Abstract
l-Homoarginine (hArg) and guanidinoacetate (GAA) are produced from l-arginine (Arg) by the catalytic action of arginine:glycine amidinotransferase. Guanidinoacetate methyltransferase methylates GAA on its non-guanidine N atom to produce creatine. Arg and hArg are converted by nitric oxide synthase (NOS) to nitric oxide (NO). NO is oxidized to nitrite and nitrate which circulate in the blood and are excreted in the urine. Asymmetric dimethylarginine (ADMA), an NOS inhibitor, is widely accepted to be exclusively produced after asymmetric N G-methylation of Arg residues in proteins and their regular proteolysis. Low circulating and urinary hArg concentrations and high circulating concentrations of ADMA emerged as risk markers in the human renal and cardiovascular systems. While ADMA’s distribution and metabolism are thoroughly investigated, such studies on hArg are sparse. The aim of the present pilot study was to investigate the distribution of exogenous hArg in plasma, liver, kidney, lung, and heart in a rat model of takotsubo cardiomyopathy (TTC). hArg hydrochloride solutions in physiological saline were injected intra-peritoneally at potentially pharmacological, non-toxic doses of 20, 220, or 440 mg/kg body weight. Vehicle (saline) served as control. As hArg has been reported to be a pro-oxidant, plasma and tissue malondialdehyde (MDA) was measured as a biomarker of lipid peroxidation. hArg administration resulted in dose-dependent maximum plasma hArg concentrations and distribution in all investigated organs. hArg disappeared from plasma with an elimination half-life ranging between 20 and 40 min. hArg administration resulted in relatively small changes in the plasma and tissue content of Arg, GAA, ADMA, creatinine, and of the NO metabolites nitrite and nitrate. Remarkable changes were observed for tissue GAA, notably in the kidney. Plasma and tissue MDA concentration did not change upon hArg administration, suggesting that even high-dosed hArg is not an oxidant. The lowest hArg dose of 20 mg/kg bodyweight increased 25-fold the mean hArg maximum plasma concentration. This hArg dose seems to be useful as the upper limit in forthcoming studies on the putative cardioprotective effects of hArg in our rat model of TTC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
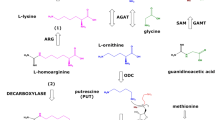
l-Arginine (Arg) is a proteinogenic amino acid and plays multiple physiological roles (Wu et al. 2009). In healthy and diseased humans, a very small fraction of less than 0.1% of whole body Arg is utilized for nitric oxide (NO) synthesis by NO synthase (NOS) isoforms in various types of cell (Gambaryan and Tsikas 2015) (see reaction 1). NO is one of the most potent endogenous vasodilators and inhibitors of platelet aggregation (Hibbs et al. 1992). Unlike for NO synthesis, about 30% of Arg is consumed daily by the body for creatine synthesis. In mitochondria, arginine:glycine amidinotransferase (AGAT, EC 2.1.4.1) catalyzes the formation of l-homoarginine (hArg; see reaction 2) and guanidinoacetate (GAA; see reaction 3) from Arg, with l-ornithine (Orn) being the second common reaction product. Subsequently, GAA is methylated on its non-guanidine N atom by hepatic guanidinoacetate methyltransferase (GAMT, EC 2.1.1.2) using S-adenosyl methionine (SAM) as the methyl donor to produce creatine (see reaction 4), an energy-related molecule. Generally, diminished NO synthesis due to Arg deficiency and decreased bioavailability of NO mainly due to elevated oxidative stress are believed to play a major role in the pathogenesis of numerous diseases in the renal and cardiovascular systems as well as in the brain
The beneficial health effects of supplementary Arg and/or l-citrulline (Citr), the precursor of Arg, are generally attributed to increases in NOS-catalyzed NO synthesis from Arg (Bode-Böger 2006). This is due to the higher concentrations of the substrate l-arginine in NO-producing cells and due to the stronger competition of Arg by endogenous NOS activity inhibitors such as asymmetric dimethylarginine (ADMA) both in the catalytic reaction and in the cellular transport (Tsikas et al. 2000).
With regard to NO generation we have to consider two important issues. First, NO can be produced from ubiquitous inorganic nitrite and nitrate by other proteins and enzymes including bacterial nitrate reductase and carbonic anhydrase (Mansuy and Boucher 2004; Hanff et al. 2016a, b; see also Schneider et al. 2015). Second, there are many serious methodological shortcomings in detecting both, NOS proteins (e.g., unspecific antibodies) and their activity regarding NO synthesis (Gambaryan and Tsikas 2015; Lacza et al. 2006a, b). NO is a short-lived gaseous radical molecule and analytically inaccessible. NO’s major metabolites, nitrite and nitrate, are measured instead, but they are abundant contaminants. The utility of nitrite and nitrate as measures of NO synthesis in vivo is compromised by several factors, most notably by dietary nitrite and nitrate (Tsikas 2015).
In recent years, hArg emerged as a novel cardiovascular risk marker (März et al. 2010; Pilz et al. 2015), but the underlying mechanisms remain unresolved. Guanidine, methyl guanidine, asymmetric dimethyl guanidine and other methyl guanidines were found to inhibit oxidative phosphorylation (Hollunger 1955; Chappell 1963). Recently, hArg and other guanidine compounds have been reported to elevate oxidative stress (Wyse et al. 2001; Sasso et al. 2015). In addition, hArg was found to attenuate NOS activity at supra-pathophysiological concentrations (i.e., 100 µM) by competing with Arg for NOS (Alesutan et al. 2016). At physiological and pathological concentrations, however, hArg is unlikely to compromise NOS activity and to promote oxidative stress (Hanff et al. 2016a, b). The biological functions of hArg itself are largely unknown. hArg and ADMA were proposed to act antagonistically in the cardiovascular system (Tsikas and Kayacelebi 2014).
hArg has been supplemented to animals (O’Neal et al. 1968; Pentyala and Rao 1999; Choe et al. 2013a, b; Stockebrand et al. 2015; Hou et al. 2016). Yet, the distribution and the metabolism of exogenous hArg have been little investigated thus far. Oral supplementation of C57BL/6 mice with 14 mg/L hArg in drinking water for 4 weeks resulted in a threefold increase in hArg plasma concentration (Choe et al. 2013a). Continuous infusion of hArg (30 mg/kg/day) in rats for 14 days resulted in a 35-fold increase of the plasma concentration of hArg (Dellera et al. 2016). In humans, oral administration of hArg to healthy young subjects (125 mg/day) for 4 weeks increased the plasma hArg plasma concentration fivefold; yet, hArg administration did not exert expected biological actions such as enhancement of flow-mediated vasodilatation (Atzler et al. 2016).
We previously reported on the utility of a rat model of stress-induced cardiomyopathy (SIC), also known as takotsubo cardiomyopathy (TTC), in elucidating underlying mechanisms of TTC (Redfors et al. 2014). In TTC patients, we measured lower plasma hArg concentrations than in healthy age- and gender-matched subjects (Kayacelebi et al. 2014). Thus, elevation of plasma hArg concentrations by supplementing hArg appears to be indicated in cardiovascular disease including TTC. However, data on potentially suitable pharmacological doses are scarce.
The aim of the present pilot study was to investigate the distribution and the metabolism of intra-peritoneally administered hArg in our rat model of TTC (Redfors et al. 2014) in order to find a potentially suitable pharmacological dose regime. hArg administration may lead to changes in concentrations of amino acids in related pathways. We, therefore, determined the concentration of Arg, GAA, ADMA, and creatinine in plasma and tissue samples of main organs of the rat. As hArg has been reported to be pro-oxidant (Wyse et al. 2001; Sasso et al. 2015), we measured the lipid peroxidation biomarker MDA (Tsikas 2017) in rat plasma and organs as well. MDA is abundantly produced in platelets by cyclooxygenase-1 and circulating MDA may also be used as a marker of platelet hypersensitivity (Tsikas 2017). We expected that this pilot study would provide us with important information about potentially suitable hArg doses for forthcoming studies in the animal model of TTC (Redfors et al. 2014) and about its putative effects on biochemical pathways related to hArg (i.e., NOS, AGAT, GAMT).
Materials and methods
Distribution and metabolism of hArg in the anesthetized rat
The distribution and the metabolism of hArg was investigated in a rat model of stress-induced cardiomyopathy (takotsubo cardiomyopathy, TTC) as described in detail elsewhere (Redfors et al. 2014). The animal experiments were performed in Gothenburg, Sweden, in June 2015 during a 1-week visit of one of the Hannover coauthors. All animals received humane care and the study protocol complied with the institutional guidelines of the Sahlgrenska University Hospital (Gothenburg, Sweden). The Sprague–Dawley rats (about 10 weeks old, 250 g body weight on average) were housed in a temperature-controlled (20–22 °C) facility with a 12-h light/dark cycle and had free access to food and water. The hArg dose regime for the present study was chosen on the basis of information available until 2015 (O’Neal et al. 1968; Pentyala and Rao 1999; Choe et al. 2013a, b). The non-toxic hArg dose regime should ensure sufficiently high blood hArg concentrations in order to receive biological response in the rat model of TTC. At the dose of 940 mg/kg body weight, which is two times higher than the highest dose we applied in the present study, hArg was found to be non-toxic in rats (O’Neal et al. 1968).
Solutions of the reference pharmacological compound, the hArg hydrochloride salt, were prepared in physiological saline for injection into the anesthetized rats at the following dosages: 0, 20, 220, and 440 mg/kg body weight (Table 1). GC–MS analyses of the stock solutions revealed no contaminating Arg, GAA or ADMA. Rat no. (R5) received 0.0 mg hArg/kg body weight (vehicle) and was killed after 70 min. This rat served as control. Its heart was not used for biochemical analyses but preserved for morphological studies. R2, R3, R13, and R18 received 20 mg hArg/kg body weight each and were killed after 64, 28, 126, and 114 min, respectively. hArg at the dose of 220 mg/kg body weight was administered to R14 and R19 which were killed after 108 and 98 min, respectively. R1, R4, and R17 received 440 mg hArg/kg body weight each and were killed after 32, 87, and 14 min, respectively.
Blood samples were drawn and immediately centrifuged. Blood volume lost due to the blood sampling was replaced with same volume of physiological saline. The rats were killed at the end of the experiment (≤126 min) with a bolus dose of pentobarbital (50 mg) (Redfors et al. 2014). Heart, lung, liver, and kidneys were obtained from the killed rats (Redfors et al. 2014). Plasma samples and organs were stored immediately at −80 °C. After transport on dry ice to the Hannover Medical School (Hannover, Germany), plasma and organs were stored at −20 °C until further process and biomarker analysis. These analyses were performed in July 2016.
Homogenization of weighed ice-cooled tissue (about 100 mg) was performed in 1-mL aliquots of ice-cooled phosphate buffered saline (PBS, pH 7.2) as described elsewhere (Kayacelebi et al. 2015a, b), yet without the use of protease inhibitors (Yang et al. 2015). Nitrate, nitrite, creatinine, and malondialdehyde (MDA) were analyzed simultaneously in 100-µL aliquots of homogenates (Hanff et al. 2016a, b). Amino acids were analyzed simultaneously in 10-µL aliquots of ultrafiltrates that were generated from 200-µL aliquots of homogenates (centrifugation at 21,000×g, 4 °C, 30 min) using 0.5-mL Vivaspin 500 cartridges (10-kDa cutoff; Sartorius, Göttingen, Germany) as described previously (Kayacelebi et al. 2015a, b).
Nitrite, nitrate, Arg, hArg, ADMA, MDA, GAA, and creatinine were quantified in rat plasma samples by using previously reported fully validated gas chromatographic-mass spectrometric (GC–MS) methods using stable-isotope labelled analogues as internal standards (Tsikas 2000; Tsikas et al. 2000; Kayacelebi et al. 2015b; Hanff et al. 2016a, b; Hanff et al. 2017). All GC–MS analyses were performed on the apparatus model ISQ from ThermoFisher (Dreieich, Germany). The content of the analytes in the tissues is reported as nmol analyte per g wet tissue.
Alongside the analysis of rat plasma samples we analyzed a human plasma sample serving as a quality control (QC) sample for the amino acids. This sample was analyzed in duplicate. The concentration was determined to be (mean ± SD) 1.64 ± 0.05 µM for hArg, 1.76 ± 0.06 µM for GAA, 59.7 ± 1.38 µM for Arg and 0.56 ± 0.018 µM for ADMA. The respective coefficients of variation were 3.2, 3.5, 2.3, and 3.2%, indicating the good precision of the method for Arg and its metabolites hArg, GAA, and ADMA.
Detection of AGAT activity in rat kidney in vitro
As the kidney is the major contributor to biological hArg, in the present study we used freshly prepared kidney homogenate from the control rat to detect AGAT activity. l-[Guanidino-15N2]-arginine ([15N2]-Arg), Lys and Gly were used as the substrates each at a concentration of 1000 µM. This concentration is considerably lower than that used by other groups (e.g., Verhoeven et al. 2003; Alessandrì et al. 2005; Yang et al. 2015). l-[Guanidino-15N2]-homoarginine ([15N2]-hArg) and [guanidino-15N2]-guanidinoacetate ([15N2]-GAA) served as the reaction products of the renal AGAT activity (see reactions 5 and 6):
[15N2]-Arg (98 atom% 15N; Cambridge Isotope Laboratories; Andover, MA, USA) was found by GC–MS not to contain [15N2]-hArg, hArg, [15N2]-GAA or GAA. Homogenate suspensions (each three 100-µL aliquots for each incubation time) were incubated at 37 °C for 0, 11, and 30 min. Reactions were stopped by adding ice-cold 2 M HCl/methanol (50 µL) after the respective incubation time and by immediate setting the samples in an ice bath. The incubation time 0 min is actually about 10–15 s which corresponds to the time needed to stop the reaction. After sample centrifugation (4500×g, 4 °C, 10 min), decantation of 40-µL aliquots of the clear supernatants and solvent evaporation to dryness using a stream of nitrogen gas, amino acids were derivatized and analyzed by GC–MS in the selected-ion monitoring mode using methane as the reactand gas for negative-ion chemical ionization (Kayacelebi et al. 2015a, b). The mass fragments m/z 588 and m/z 586 for [15N2]-Arg and [14N2]-Arg, m/z 602 and m/z 600 for [15N2]-hArg and [14N2]-hArg, and m/z 385 and m/z 383 for [15N2]-GAA and [14N2]-GAA, respectively, were monitored alternately within the same analysis. The area values of the peaks of the amino acid derivatives were used to calculate the peak area ratio (PAR) values which in turn were used as a measure of AGAT activity. Because of the natural occurrence of the 15N isotope of the N element (abundance, 0.36%), the PAR values were determined in kidney homogenate suspensions in triplicate before the addition of [15N2]-Arg in order to receive the blank values. They were determined to be (mean ± SD) 0.029 ± 0.005 for [15N2]-Arg/[14N2]-Arg, 0.032 ± 0.006 for [15N2]-hArg/[14N2]-hArg, and 0.05 ± 0.01 for [15N2]-GAA/[14N2]-GAA. The PAR [14N2]-Arg/[15N2]-Arg in a 1-mM stock solution of [15N2]-Arg was determined to be 0.029 ± 0.005 indicating virtually no contamination of [15N2]-Arg by [14N2]-Arg. Because of the lack of [15N2]-hArg and [15N2]-GAA reference compounds no PAR values are available for [14N2]-hArg/[15N2]-hArg and [15N2]-GAA/[14N2]-GAA.
Graphs were constructed by GraphPad Prism 5.04. This software was used to determine means, standard deviation (SD), and standard error of the mean. Data are presented as mean ± SD, mean ± SEM or median with interquartile range as specified in the text and in the Figure legends. Given the small number of data points, the pharmacokinetics of hArg was not computed.
Results
Distribution and metabolism of hArg
Intra-peritoneal injection of hArg in the anesthetized rats at dosages of 0, 20, 220, and 440 mg/kg body weight resulted in dose-dependent increases in hArg plasma concentrations. The highest hArg plasma concentrations occurred at different time points in the individual rats and were 3, 76, 515, and 1338 µM, respectively (Fig. 1a). The corresponding highest plasma concentration values of Arg (257, 159, 240, 210 µM), GAA (15, 6.4, 7.8, 11 µM), and ADMA (2, 1.3, 1.5, 1.7 µM) seemed to slightly decrease upon hArg injection (Fig. 1a). The highest plasma concentration value of the lipid peroxidation/platelet hypersensitivity biomarker MDA increased only at the highest hArg dose (10.7, 10.5, 6.3, 31.3 µM) (Fig. 1a).
Homoarginine metabolism in the anesthetized rat. a Highest measured plasma concentrations of homoarginine (hArg), arginine (Arg), guanidinoacetate (GAA), asymmetric dimethylarginine (ADMA), and malondialdehyde (MDA) upon infusion of hArg at the indicated dose (0, 20, 220, and 440 mg/kg body weight) in anesthetized rats (n = 3–4 per dose). As the same dose of hArg resulted in different maximum concentrations, each the greatest of the maximum amino acid concentration is depicted. b Plasma concentration of hArg, Arg, nitrate, creatinine, GAA, and ADMA upon infusion of hArg into the rat no. 4 at the dose of 440 mg/kg body weight. Note the decadic logarithmic scale on the y axis in both panels
In the rat no. 4 (R4), samples were available from all time points and we were, therefore, able to determine the concentration of several members of the Arg/hArg/NO pathway before (0 min) and 15, 30, and 60 min after the injection of hArg at the highest dose of 440 mg/kg body weight. Injection of hArg at this dose resulted in increases in the plasma concentrations of hArg (2700-fold after 15 min), Arg, nitrate, creatinine, GAA, and ADMA (Fig. 1b). The highest amino acid plasma concentrations were observed 15 or 30 min after hArg injection. Arg, GAA, and ADMA plasma concentrations increased from 107, 7.4, and 0.89 µM at baseline to 208 (1.9-fold), 10.9 (1.5-fold), and 1.68 (1.9-fold) µM after 15 min, respectively.
We tested for potential correlations among the plasma parameters. Plasma nitrite and nitrate concentrations did not correlate with any amino acid concentration (data not shown). Given the very wide hArg plasma concentration range, we tested potential correlations between the concentrations of plasma creatinine and the amino acids in four ranges (Table 2). Plasma creatinine correlated strongly (r = −0.87, P = 0.01, n = 7) inversely with the hArg plasma concentration in the highest range (400–1400 µM) (Fig. 2). Such a correlation could also exist in the lower concentration range (3–110 µM), yet the number of concentration was only 4 in this group (Table 2). This finding suggests that exogenous hArg altered the creatinine homeostasis.
Figure 3 and Table 3 summarize the tissue content of free (soluble) hArg, Arg, GAA, ADMA, creatinine, nitrite, nitrate, and MDA in the organs of the rats being injected either hArg-free physiological saline (control) or hArg-containing physiological saline corresponding to dosages of 20, 220, and 440 mg hArg/kg body weight. hArg was distributed in all organs (Fig. 3a). At lower doses, hArg seems to accumulate in the kidney compared to the other organs, whereas at the higher hArg doses the liver rather than the kidney appears to be the preferred organ. In the control rat (dose, 0 mg hArg/kg body weight), the hArg content of kidney and liver is of the same order of magnitude as reported in the literature (Yang et al. 2015; for review see Bernstein et al. 2015). The content of free, non-protein associated Arg did not change appreciably with increasing hArg dose (Fig. 3b). The content of free GAA increased in the kidney to a similar degree at 20 and 220 mg hArg/kg body weight and was lower at the highest hArg dose applied in this study (Fig. 3c). The content of free, non-protein associated ADMA seems to have increased only in the kidney (Fig. 3d). Doubling the hArg dose from 220 to 440 mg/kg body weight increased the creatinine content of the lung 33 severalford, i.e., from 345 ± 24 to 11,380 ± 1562 nmol/g wet tissue (Table 3). No appreciable increases in the organ contents were observed for creatinine, (Fig. 3e). No appreciable increases in the organ contents were observed for creatinine, nitrite (Fig. 3f), and nitrate (Fig. 3g). The tissue content of free MDA seems to have increased at the two highest hArg doses (Fig. 3h).
Homoarginine metabolism in the anesthetized rat. Distribution of hArg, Arg, GAA, ADMA, creatinine, nitrite, nitrate, and MDA in heart, lung, kidney, and liver after injection of hArg at the indicated dose values. Tissue content values are presented as mean ± SD for 1 rat (0 mg/kg body weight), for ≤4 rats (20 mg/kg body weight), for ≤2 rats (220 mg/kg body weight), and for ≤3 rats (440 mg/kg body weight). Note the decadic logarithmic scale on the y axis in all panels. NA not available
Detection of AGAT activity in rat kidney in vitro
Addition of [15N2]-Arg (1000 µM) to the kidney homogenate increased the PAR of [15N2]-Arg/[14N2]-Arg from (mean ± SD) 0.029 ± 0.005 to 10.3 ± 1.6 at the incubation time 0 min, indicating an endogenous Arg concentration of about 100 µM in the kidney homogenate (Fig. 4a). The PAR of [15N2]-Arg/[14N2]-Arg changed only little during incubation (10.9 ± 1.5 at 10 min, 9.96 ± 0.30 at 30 min). Immediately after the addition of [15N2]-Arg to the kidney homogenate the PAR [15N2]-hArg/[14N2]-hArg increased abruptly to 0.192 ± 0.014, but did not further change after 10 min (0.187 ± 0.014) and 30 min (0.217 ± 0.014) of incubation (Fig. 4b). After the addition of [15N2]-Arg to the kidney homogenate the PAR of [15N2]-GAA/[14N2]-GAA did not increase immediately, but it increased from 0.046 ± 0.006 to 0.087 ± 0.019 after 10 min and to 0.092 ± 0.014 after 30 min of incubation (Fig. 4b). The increases in the PAR values of hArg and GAA indicate formation of [15N2]-hArg and [15N2]-GAA from [15N2]-Arg.
Detection of AGAT activity in rat kidney tissue homogenate. Peak area ratio (PAR) of a l-[guanidino-15N2]-arginine ([15N2]-Arg) to unlabeled Arg ([14N2]-Arg) and of b l-[guanidino-15N2]-homoarginine ([15N2]-hArg) to unlabeled hArg ([14N2]-hArg) or l-[guanidino-15N2]-guanidinoacetate ([15N2]-GAA) to unlabeled GAA ([14N2]-GAA) measured in kidney homogenate upon addition of l-[guanidino-15N2]-arginine, l-lysine, and glycine (each at 200 µM) and incubation at 37 °C for the indicated incubation times
Discussion
In the present study, bolus intra-peritoneal injection of hArg in anesthetized rats resulted in dose-dependent increases in the plasma hArg concentration. Even the lowest hArg dose of 20 mg/kg body weight resulted in a 25-fold increase of the plasma hArg concentration. Continuous infusion of hArg (30 mg/kg/day) in rats for 14 days via osmotic pumps was reported to increase the plasma concentration of hArg by a factor of 35 (Dellera et al. 2016). In mice, both continuous infusion (0.7 mg/kg/day) and oral administration (14 mg/L in drinking water) of hArg were reported to increase moderately the plasma hArg concentration (0.07 vs 0.23, and 0.15 vs 0.20 µM, respectively; Choe et al. 2013a).
In vitro and in vivo in animals and humans, hArg was found to be produced from l-arginine and l-lysine by the catalytic action of AGAT; this enzyme was found to catalyze the synthesis of GAA from l-arginine and glycine as well (Ryan and Wells 1964; Ryan et al. 1968, 1969; Cathelineau et al. 1974; Levin et al. 1974; Kato et al. 1988; Cullen et al. 2006; Davids et al. 2012; Choe et al. 2013a, b; Kayacelebi et al. 2015a, b) (Fig. 5). To the best of our knowledge, these reactions seem not to be reversible and to proceed without cofactors.
Simplified schematic of the arginine:glycine amidinotransferase (AGAT) activity assay used in the present work. l-[Guanidino-15N2]-Arginine (l-[15N2]-arginine) is the substrate of AGAT. a When glycine serves as a co-substrate, l-[15N2]-guanidinoacetate and l-ornithine are formed. b When l-lysine serves as a co-substrate l-[15N2]-homoarginine and l-ornithine are formed. l-[15N2]-Arginine and l-[15N2]-homoarginine are substrates for arginase 1 which hydrolyzes them to [15N2] urea/l-ornithine and [15N2] urea/l-lysine, respectively. l-[15N2]-Guanidinoacetate methyltransferase (GAMT) methylates guanidinoacetate on the non-guanidine N atom to form l-[15N2]-creatine. The catalytic step involves nucleophilic attack of the SH group of a particular cysteine moiety of AGAT on the C atom of the guanidine group of l-arginine
At the highest hArg dose of 440 mg/kg body weight used in our study, the plasma concentration of Arg, GAA, and ADMA increased slightly and temporarily. These findings are in agreement with the changes seen in amino acid profiles in plasma upon supplementation of Arg in rats (Holecek and Sispera 2016). Injected hArg seems not to have been utilized for the synthesis of ADMA upon hydrolysis to Arg or for the release of ADMA from proteolyzed tissue proteins in our study. We observed only small changes in free Arg in heart, lung, and liver upon hArg injection. The content of free Arg in the kidney increased fourfold at all doses applied. This observation suggests that injected hArg was utilized by the rats even at the lowest hArg dose to generate and/or accumulate Arg in the kidneys and to a lower extent in the liver. In the highest hArg plasma concentration range (400–1400 µM), plasma creatinine correlated inversely with the plasma hArg concentration, suggesting plasma creatinine concentration reduction in a manner depending on hArg. Interestingly, in this hArg concentration range there was a strongly positive correlation between hArg and GAA plasma concentrations (r = 0.89, P = 0.007, n = 7), but not with Arg (r = 0.33, P = 0.47) or ADMA (r = 0.53, P = 0.22) plasma concentration. It is remarkable that doubling the hArg dose from 220 to 440 mg/kg body weight increased the lung creatinine content by a factor of 33 (Table 3). A creatinine content of 11.4 µmol/g wet lung tissue is likely to correspond to a creatinine concentration of a few mM, whereas the plasma creatinine was only 30–50 µM (Fig. 2). Possible explanations for the latter observation could be enhanced excretion of creatinine, its redistribution, and a local biosynthesis and accumulation of creatinine in the lung (Table 3). AGAT is expressed in rat and human lung, albeit to a very minor extent compared to kidney and liver (Cullen et al. 2006). Yet, the very high hArg in lung tissue could have resulted in higher AGAT and GAMT activities as has been observed for the myocardium in case of creatine deficiency (Cullen et al. 2006). In a mice model of chronic allergic lung inflammation, creatine supplementation exacerbated allergic inflammation and airway responsiveness (Vieira et al. 2007). hArg, at the toxic dose of 1880 mg/kg body weight, has been reported to cause hypersensitivity, yet the possible involvement of creatine/creatinine had not been investigated in that study (O’Neal et al. 1968).
Because of the limited number of rats, the small number of concentration and time points, and the lack of analyses in urine, brain and other organs such as the gut which is known to metabolize/catabolize creatinine (Jones and Burnett 1972; Yamashita et al. 2010), the distribution, the metabolism, and the pharmacokinetic data obtained in the present study should be considered as preliminary and incomplete with respect to hArg. Unfortunately, the time points for blood sampling and killing varied considerably during the experiments. Yet, we were able to measure plasma concentrations of many analytes at few time points within the time interval of 90 min. This time interval is generally used in our studies on experimentally induced TTC (Redfors et al. 2014). As an approximation, our results suggest that the elimination half-life time of exogenous hArg is of the order of 20–40 min in the investigated hArg dose regime. In healthy young volunteers the half-life of supplemented hArg could not be determined (Atzler et al. 2016). In pigs and rats given a single dose of hArg in drinking water (1 and 10 mg/kg body weight) no changes were observed in plasma hArg concentrations, while administered hArg was quantitatively recovered in urine, suggesting no hArg catabolism in the animals (Hou et al. 2016). Oral Arg administration for 13 weeks to pigs and rats increased plasma and urinary hArg concentration in a dose-dependent manner (Hou et al. 2016). As we did not collect urine samples in our study, we have no information whether the decrease of plasma hArg concentration observed in our study is due to excretion of non-metabolized hArg. In the rat given the highest hArg dose (440 mg/kg body weight) we observed increases in Arg, GAA, and creatinine plasma concentrations, suggesting partial utilization of hArg in the biosynthesis of Arg and GAA. At the dose of 440 mg/kg body weight, hArg plasma concentration increased (from 0.5 µM) to 1338 µM (15 min), 1176 µM (30 min) and 498 µM (60 min). At these very high hArg plasma concentrations inhibition of the activity of enzymes, such as arginase (Ryan et al. 1968), alkaline phosphatase, and possibly of other related enzymes including AGAT, GAMT, and dimethylarginine dimethylaminohydrolase (DDAH), is likely to have occurred and to have led to temporal increases in Arg, GAA, and ADMA plasma concentrations.
Our study appears to suggest some increase in NO synthesis upon hArg supplementation. Thus, injection of 440 mg hArg per kg body weight increased the nitrate plasma concentration from 54 µM (before injection) to 101 µM (15 min) and even to 130 µM (60 min). Other groups have also reported increases in nitrite and nitrate upon hArg administration (Yang et al. 2015; Dellera et al. 2016; Hou et al. 2016). For instance, oral Arg supplementation (3.6 g/kg body weight/day for 3 months) increased the plasma concentration of Arg (1.8-fold), Orn (1.7-fold), hArg (1.2-fold), and nitrite + nitrate (1.6-fold) (Yang et al. 2015). We agree with these groups on their proposed explanation that the increases in plasma nitrite and nitrate concentrations observed in their studies are due to elevation of Arg rather than of hArg concentration. This thought is supported by observations that hArg diminishes NOS-catalyzed synthesis from Arg when applied at high concentrations (i.e., 100 µM), most likely due to a lower affinity of hArg to NOS compared Arg (Alesutan et al. 2016).
Comprehensive studies in rats indicated that long-term dietary supplementation of Arg (as hydrochloride salt) to rats is safe and induces considerable changes in profiles of activity of particular amino acids and enzymes (Yang et al. 2015). At a dose of 5 mmol/kg body weight, i.e., 940 mg/kg body weight, hArg caused no appreciable symptoms of toxicity in rats (O’Neal et al. 1968). However, hArg administration of 10 mmol/kg body weight, i.e., 1880 mg/kg body weight, which is four times higher than the highest dose we applied in our study, may cause hypersensitivity, and even death may occur (O’Neal et al. 1968). Thus, the hArg dose regime we used in the present study is likely not to have exerted toxic effects in the rats.
Platelets are the major source of MDA in humans (Tsikas 2017; Tsikas et al. 2017). MDA correlates very closely with other biomarkers of lipid peroxidation including 4-hydroxy-2-nonenal and 8-iso-prostaglandin F2α and seems to be a dependable representative of lipid peroxidation biomarkers (Tsikas 2017; Tsikas et al. 2017). As activated platelets may produce large amounts of MDA from arachidonic acid by cyclooxygenase-1, plasma MDA concentration may also be useful to measure platelet hypersensitivity. The plasma concentration of MDA increased slightly only at the highest hArg dose of 440 mg/kg (2.3 mmol/kg) body weight. In contrast to studies from other groups (Wyse et al. 2001; Sasso et al. 2015), previous in vitro studies from our group and the present study suggest that hArg lacks such effects in vivo in the rat at doses below 220 mg/kg body weight, as well as in vitro at concentrations up to 50 µM (Hanff et al. 2016a, b).
In a recent study, Arg supplementation to rats resulted in enhanced tissue hArg content and AGAT activity in the kidney but not in the liver or small intestine, suggesting that the kidney is a major contributor to circulating hArg (Yang et al. 2015). This finding prompted us to detect AGAT activity in the kidney of the control rat which had been administered with hArg-free physiological saline by measuring formation of [15N2]-hArg and [15N2]-GAA from externally added [15N2]-Arg, Lys, and Gly. Unlike other assays which use very high substrate concentrations and very long incubation times (Verhoeven et al. 2003; Alessandrì et al. 2005; Yang et al. 2015), we used in the present study relatively low concentrations of the substrates [15N2]-Arg, Gly, and Lys, i.e., 1 mM each, and shorter incubation times. Under these conditions we observed formation of [15N2]-hArg and [15N2]-GAA in the kidney. The relatively low changes in [15N2]-hArg and [15N2]-GAA upon longer incubation times may suggest a steady state between their formation from [15N2]-Arg and their subsequent metabolism/catabolism and/or inhibition of AGAT activity by Orn (Verhoeven et al. 2003) and creatine (Tsikas and Wu 2015). The use of [15N2]-Arg and the measurement of [15N2]-hArg and [15N2]-GAA by mass spectrometry-based methods such as GC–MS assays of AGAT activity in vitro and in vivo (Kayacelebi et al. 2015b; see also Verhoeven et al. 2003) offers considerable advantages, most notably higher specificity, over other methods that use unlabeled Arg and measure unlabeled hArg and/or GAA (Alessandrì et al. 2005; Yang et al. 2015) or even Orn (McGuire et al. 1980). Yet, tissue AGAT activity assays based on GC–MS (Verhoeven et al. 2003; Alessandrì et al. 2005; present study) or on other techniques warrant further improvement and full characterization of their analytical characteristics. Of particular importance are the linearity of the assays and the concomitant inhibition of the activity of enzymes such as guanidinoacetate methyl transferase (GAMT) and arginase that are known to be abundantly present in kidney and other organs and to possess much higher activity that NOS isoforms towards Arg.
In conclusion, using the animal model of TTC, developed and validated by one of our groups (Redfors et al. 2014), intra-peritoneal injection of hArg into anesthetized rats resulted in remarkable dose-dependent increases of plasma hArg concentration, but in small changes of the plasma concentrations of Arg, ADMA, and GAA. In this TTC model, the plasma half-life of exogenous hArg ranges between 20 and 40 min in the anesthetized rat. Injected hArg is distributed in liver, kidney, lung, and heart and seems to accumulate preferably in the kidneys. As we did not collect urine samples, the present study does not provide information about renal elimination of non-metabolized hArg which was found to be remarkably high in pigs (Hou et al. 2016). In forthcoming experiments in our rat model of TTC (Redfors et al. 2014), we will test putative cardiovascular protective effects of hArg by using doses not higher than 20 mg (≈0.1 mmol) hArg per kg body weight. At hArg bolus intra-peritoneal doses up to 20 mg/kg body weight, side-effects due to changes in related amino acids and enzymes are not expectable. In this pilot study, we provided evidence that l-[guanidino-15N2]-arginine is a useful substrate to assay specifically AGAT activity in rat kidney in vitro by measuring l-[guanidino-15N2]-homoarginine and l-[guanidino-15N2]-guanidinoacetate. Yet, this assay and other reported AGAT assays require further improvement, validation, and characterization of their analytical performance. A recent study demonstrated a link between hArg and NO formation in hippocampus but not in frontal lobe of the rat (Sase et al. 2016; see also Choe et al. 2013a; Bernstein et al. 2015). In forthcoming studies we will include rat brain to investigate distribution, metabolism, and pharmacology of hArg.
Abbreviations
- ADMA:
-
Asymmetric dimethylarginine (N G,N G-dimethyl-l-arginine)
- AGAT:
-
Arginine:glycine amidinotransferase
- DDAH:
-
Dimethylarginine dimethylaminohydrolase
- GAA:
-
Guanidinoacetate
- GAMT:
-
Guanidinoacetate methyltransferase
- GC–MS:
-
Gas chromatography–mass spectrometry
- hArg:
-
Homoarginine
- MDA:
-
Malondialdehyde
- N G :
-
Guanidine nitrogen
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- PBS:
-
Phosphate buffered saline
- SIC:
-
Stress-induced cardiomyopathy
- TTC:
-
Takotsubo cardiomyopathy
References
Alessandrì MG, Celati L, Battini R, Casarano M, Cioni G (2005) Gas chromatography/mass spectrometry assay for arginine: glycine-amidinotransferase deficiency. Anal Biochem 343:356–358
Alesutan I, Feger M, Tuffaha R, Castor T, Musculus K, Buehling SS et al (2016) Augmentation of phosphate-induced osteo-/chondrogenic transformation of vascular smooth muscle cells by homoarginine. Cardiovasc Res 110:408–418
Atzler D, Schönhoff M, Cordts K, Ortland I, Hoppe J, Hummel FC, Gerloff C, Jaehde U, Jagodzinski A, Böger RH, Choe CU, Schwedhelm E (2016) Oral supplementation with l-homoarginine in young volunteers. Br J Clin Pharmacol 82:1477–1485
Bernstein HG, Jäger K, Dobrowolny H, Steiner J, Keilhoff G, Bogerts B, Laube G (2015) Possible sources and functions of l-homoarginine in the brain: review of the literature and own findings. Amino Acids 47:1729–1740
Bode-Böger SM (2006) Effect of l-arginine supplementation on NO production in man. Eur J Clin Pharmacol 62:91–99
Cathelineau L, Saudubray JM, Charpentier C, Polonovski C (1974) Letter: the presence of the homoanalogues of substrates of the urea cycle in the presence of argininosuccinate synthetase deficiency. Pediatr Res 8:857
Chappell JB (1963) The effect of alkylguanidines on mitochondrial metabolism. J Biol Chem 238:410–417
Choe CU, Atzler D, Wild PS, Carter AM, Boger RH, Ojeda F et al (2013a) Homoarginine levels are regulated by arginine:glycine amidinotransferase and affect stroke outcome: results from human and murine studies. Circulation 128:1451–1461
Choe CU, Nabuurs C, Stockebrand MC, Neu A, Nunes P, Morellini F et al (2013b) Arginine:glycine amidinotransferase deficiency protects from metabolic syndrome. Hum Mol Genet 22:110–123
Cullen ME, Yuen AH, Felkin LE, Smolenski RT, Hall JL, Grindle S et al (2006) Myocardial expression of the arginine:glycine amidinotransferase gene is elevated in heart failure and normalized after recovery: potential implications for local creatine synthesis. Circulation 114:I16–I20
Davids M, Ndika JD, Salomons GS, Blom HJ, Teerlink T (2012) Promiscuous activity of arginine:glycine amidinotransferase is responsible for the synthesis of the novel cardiovascular risk factor homoarginine. FEBS Lett 586:3653–3657
Dellera F, Ganzetti GS, Froio A, Manzini S, Busnelli M, Meinitzer A, Sirtori CR, Chiesa G, Parolini C (2016) l-Homoarginine administration reduces neointimal hyperplasia in balloon-injured rat carotids. Thromb Haemost 116:400–402
Gambaryan S, Tsikas D (2015) A review and discussion of platelet nitric oxide and nitric oxide synthase: do blood platelets produce nitric oxide from l-arginine or nitrite? Amino Acids 47:1779–1793
Hanff E, Böhmer A, Zinke M, Gambaryan S, Schwarz A, Supuran CT et al (2016a) Carbonic anhydrases are producers of S-nitrosothiols from inorganic nitrite and modulators of soluble guanylyl cyclase in human platelets. Amino Acids 48:1695–1706
Hanff E, Kayacelebi AA, Yanchev GR, Maassen N, Haghikia A, Tsikas D (2016b) Simultaneous stable-isotope dilution GC–MS measurement of homoarginine, guanidinoacetate and their common precursor arginine in plasma and their interrelationships in healthy and diseased humans. Amino Acids 48:721–732
Hanff E, Lützow M, Kayacelebi AA, Finkel A, Maassen M, Yanchev GR, Haghikia A, Bavendiek U, Buck A, Lücke T, Maassen N, Tsikas D (2017) Simultaneous GC–ECNICI–MS measurement of nitrite, nitrate and creatinine in human urine and plasma in clinical settings. J Chromatogr B 1047:207–2014
Hibbs JB Jr, Westenfelder C, Taintor R, Vavrin Z, Kablitz C, Baranowski RL, Ward JH, Menlove RL, McMurry MP, Kushner JP, Samlowski WE (1992) Evidence for cytokine-inducible nitric oxide synthesis from l-arginine in patients receiving interleukin-2 therapy. J Clin Invest 89:867–877
Holecek M, Sispera L (2016) Effects of arginine supplementation on amino acid profiles in blood and tissues in fed and overnight-fasted rats. Nutrients 8:206
Hollunger G (1955) Guanidines and oxidative phosporylations. Acta Pharmacol Toxicol 11(Suppl 1):1–84
Hou Y, Hu S, Jia S, Nawaratna G, Che D, Wang F et al (2016) Whole-body synthesis of l-homoarginine in pigs and rats supplemented with l-arginine. Amino Acids 48:993–1001
Jones JD, Burnett PC (1972) Implication of creatinine and gut flora in the uremic syndrome: induction of “creatininase” in colon contents of the rat by dietary creatinine. Clin Chem 18:280–284
Kato T, Sano M, Mizutani N, Hayakawa C (1988) Homocitrullinuria and homoargininuria in hyperargininaemia. J Inherit Metab Dis 11:261–265
Kayacelebi AA, Nguyen TH, Neil C, Horowitz JD, Jordan J, Tsikas D (2014) Homoarginine and 3-nitrotyrosine in patients with takotsubo cardiomyopathy. Int J Cardiol 173:546–547
Kayacelebi AA, Langen J, Weigt-Usinger K, Chobanyan-Jurgens K, Mariotti F, Schneider JY et al (2015a) Biosynthesis of homoarginine (hArg) and asymmetric dimethylarginine (ADMA) from acutely and chronically administered free l-arginine in humans. Amino Acids 47:1893–1908
Kayacelebi AA, Knöffel AK, Beckmann B, Hanff E, Warnecke G, Tsikas D (2015b) Measurement of unlabeled and stable isotope-labeled homoarginine, arginine and their metabolites in biological samples by GC–MS and GC–MS/MS. Amino Acids 47:2023–2034
Lacza Z, Kozlov AV, Pankotai E, Csordás A, Wolf G, Redl H, Kollai M, Szabó C, Busija DW, Horn TF (2006a) Mitochondria produce reactive nitrogen species via an arginine-independent pathway. Free Radic Res 40:369–378
Lacza Z, Pankotai E, Csordás A, Gero D, Kiss L, Horváth EM, Kollai M, Busija DW, Szabó C (2006b) Mitochondrial NO and reactive nitrogen species production: does mtNOS exist? Nitric Oxide 14:162–168
Levin B, Oberholzer VG, Palmer T (1974) Letter: the high levels of lysine, homocitrulline, and homoarginine found in argininosuccinate synthetase deficiency. Pediatr Res 8:857–858
Mansuy D, Boucher JL (2004) Alternative nitric-oxide producing substrates for NO synthases. Free Radic Biol Med 37:1105–1121
März W, Meinitzer A, Drechsler C, Pilz S, Krane V, Kleber ME, Fischer J, Winkelmann BR, Böhm BO, Ritz E, Wanner C (2010) Homoarginine, cardiovascular risk, and mortality. Circulation 122:967–975
McGuire DM, Tormanen CD, Segal IS, Van Pilsum JF (1980) The effect of growth hormone and thyroxine on the amount of l-arginine:glycine amidinotransferase in kidneys of hypophysectomized rats. Purification and some properties of rat kidney transamidinase. J Biol Chem 255:1152–1159
O’Neal RM, Chen CH, Reynolds CS, Meghal SK, Koeppe RE (1968) The ‘neurotoxicity’ of l-2,4-diaminobutyric acid. Biochem J 106:699–706
Pentyala J, Rao SLN (1999) Sustained nitric oxide generation with l-homoarginine. Res Commun Biochem Cell Mol Biol 3:223–232
Pilz S, Meinitzer A, Gaksch M, Grübler M, Verheyen N, Drechsler C, Hartaigh BÓ, Lang F, Alesutan I, Voelkl J, März W, Tomaschitz A (2015) Homoarginine in the renal and cardiovascular systems. Amino Acids 47:1703–1713
Redfors B, Oras J, Shao Y, Seemann-Lodding H, Ricksten SE, Omerovic E (2014) Cardioprotective effects of isoflurane in a rat model of stress-induced cardiomyopathy (takotsubo). Int J Cardiol 176:815–821
Ryan WL, Wells IC (1964) Homocitrulline and homoarginine synthesis from lysine. Science 144:1122–1127
Ryan WL, Barak AJ, Johnson RJ (1968) Lysine, homocitrulline, and homoarginine metabolism by the isolated perfused rat liver. Arch Biochem Biophys 123:294–297
Ryan WL, Johnson RJ, Dimari S (1969) Homoarginine synthesis by rat kidney. Arch Biochem Biophys 131:521–526
Sase A, Nawaratna G, Hu S, Wu G, Lubec G (2016) Decreased hippocampal homoarginine and increased nitric oxide and nitric oxide synthase levels in rats parallel training in a radial arm maze. Amino Acids 48:2197–2204
Sasso S, Dalmedico L, Magro DD, Pereira EM, Wyse AT, de Lima DD (2015) Differential in vitro effects of homoarginine on oxidative stress in plasma, erythrocytes, kidney and liver of rats in the absence and in the presence alpha-tocopherol, ascorbic acid or l-NAME. Amino Acids 47:1931–1939
Schneider JY, Rothmann S, Schröder F, Langen J, Lücke T, Mariotti F, Huneau JF, Frölich JC, Tsikas D (2015) Effects of chronic oral l-arginine administration on the l-arginine/NO pathway in patients with peripheral arterial occlusive disease or coronary artery disease: l-arginine prevents renal loss of nitrite, the major NO reservoir. Amino Acids 47:1961–1974
Stockebrand M, Hornig S, Neu A, Atzler D, Cordts K, Böger RH et al (2015) Homoarginine supplementation improves blood glucose in diet-induced obese mice. Amino Acids 47:1921–1927
Tsikas D (2000) Simultaneous derivatization and quantification of the nitric oxide metabolites nitrite and nitrate in biological fluids by gas chromatography/mass spectrometry. Anal Chem 72:4064–4072
Tsikas D (2015) Circulating and excretory nitrite and nitrate: their value as measures of nitric oxide synthesis, bioavailability and activity is inherently limited. Nitric Oxide 45:1–3
Tsikas D (2017) Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem 524:13–30
Tsikas D, Kayacelebi AA (2014) Do homoarginine and asymmetric dimethylarginine act antagonistically in the cardiovascular system? Circ J 78:2094–2095
Tsikas D, Böger RH, Sandmann J, Bode-Böger SM, Frölich JC (2000) Endogenous nitric oxide synthase inhibitors are responsible for the l-arginine paradox. FEBS Lett 478:1–3
Tsikas D, Rothmann S, Schneider JY, Gutzki FM, Beckmann B, Frölich JC (2017) Simultaneous measurement of malondialdehyde (MDA) and 4-hydroxy-2-nonenal in human plasma: effects of long-term l-arginine addministration. Anal Biochem 524:31–44
Tsikas D, Wu G (2015) Homoarginine, arginine, and relatives: analysis, metabolism, tcharansport, physiology, and pathology. Amino Acids 47:1697–1702
Verhoeven NM, Schor DS, Roos B, Battini R, Stöckler-Ipsiroglu S, Salomons GS, Jakobs C (2003) Diagnostic enzyme assay that uses stable-isotope-labeled substrates to detect l-arginine:glycine amidinotransferase deficiency. Clin Chem 49:803–805
Vieira RP, Duarte AC, Claudino RC, Perini A, Santos AB, Moriya HT, Arantes-Costa FM, Martins MA, Carvalho CR, Dolhnikoff M (2007) Creatine supplementation exacerbates allergic lung inflammation and airway remodeling in mice. Am J Respir Cell Mol Biol 37:660–667
Wu G, Bazer FW, Davis TA, Kim SW, Li P, Marc Rhoads J et al (2009) Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37:153–168
Wyse AT, Bavaresco CS, Hagen ME, Delwing D, Wannmacher CM, Severo Dutra-Filho C et al (2001) In vitro stimulation of oxidative stress in cerebral cortex of rats by the guanidino compounds accumulating in hyperargininemia. Brain Res 923:50–57
Yamashita K, Nakajima Y, Matsushita H, Nishiya Y, Yamazawa R, Wu YF, Matsubara F, Oyama H, Ito K, Yoshimoto T (2010) Substitution of Glu122 by glutamine revealed the function of the second water molecule as a proton donor in the binuclear metal enzyme creatininase. J Mol Biol 396:1081–1096
Yang Y, Wu Z, Jia S, Dahanayaka S, Feng S, Meininger CJ, McNeal CJ, Wu G (2015) Safety of long-term dietary supplementation with l-arginine in rats. Amino Acids 47:1909–1920
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical statement
Rats received humane care and the study protocol complied with the institutional guidelines of the Sahlgrenska University Hospital.
Additional information
Handling Editors: C.-A. A. Hu, Y. Yin, Y. Hou, G. Wu, Y. Teng.
Rights and permissions
About this article
Cite this article
Günes, D.N., Kayacelebi, A.A., Hanff, E. et al. Metabolism and distribution of pharmacological homoarginine in plasma and main organs of the anesthetized rat. Amino Acids 49, 2033–2044 (2017). https://doi.org/10.1007/s00726-017-2465-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-017-2465-7