There was no significant reduction observed in TS IL-4 mRNA expression and there were no significant differences in IL-4 mRNA expression between CS and TS (Fig. 4c).
Regulatory T cells in rat peripheral blood
As shown in Table 4, there were no significant differences in the percentage of CD25+ CD4+/lymphocytes in both CS and TS. However, the percentage of Foxp3+ CD4+/lymphocytes and Foxp3+ CD25+ CD4+/lymphocytes (Tregs) were dramatically increased (P < 0.05) when compared to the 0 h controls for both groups following S. uberis injection. Pre-treatment further increased the percentage of Foxp3+ CD4+/lymphocytes and Tregs in rat peripheral blood at 24 h PI (P < 0.05).
Discussion
In the current study, histologic observation indicated that an acute inflammatory response was induced in rat mammary tissue by S. uberis infection with 100 cfu per gland. This response was characterized by PMN infiltration, increased bleeding, epithelial cell degeneration, and excess adipose tissue. These results are consistent with the results obtained in a previous study (Trinidad et al. 1990; Thomas et al. 1994).
Tau is the most abundant free amino acid in most animal tissues. A large number of reports have demonstrated the key role of Tau and its derivatives in the innate immune response and suggest its use in the treatment of various topical infections and chronic inflammatory diseases (Nagl et al. 2000; Erdem et al. 2008; Verdrengh and Tarkowski 2005). In this study, we investigated the immunoregulatory and protective effects of Tau on the lactating rat mammary gland after S. uberis injection. Histologic observation showed that there was a significant reduction in PMN infiltration at 16 h, 24 h, 48 h, and 72 h PI, adipose tissue reduction at 48 h PI and a reduction in bleeding and epithelial cell degeneration at 48 h and 72 h PI compared to CS. This indicates that Tau minimized the damage caused by S. uberis in the mammary gland, especially two days after S. uberis injection.
NAGase is a lysosomal enzyme widely distributed in animal tissue. It is released from cells by exocytosis or from the breakdown of cells (Welman et al. 1978). NAGase elevation in mammary tissue may have been induced by leakage of NAGase from the damaged secretory epithelium (Urech et al. 1999). The use of NAGase as a marker enzyme to determine the extent of damage to epithelial cells of the mammary gland has been previously reported (Leitner et al. 2001). In the current study, the activity of NAGase in mammary tissue was lower after S. uberis injection in TS than that in CS at 48 h PI. The results suggest that epithelial cell damage was attenuated by the administration of Tau. This finding is in concordance with the study of Tau in other disease processes. Venkatesan et al. (1997) reported that treatment with Tau significantly suppressed adriamycin-induced urinary excretion of NAGase and had beneficial effects against proteinuria and hyperlipidemia associated with the nephrotic syndrome.
Nakajima et al. (1993) found that NAGase originating from white blood cells increased in milk as a result of mastitis, and PMN accumulation induced the elevation of NAGase in the mammary gland. This might explain why the activity of serum NAGase and MPO (a surrogate marker for PMNs) (Teixeira et al. 2006) declined after S. uberis injection. It may have been due to the recruitment of immune cells (mainly PMNs) from the peripheral blood supply into the mammary gland in the initial stages of inflammation.
PMN infiltration was not observed in rat mammary tissue prior to injection of S. uberis. PMNs were present in mammary alveoli at 8 h PI, reached maximum levels at 16 h PI both in CS and TS, and subsequently decreased. This change in PMN numbers is consistent with MPO activity in mammary tissue, which also peaked at 16 h PI. Treatment with Tau significantly decreased the MPO activity at 16 h, 48 h and 72 h PI suggesting that PMN infiltration and function are modulated by Tau. A similar result was observed by Akdemir et al. (Akdemir et al. 2011) regarding reperfusion injury in which the numbers of PMNs and amount of tissue necrosis in the Tau treatment group was significantly lower than the untreated control group.
IL-2 and INF-γ are produced by Th1 cells (Abbas et al. 1996). Our data demonstrates that IL-2 and INF-γ mRNA levels are increased after S. uberis challenge. Interestingly, the tissue injury in CS was most pronounced at 24 PI and corresponded to the peak expressions of IL-2 and IFN-γ mRNA. This suggests that a major immune response mediated by Th1 cells might be related to the damage within the mammary gland. It is reported that Tau has the ability to suppress Th1-type immunity (Wirleitner et al. 2004). In our study, intragastric administration of Tau to rats significantly decreased the expression of IL-2 mRNA in mammary tissue at 24 h, and IFN-γ at 24 h, 48 h and 72 h PI. This indicates that Tau may moderate the over expression of Th1 cytokines and thus protect the mammary gland.
IL-4 is secreted by Th2 cells (Abbas et al. 1996). It is reported that it could antagonize Th1 cell proliferation (Schulze-Koops and Kalden 2001). In the current study, IL-4 mRNA decreased after S. uberis challenge in CS, whereas it increased in TS. This indicates that Tau might induce the differentiation of CD4+ T cells to Th2 cells which secrete IL-4 and then inhibit the proliferation of Th1 cells, further reducing the secretion of IL-2 and IFN-γ (Schulze-Koops and Kalden 2001). However, the changes in mRNA levels do not necessarily reflect the changes in the encoded protein levels or their activity. Whether the changes of mRNA reflect protein changes needs to be further studied.
T Regulatory cells(Tregs)recognized by specific marker Foxp3+ CD25+ CD4+ is another subset of T cells (Fontenot and Rudensky 2005). They prevent the over activation of effector cells, help in maintaining homeostasis and thus prevent or mitigate inflammatory disease via their immunosuppressive properties (Golovina and Vonderheide 2010). It has been reported that Tregs play important roles in regulating immune responses during infection and in preventing tissue damage to the host (Kohm et al. 2002; Frey et al. 2005). Our results show that the percentage of Tregs in CS was dramatically increased in rat peripheral blood at 24 h and 72 h PI. Treatment with Tau further increases the percentage of Tregs in rat peripheral blood after S. uberis injection and significant differences between CS and TS are evident in 24 h PI. At this time point, the levels of IL-2 and IFN-γ mRNA, NAGase and MPO activities, and the damage caused by S. uberis in mammary tissue are also minimized. These results indicate that all of the above minimized changes might be a consequence of increased Tregs. The mechanisms of how Tau regulates the Tregs and affects the immune response require further study.
Previous studies have shown that the time interval for effector cell phenotypic changes may be a “day” (Ko et al. 2005; McGeachy et al. 2005; Kohm et al. 2006; Miao et al. 2007). In the current study, we detected the Tregs in rat peripheral blood only at the 0, 24, and 72 h time points. In conclusion, our data indicate that Tau may attenuate S. uberis-induced mastitis in rats by increasing T regulatory cells. These data provide a better understanding on the mechanism of S. uberis mastitis. However, applying rat data to dairy cows should be carefully considered. Whether Tau could be used to attenuate bovine S. uberis mastitis need to be further studied.
Abbreviations
- Tau:
-
Taurine.
- S. uberis :
-
Streptococcus uberis
- Tregs:
-
T regulatory cells
- NAGase:
-
N-acetyl-β-D-glucosaminidase
- MPO:
-
Myeloperoxidase
- PMN:
-
Neutrophil
References
Abbas AK, Murphy KM, Sher A (1996) Functional diversity of helper T lymphocytes. Nature 31:787–793
Akdemir O, Hede Y, Zhang F, Lineaweaver WC, Arslan Z, Songur E (2011) Effects of taurine on reperfusion injury. J Plast Reconstr Aesthet Surg 64(7):921–928
Bannerman DD, Paape MJ, Goff JP, Kimura K, Lippolis JD, Hope JC (2004) Innate immune response to intramammary infection with Serratia marcescens and Streptococcus uberis. Vet Res 35:681–700
Barrio MB, Rainard P, Poutrel B (2003) Milk complement and the opsonophagocytosis and killing of Staphylococcus aureus mastitis isolates by bovine neutrophils. Microb Pathog 34:1–9
Bradley A (2002) Bovine mastitis: an evolving disease. Vet J 164:116–128
Cheng X, Liao YH, Ge H, Li B, Zhang J, Yuan J, Wang M, Liu Y, Guo Z, Chen J, Zhang J, Zhang L (2005) TH1/TH2 functional imbalance after acute myocardial infarction: coronary arterial inflammation or myocardial inflammation. J Clin Immunol 25:246–253
Diarra MS, Petitclerc D, Deschênes E, Lessard N, Grondin G, Talbot BG, Lacasse P (2003) Lactoferrin against Staphylococcus aureus Mastitis. Lactoferrin alone or in combination with penicillin G on bovine polymorphonuclear function and mammary epithelial cells colonisation by Staphylococcus aureus. Vet Immunol Immunopathol 95:33–42
Erdem A, Sevgili AM, Akbiyik F, Atilla P, Cakar N, Balkanci ZD, Iskit AB, Guc MO (2008) The effect of Tau on mesenteric blood flow and organ injury in sepsis. Amino Acids 35:403–410
Field TR, Ward PN, Pedersen LH, Leigh JA (2003) The hyaluronic acid capsule of Streptococcus uberis is not required for the development of infection and clinical mastitis. Infect Immun 71:132–139
Fontenot JD, Rudensky AY (2005) A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol 6:331–337
Frey O, Petrow PK, Gajda M, Siegmund K, Huehn J, Scheffold A, Hamann A, Radbruch A, Bräuer R (2005) The role of regulatory T cells in antigen-induced arthritis: aggravation of arthritis after depletion and amelioration after transfer of CD4+ CD25+ T cells. Arthritis Res Ther 7:R291–R301
Golovina TN, Vonderheide RH (2010) Regulatory T cells: overcoming suppression of T-cell immunity. Cancer J 16:342–347
Grimble RF (1994) Sulphur amino acids and the metabolic response to cytokines. Adv Exp Med Biol 359:41–49
Grimble RF (2006) The effects of sulfur amino acid intake on immune function in humans. J Nutr 136:1660S–1665S
Gu B, Miao J, Fa Y, Lu J, Zou S (2010) Retinoic acid attenuates lipopolysaccharide-induced inflammatory responses by suppressing TLR4/NF-kappaB expression in rat mammary tissue. Int Immunopharmacol 10:799–805
Hill AW, Shears AL, Hibbitt KG (1978) The elimination of serum-resistant Escherichia coli from experimentally infected single mammary glands of healthy cows. Res Vet Sci 25:89–93
Hillerton JE, Berry EA (2005) Treating mastitis in the cow–a tradition or an archaism. J Appl Microbiol 98:1250–1255
Hillerton JE, Shearn MF, Teverson RM, Langridge S, Booth JM (1993) Effect of pre-milking teat dipping on clinical mastitis on dairy farms in England. J Dairy Res 60:31–41
Hossain A, Zheng CL, Kukita A, Kohashi O (2001) Balance of Th1/Th2 cytokines associated with the preventive effect of incomplete Freund’s adjuvant on the development of adjuvant arthritis in LEW rats. J Autoimmun 17:289–295
Huxtable RJ (1996) Tau past, present, and future. Adv Exp Med Biol 403:641–650
Kawai K, Nagahata H, Lee NY, Anri A, Shimazaki K (2003) Effect of infusing lactoferrin hydrolysate into bovine mammary glands with subclinical mastitis. Vet Res Commun 27:539–548
Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, Shimizu J, Nomura T, Chiba T, Sakaguchi S (2005) Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+ CD25+ CD4+ regulatory T cells. J Exp Med 202:885–891
Kohm AP, Carpentier PA, Anger HA, Miller SD (2002) CD4+ CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol 169:4712–4716
Kohm AP, McMahon JS, Podojil JR, Begolka WS, DeGutes M, Kasprowicz DJ, Ziegler SF, Miller SD (2006) Cutting Edge: anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+ CD25+ T regulatory cells. J Immunol 176:3301–3305
Kontny E, Szczepanska K, Kowalczewski J, Kurowska M, Janicka I, Marcinkiewicz J, Maslinski W (2000) The mechanism of Tau chloramine inhibition of cytokine (interleukin-6, interleukin-8) production by rheumatoid arthritis fibroblastlike synoviocytes. Arthritis Rheum 43:2169–2177
Leitner G, Chaffer M, Zamir S, Mor T, Glickman A, Winkler M, Weisblit L, Saran A (2001) Udder disease etiology, milk somatic cell counts and NAGase activity in Israeli Assaf sheep throughout lactation. Small Rumin Res 39:107–112
McGeachy MJ, Stephens LA, Anderton SM (2005) Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+ CD25+ regulatory cells within the central nervous system. J Immunol 175:3025–3032
Miao JF, Zhu YM, Gu BB, Wang XB, Zou SX, Deng YE (2007) Evaluation of the changes of immune cells during lipopolysaccharide-induced mastitis in rats. Cytokine 40:135–143
Miao JF, Zhang YS, Huang GQ, Ma HT, Zou SX, Zhu YM (2009) Polysaccharide nucleic acid of Bacillus Guerin modulates Th1/Th2 cytokine gene expression and tissue injure of lipopolysaccharide-induced mastitis in rats. Agricultural Sciences in China 8:1010–1018
Moyes KM, Drackley JK, Morin DE, Bionaz M, Rodriguez-Zas SL, Everts RE, Lewin HA, Loor JJ (2009) Gene network and pathway analysis of bovine mammary tissue challenged with Streptococcus uberis reveals induction of cell proliferation and inhibition of PPARgamma signaling as potential mechanism for the negative relationships between immune response and lipid metabolism. BMC Genomics 10:542
Nagl M, Hess MW, Pfaller K, Hengster P, Gottardi W (2000) Bactericidal activity of micromolar N-chlorotaurine: evidence for its antimicrobial function in the human defense system. Antimicrob Agents Chemother 44:2507–2513
Nakajima Y, Momotani E, Murakami T, Ishikawa Y, Morimatsu M, Saito M, Suzuki H, Yasukawa K (1993) Induction of acute phase protein by recombinant human interleukin-6 (IL-6) in calves. Vet Immunol Immunopathol 35:385–391
Ohtsuka R, Shutoh Y, Fujie H, Yamaguchi S, Takeda M, Harada T, Doi K (2005) Changes in histology and expression of cytokines and chemokines in the rat lung following exposure to ovalbumin. Exp Toxicol Pathol 56:361–368
Ricciardelli I, Lindley KJ, Londei M, Quaratino S (2008) Anti tumour necrosis-alpha therapy increases the number of FOXP3 regulatory T cells in children affected by Crohn’s disease. Immunology 125:178–183
Schulze-Koops H, Kalden JR (2001) The balance of Th1/Th2 cytokines in rheumatoid arthritis. Best Pract Res Clin Rheumatol 15:677–691
Tamilselvam B, Almeida RA, Dunlap JR, Oliver SP (2006) Streptococcus uberis internalizes and persists in bovine mammary epithelial cells. Microb Pathog 40:279–285
Teixeira AS, Araújo FA, Ferreira MA, Barcelos LS, Teixeira MM, Andrade SP (2006) Angiogenesis and inflammation in skeletal muscle in response to ascites tumor in mice. Life Sci 78:1637–1645
Thomas LH, Haider W, Hill AW, Cook RS (1994) Pathologic findings of experimentally induced Streptococcus uberis infection in the mammary gland of cows. Am J Vet Res 55:1723–1728
Trinidad P, Nickerson SC, Adkinson RW (1990) Histopathology of staphylococcal mastitis in unbred dairy heifers. J Dairy Sci 73:639–647
Urech E, Puhan Z, Schällibaum M (1999) Changes in milk protein fraction as affected by subclinical mastitis. J Dairy Sci 82:2402–2411
Venkatesan N, Venkatesan P, Karthikeyan J, Arumugam V (1997) Protection by taurine against adriamycin-induced proteinuria and hyperlipidemia in rats. Proc Soc Exp Biol Med 215:158–164
Verdrengh M, Tarkowski A (2005) Inhibition of septic arthritis by local administration of Tau chloramine, a product of activated neutrophils. J Rheumatol 32:1513–1517
Wei HX, Chuang YH, Li B, Wei H, Sun R, Moritoki Y, Gershwin ME, Lian ZX, Tian Z (2008) CD4+ CD25+ Foxp3+ regulatory T cells protect against T cell-mediated fulminant hepatitis in a TGF-beta-dependent manner in mice. J Immunol 181:7221–7229
Welman E, Selwyn AP, Peters TJ, Colbeck JF, Fox KM (1978) Plasma lysosomal enzyme activity in acute myocardial infarction. Cardiovasc Res 12:99–105
Wirleitner B, Neurauter G, Nagl M, Fuchs D (2004) Down-regulatory effect of N-chlorotaurine on tryptophan degradation and neopterin production in human PBMC. Immunol Lett 93:143–149
Zhu YM, Miao JF, Fan HJ, Zou SX, Chen WH (2007a) Protective effect of CpG-DNA against mastitis induced by Staphylococcus aureus infection in a rat model. Int Immunopharmacol 7:435–443
Zhu YM, Miao JF, Zhang YS, Li Z, Zou SX, Deng YE (2007b) CpG-ODN enhances mammary gland defense during mastitis induced by Escherichia coli infection in goats. Vet Immunol Immunopathol 120:168–176
Acknowledgments
This project was supported by grants from the China National Science Foundation (No. 31072104), the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20100097120005), the Natural Science Foundation of Jiangsu Province, China (No. BK2010444) and the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions. The authors wish to express their thanks to Dr. William W. Riley (Hinapharn Pharmaceutical Co., Ltd., Foshan) and Dr. Howard Gelberg (Oregon State University) for manuscript editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miao, J., Zhang, J., Zheng, L. et al. Taurine attenuates Streptococcus uberis-induced mastitis in rats by increasing T regulatory cells. Amino Acids 42, 2417–2428 (2012). https://doi.org/10.1007/s00726-011-1047-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-1047-3