Abstract
l-Homoarginine (hArg) is an endogenous amino acid which has emerged as a novel biomarker for stroke and cardiovascular disease. Low circulating hArg levels are associated with increased mortality and vascular events, whereas recent data have revealed positive correlations between circulating hArg and metabolic vascular risk factors like obesity or blood glucose levels. However, it is unclear whether hArg levels are causally linked to metabolic parameters. Therefore, the aim of our study was to investigate whether hArg directly influences body weight, blood glucose, glucose tolerance or insulin sensitivity. Here, we show that hArg supplementation (14 and 28 mg/mL orally per drinking water) ameliorates blood glucose levels in mice on high-fat diet (HFD) by a reduction of 7.3 ± 3.7 or 13.4 ± 3.8 %, respectively. Fasting insulin concentrations were slightly, yet significantly affected (63.8 ± 11.3 or 162.1 ± 39.5 % of control animals, respectively), whereas body weight and glucose tolerance were unaltered. The substantial augmentation of hArg plasma concentrations in supplemented animals (327.5 ± 40.4 or 627.5 ± 60.3 % of control animals, respectively) diminished profoundly after the animals became obese (129.9 ± 16.6 % in control animals after HFD vs. 140.1 ± 8.5 or 206.3 ± 13.6 %, respectively). This hArg-lowering effect may contribute to the discrepancy between the inverse correlation of plasma hArg levels with stroke and cardiovascular outcome, on the one hand, and the direct correlation with cardiovascular risk factors like obesity and blood glucose, on the other hand, that has been observed in human studies. Our results suggest that the glucose-lowering effects of hArg may reflect a compensatory mechanism of blood glucose reduction by hArg upregulation in obese individuals, without directly influencing body weight or glucose tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Homoarginine (hArg) is an endogenous non-proteinogenic cationic amino acid in humans, which has been identified as a novel risk factor for stroke and cardiovascular disease (Atzler et al. 2015). Low circulating hArg levels are associated with cerebrovascular and cardiovascular outcome and mortality (März et al. 2010; Pilz et al. 2011a; Choe et al. 2013a). Although the underlying mechanism remains still unclear, previous studies have suggested that hArg is involved in nitric oxide (NO) metabolism and might therefore alter vascular function. hArg can serve as substrate for NO synthase (NOS) to produce NO (Lambert et al. 1992; Moali et al. 1998, 2000; Bretscher et al. 2003). Furthermore, hArg can competitively inhibit arginase activity, and could thereby indirectly increase arginine levels and facilitate NO production (Hrabak et al. 1994; Reczkowski et al. 1994; Pentyala and Rao 1999). Physiological functions of hArg, like inhibition of platelet aggregation and augmentation of flow-mediated dilatation, have been suggested, but have not been confirmed so far (Radomski et al. 1990; Valtonen et al. 2008; Choe et al. 2013a). In this context, it should be considered that circulating hArg concentrations are several fold lower than those of its analog l-arginine and the affinity of hArg to NOS is also remarkably lower compared to arginine (Lambert et al. 1992; Moali et al. 1998; Moali et al. 2000; Bretscher et al. 2003).
Circulating hArg concentrations are inversely correlated with stroke and cardiovascular outcome. Conversely, some classical risk factors have been positively linked with hArg levels. In particular, the body mass index (BMI) positively correlated with hArg in several clinical studies, (März et al. 2010; Ravani et al. 2013; van der Zwan et al. 2013; Atzler et al. 2014). Furthermore, fasting blood glucose levels, hemoglobin A1c and presence of diabetes mellitus were positively correlated with circulating hArg (Pilz et al. 2014; Ravani et al. 2013; van der Zwan et al. 2013; Atzler et al. 2014). At present, it is unclear whether hArg directly influences body weight or other metabolic parameters like glucose homeostasis.
Previously, we have shown that l-arginine:glycine amidinotransferase (AGAT) is required in human embryonic kidney cells and mice to synthesize hArg (Choe et al. 2013a), and hArg was not detectable in AGAT-deficient lymphoblasts (Davids et al. 2012). Given that AGAT is the first and rate-limiting enzyme of creatine synthesis, it is not surprising that in AGAT-deficient mice creatine and hArg levels were below the detection limits of the methods used (Choe et al. 2013a, b). AGAT-deficient mice revealed reduced body weight, BMI, fasting blood glucose levels and improved glucose tolerance; these effects were all completely reversible upon creatine supplementation and independent of hArg (Choe et al. 2013b). Effects of hArg on glucose metabolism in wild-type (wt) animals have not been studied so far. Therefore, we aimed to elucidate the role of hArg in body weight and glucose homeostasis. For this purpose, we supplemented C57BL/6J wt mice with hArg and analyzed body weight and glucose handling on normal- and high-fat diet (HFD).
Materials and methods
Care and treatment of mice
Animals used in this study were C57BL/6J male mice. Mice (<5 per cage) were kept in standard cages under a 12–12 h light–dark cycle, constant temperature and humidity, and received standard food and water ad libitum. Chronic hArg supplementation was achieved by adding to drinking water 0 mg/L (control), 14 or 28 mg/L hArg (Sigma-Aldrich, Steinheim, Germany) to reach final concentrations of 0, ~75 and 150 µM, respectively. After 8 weeks of hArg supplementation on normal diet, mice received HFD for a minimum of additional 8 weeks with continuous hArg supplementation. Normal diet (R/M-H, Ssniff, Soest, Germany) was essentially hArg free (165.6 ng/kg) and contained 11.4 g/kg l-arginine (arg). It was composed of 19 % (w/w) protein [33 % metabolisable energy (kJ %)], 3.3 % (w/w) fat (9 kJ %) and 41 % (w/w) carbohydrate (58 kJ %). HFD contained 60 kJ % fat, 19 kJ % protein and 21 kJ % carbohydrates (Ssniff E15741-34, acc. D12492 (II) mod., Ssniff, Soest, Germany). It contained 8.8 g/kg arg and was essentially hArg free (21.6 ng/kg). Mice were 12.1 ± 0.5 weeks at the beginning of the hArg supplementation, with no difference of age between hArg treatment groups (P > 0.92 by one-way ANOVA), n = 19–20 animals per hArg supplementation group. For calculation of the BMI (body weight/body length2 expressed in kg/m2) the snout–anus length of the animals was measured. All experimental procedures were in accordance with institutional guidelines and approved by the local animal Ethics Committee.
Glucose and insulin tolerance tests
Mice were fasted for 14–16 h and intraperitoneally injected with glucose (2 g per kg body weight) or insulin (0.5 U per kg body weight) (Sigma-Aldrich) as previously described (Choe et al. 2013b). Blood glucose was measured from tail capillary blood and collected at the indicated times.
Blood collection and biochemical analyses
Venous EDTA-treated blood was retrieved by submandibular puncture. Heparinized blood was obtained by direct cardiac puncture in isoflurane anesthesia. Leptin, insulin, C-peptide and glucagon plasma levels were measured from plasma of heparinized blood by ELISA according to the manufacturers’ protocols (Crystal Chemistry, CusaBio) as previously described (Choe et al. 2013b). hArg measurements in plasma were performed using a LC–MS/MS assay as described previously (Atzler et al. 2011) on a Varian 1200L Triple Quadrupole MS equipped with two Varian ProStar model 210 HPLC pumps (Agilent Technologies, Santa Clara, CA, USA). After addition of the stable isotope labeled internal standard [13C6]-hArg (to a final concentration of 10 µM), protein precipitation with methanol, and derivatization of hArg and [13C6]-hArg to their butyl ester derivatives, the analytes were dissolved in sample buffer (methanol–water, 25/75 v/v, containing 0.1 vol% ammonium formate, adjusted to pH 5 with formic acid).
Statistical analyses
Data are given as mean ± SEM. The following statistical tests were applied: one-way ANOVA, two-way ANOVA or two-way ANOVA for repeated measurements, followed by Newman–Keuls post hoc comparisons (Graphpad Prism 5, Statistica 5, Statsoft). All tests were two-tailed and the level of significance was set at P < 0.05.
Results
Effect of chronic hArg supplementation on blood glucose in diet-induced obese animals
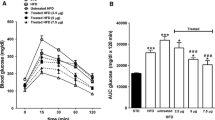
To investigate a possible causal relation between plasma hArg and glucose, we determined blood glucose levels in mice supplemented with hArg on normal diet or HFD. Fasting blood glucose increased in all animals upon HFD. Following 8 weeks of HFD, however, chronic hArg supplementation ameliorated fasting blood glucose in a dose-dependent manner (Fig. 1a). Blood glucose levels in ad libitum fed state were similarly improved by hArg supplementation in obese mice (0 mg/L hArg group: 181 ± 8 mg/dL; 14 mg/L hArg group: 170 ± 7 mg/dL; 28 mg/L hArg group: 143 ± 5 mg/dL, n = 10, P < 0.01).
Chronic hArg supplementation ameliorates blood glucose levels in diet-induced obese mice, without measurable effects on body weight. Comparison of fasted blood glucose levels (a) and body weight (b) before and during oral hArg supplementation (0, 14, or 28 mg/L hArg in drinking water) on normal diet and on HFD (n = 19–20). Data are expressed as mean ± SEM. **P < 0.01, ***P < 0.001 by two-way ANOVA with repeated measurements
We have previously shown, that the HFD regime utilized here results in pronounced obesity, impaired glucose handling and a diabetes-like phenotype in C57Bl/6J wt mice (Choe et al. 2013a). As expected, HFD induced pronounced obesity in all animals; however, hArg supplementation did not change the body weight of animals under either normal or HFD (Fig. 1b). Also, the BMI of animals after HFD were unaltered by hArg supplementation (0 mg/L hArg group: 3.84 ± 0.16 kg/m2; 14 mg/L hArg group: 3.75 ± 0.13 kg/m2; 28 mg/L hArg group: 3.85 ± 0.7 kg/m2, n = 7–10, P = 0.80).
Effect of hArg supplementation on glucose handling and insulin responses
We next analyzed the effect of hArg supplementation on glucose handling and insulin responses. Glucose tolerance decreased in obese animals. hArg supplementation, however, did not influence glucose tolerance (Fig. 2a, c, d). Consistent with progressing insulin resistance, glucose responses to insulin injections were slower and less pronounced in obese animals, but no significant difference was observed between the hArg treatment groups (Fig. 2b, e, f).
Obesity-related changes of glucose and insulin tolerance were unaffected by chronic hArg supplementation. Glucose tolerance (a, c, e) (n = 19–21) and insulin tolerance (b, d, f) (n = 9–10) tests in mice on normal diet before (a, b) or after 8 weeks of hArg supplementation (0, 14, or 28 mg/L in drinking water) (c, d) or after additional 8 weeks on HFD with hArg supplementation (e, f), data are given as mean ± SEM
Effect of hArg supplementation on blood levels of hArg
To analyze the correlation between hArg supplementation and hArg blood levels, we determined hArg plasma concentrations in supplemented animals before and after diet-induced obesity (DIO). Oral hArg supplementation via drinking water with 14 or 28 mg/L for 8 weeks resulted in 3- or 6-fold increased plasma hArg concentrations, respectively: 0 mg/L hArg group, 0.14 ± 0.02 µM; 14 mg/L hArg group, 0.46 ± 0.06 µM; 28 mg/L hArg group, 0.88 ± 0.08 µM, (n = 18–20) (Fig. 3a). DIO had no impact on hArg levels in non-supplemented animals. However, HFD completely blunted any elevation of hArg plasma concentration by 14 mg/L hArg supplementation and reduced the hArg plasma concentration of animals supplemented with 28 mg/L hArg by 67 % to only about 1.5-fold increased levels compared with non-supplemented animals: 0 mg/L hArg group, 0.19 ± 0.02 µM; 14 mg/L hArg group, 0.20 ± 0.01 µM; 28 mg/L hArg group, 0.29 ± 0.02 µM, (n = 15–19) (Fig. 3b).
Plasma hArg levels are decreased in adipose animals. Plasma hArg concentrations after 8 weeks of hArg supplementation (0, 14, or 28 mg/L hArg in drinking water), before (a) or after additional 8 weeks on HFD and hArg supplementation (b), data are expressed as mean ± SEM (n = 15–20), ***P < 0.001 by one-way ANOVA
hArg effects on insulin, C-peptide, leptin and glucagon levels in diet-induced obese mice
With different glucose levels between hArg treatment groups, yet equal insulin responsiveness, we wondered whether secretion of glucose or lipid regulating hormones differed between hArg treatment groups. Therefore, we determined plasma levels of insulin, and glucagon, and as an independent measure for insulin secretory rate we also determined the pro-insulin connecting peptide (C-peptide) of diet-induced obese animals from different hArg supplementation groups (Fig. 4a–c). Because leptin has been positively associated with hArg in clinical and population-based studies (Atzler et al. 2014) and leptin and insulin influence each other in the regulation of body weight and glucose homeostasis (Barr et al. 1997; Covey et al. 2006) we also measured leptin plasma levels (Fig. 4d). Plasma insulin (P = 0.03) (Fig. 4a), C-peptide (P = 0.03) (Fig. 4b) and leptin (P < 0.01) (Fig. 4d) levels were significantly influenced by hArg, with post hoc group comparison revealing significant differences between the two animal groups supplemented with different hArg dosages. In contrast, glucagon levels (Fig. 4c) were not significantly influenced by hArg supplementation (P > 0.23).
Discussion
In the current study, we have investigated for the first time the effects of chronic hArg supplementation on body weight and glucose homeostasis in mice before and after diet-induced obesity. In human studies, circulating hArg has emerged as marker for cardiovascular disease (Atzler et al. 2015). Furthermore, genome-wide association analyses revealed hArg levels to be tightly linked with variants of the AGAT genomic locus (Choe et al. 2013a; Kleber et al. 2013). Given that AGAT synthesizes hArg and also guanidinoacetate (Choe et al. 2013a, b), which is the precursor of creatine, we used an oral supplementation strategy to investigate AGAT-independent hArg effects on glucose handling and body weight homeostasis.
Population-based studies and clinical cohorts of cardiovascular or dialysis patients suggested that hArg might increase BMI (März et al. 2010; Ravani et al. 2013; van der Zwan et al. 2013; Atzler et al. 2014). In this study, we show that a 3- to 6-fold increase of hArg plasma concentrations did not significantly increase body weight. Recently, it was reported that weight loss of more than 30 kg in four obese subjects after bariatric surgery had no influence on hArg plasma concentration, and no correlation was found between hArg plasma concentration and BMI (May et al. 2015). Moreover, our data rather suggest that increased body weight and fat mass reduce hArg plasma levels, because the increased hArg concentrations observed after 8 weeks of hArg supplementation were blunted after additional 8 weeks of HFD combined with hArg supplementation. Given that hArg in adipose tissue seems to be independent of hArg plasma concentration (May et al. 2015), it is plausible that increased adipose tissue mass could cause a reduction in hArg plasma concentration. In this context, cationic amino acid transporters might contribute to reduced hArg plasma concentrations by carrying hArg into different tissues (White and Christensen 1982; White et al. 1982). Glucosuria has been suggested to impair renal amino acid reabsorption (Bingham et al. 2001). Therefore, reduced renal hArg resaborption may also contribute to a reduction of hArg plasma levels after DIO. Increased levels of arginase have been described in obese adolescents (Kim et al. 2012; Jung et al. 2014), which might suggest another mechanism leading to reduced hArg plasma levels in obese subjects.
In addition to body weight, parameters of glucose homeostasis have been positively associated with hArg plasma concentrations in human studies (Pilz et al. 2014; Ravani et al. 2013; van der Zwan et al. 2013; Atzler et al. 2014). In contrast, diet-induced obese mice with elevated hArg plasma levels had significantly lower blood glucose levels. This result is in opposition to correlations of clinical studies, but suggests a compensatory mechanism—i.e., impaired glucose homeostasis triggering increased hArg plasma levels to reduce blood glucose. In our study, hArg did not influence fasting blood glucose in mice on normal diet. Therefore, the blood glucose-lowering effect of hArg does not seem to represent a generally applicable mechanism. Furthermore, circulating hArg levels are lower in patients with long-lasting type 2 diabetes mellitus (Drechsler et al. 2011), which might suggest adaptive mechanisms under conditions of chronic glucose intolerance. hArg supplementation did not alter glucose and insulin tolerance in our study, but increased fasting insulin concentrations were detected in animals supplemented with the higher dose of hArg as compared to the lower hArg dose. A similar increase of C-peptide plasma concentration, which is an independent measure of insulin secretion, that is not influenced by insulin degradation and undisturbed by exogenous insulin application (like in insulin tolerance tests), also indicated an elevation of insulin secretion. This could explain reduced fasting blood glucose. In line with this observation, a positive correlation between insulin and hArg has also been observed in population-based studies (Atzler et al. 2014). And previous studies with isolated pancreatic islet cells have shown that hArg can dose-dependently stimulate insulin release (Blachier et al. 1989; Weinhaus et al. 1997; Henningsson and Lundquist 1998), which is consistent with our findings in hArg-supplemented mice and in clinical correlations. In our study, hArg lowered blood glucose levels only in diet-induced obese mice, therefore suggesting a compensatory mechanism under conditions of severely impaired glucose tolerance. Although cell-based experiments have suggested that hArg also increases glucagon secretion, glucagon levels were unaltered in our hArg treatment groups (Henningsson and Lundquist 1998).
This is the first report of an intervention testing the influence of hArg on glucose homeostasis and the study has been designed for analyzing hArg effects on DIO and associated changes in glucose handling, with repeated measurements before and after HFD. Therefore, this study lacks a control group of age-matched lean animals on normal, low-fat diet for the entire duration of the experiment. There was no hArg effect on the induction of adiposity with regard to body weight gain and BMI, and all animals became obviously obese. Yet a comparison with age-matched lean animals on normal low-fat diet or a careful analysis of body fat content might reveal additional interactions between hArg supplementation and HFD which we may have missed in our study. Also, pharmacokinetic data of oral hArg supplementation are still missing, and this can impede the interpretation of differences in plasma hArg levels between the lean and obese state. However, preliminary experiments with small-sized supplementation groups indicated the adjustment of stable circulating hArg levels already within 2 weeks of treatment.
Given the positive correlation of hArg with creatine in clinical studies, it was argued that hArg levels might indicate intracellular energy stores, i.e. the phosphocreatine pool (März et al. 2010; Pilz et al. 2011b). Previously, we have shown that hArg itself reduces infarct size and neurological outcome in a murine experimental stroke model (Choe et al. 2013a). The data of the presented study suggest that the metabolic effects of hArg are also independent of AGAT expression and AGAT-related products such as guanidinoacetate and creatine.
In summary, our data reveal glucose-lowering effects of hArg in diet-induced obese mice (summarized in Fig. 5). This observation may suggest a compensatory mechanism under conditions of glucose intolerance and in patients with type 2 diabetes mellitus. Although clinical correlative data revealed a positive correlation between hArg and body weight, our results do not support a direct role of hArg in body weight regulation in humans.
Schematic summary of hArg supplementation and diet-induced obesity. hArg supplementation increases circulating hArg on normal low-fat diet (LFD), whereas after HFD only high dosage supplementation (28 mg/L drinking water) (++hArg++hArg) yields increased hArg levels, but low dosage supplementation (14 mg/L) (+hArg) does not. Arrows indicate the direction and strength of hArg effects on diabetes, glucose tolerance, blood glucose and DIO
Abbreviations
- AGAT:
-
Arginine:glycine amidinotransferase
- BMI:
-
Body mass index
- DIO:
-
Diet-induced obesity
- ELISA:
-
Enzyme-linked immunosorbent assay
- hArg:
-
Homoarginine
- HFD:
-
High-fat diet
- HPLC:
-
High-performance liquid chromatography
- LC–MS/MS:
-
Liquid chromatography–tandem mass spectrometry
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- Wt:
-
Wild-type
References
Atzler D, Mieth M, Maas R, Böger RH, Schwedhelm E (2011) Stable isotope dilution assay for liquid chromatography-tandem mass spectrometric determination of l-homoarginine in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 879:2294–2298
Atzler D, Gore MO, Ayers CR, Choe CU, Böger RH, de Lemos JA, McGuire DK, Schwedhelm E (2014) Homoarginine and cardiovascular outcome in the population-based Dallas Heart Study. Arterioscler Thromb Vasc Biol 34:2501–2507
Atzler D, Schwedhelm E, Choe CU (2015) l-Homoarginine and cardiovascular disease. Curr Opin Clin Nutr Metab Care 18:83–88
Barr VA, Malide D, Zarnowski MJ, Taylor SI, Cushman SW (1997) Insulin stimulates both leptin secretion and production by rat white adipose tissue. Endocrinology 138:4463–4472
Bingham C, Ellard S, Nicholls AJ, Pennock CA, Allen J, James AJ, Satchell SC, Salzmann MB, Hattersley AT (2001) The generalized aminoaciduria seen in patients with hepatocyte nuclear factor-1 alpha mutations is a feature of all patients with diabetes and is associated with glucosuria. Diabetes 50:2047–2020
Blachier F, Mourtada A, Sener A, Malaisse WJ (1989) Stimulus-secretion coupling of arginine-induced insulin release. Uptake of metabolized and nonmetabolized cationic amino acids by pancreatic islets. Endocrinology 124:134–141
Bretscher LE, Li H, Poulos TL, Griffith OW (2003) Structural characterization and kinetics of nitric-oxide synthase inhibition by novel N5-(iminoalkyl)- and N5- (iminoalkenyl)-ornithines. J Biol Chem 278:46789–46797
Choe CU, Atzler D, Wild PS, Carter AM, Böger RH, Ojeda F, Simova O, Stockebrand M, Lackner K, Nabuurs C, Marescau B, Streichert T, Muller C, Luneburg N, De Deyn PP, Benndorf RA, Baldus S, Gerloff C, Blankenberg S, Heerschap A, Grant PJ, Magnus T, Zeller T, Isbrandt D, Schwedhelm E (2013a) Homoarginine levels are regulated by l-arginine:glycine amidinotransferase and affect stroke outcome: results from human and murine studies. Circulation 128:1451–1461
Choe CU, Nabuurs C, Stockebrand MC, Neu A, Nunes P, Morellini F, Sauter K, Schillemeit S, Hermans-Borgmeyer I, Marescau B, Heerschap A, Isbrandt D (2013b) l-arginine:glycine amidinotransferase deficiency protects from metabolic syndrome. Hum Mol Genet 22:110–123
Covey SD, Wideman RD, McDonald C, Unniappan S, Huynh F, Asadi A, Speck M, Webber T, Chua SC, Kieffer TJ (2006) The pancreatic β cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell Metab 4:291–302
Davids M, Ndika JDT, Salomons GS, Blom HJ, Teerlink T (2012) Promiscuous activity of arginine-glycine amidinotransferase is responsible for the synthesis of the novel cardiovascular risk factor homoarginine. FEBS Lett 586:3653–3657
Drechsler C, Meinitzer A, Pilz S, Krane V, Tomaschitz A, Ritz E, März W, Wanner C (2011) Homoarginine, heart failure, and sudden cardiac death in haemodialysis patients. Eur J Heart Fail 13:852–859
Henningsson R, Lundquist I (1998) Arginine-induced insulin release is decreased and glucagon increased in parallel with islet NO production. Am J Physiol 275:E500–E506
Hrabák A, Bajor T, Temesi A (1994) Comparison of substrate and inhibitor specificity of arginase and nitric oxide (NO) synthase for arginine analogues and related compounds in murine and rat macrophages. Biochem Biophys Res Commun 198:206–212
Jung C, Figulla HR, Lichtenauer M, Franz M, Pernow J (2014) Increased levels of circulating arginase I in overweight compared to normal weight adolescents. Pediatr Diabetes 151:51–56
Kim OY, Lee SM, Chung JH, Do HJ, Moon J, Shin MJ (2012) Arginase I and the very low-density lipoprotein receptor are associated with phenotypic biomarkers for obesity. Nutrition 28:635–639
Kleber ME, Seppälä I, Pilz S, Hoffmann MM, Tomaschitz A, Oksala N, Raitoharju E, Lyytikäinen LP, Mäkelä KM, Laaksonen R, Kähönen M, Raitakari OT, Huang J, Kienreich K, FahrleitnerPammer A, Drechsler C, Krane V, Boehm BO, Koenig W, Wanner C, Lehtimäki T, März W, Meinitzer A (2013) Genome-wide association study identifies 3 genomic loci significantly associated with serum levels of homoarginine: the AtheroRemo Consortium. Circ Cardiovasc Genet 6:505–513
Lambert LE, French JF, Whitten JP, Baron BM, McDonald IA (1992) Characterization of cell selectivity of two novel inhibitors of nitric oxide synthesis. Eur J Pharmacol 216:131–134
März W, Meinitzer A, Drechsler C, Pilz S, Krane V, Kleber ME, Fischer J, Winkelmann BR, Böhm BO, Ritz E, Wanner C (2010) Homoarginine, cardiovascular risk, and mortality. Circulation 122:967–975
May M, Kayacelebi AA, Batkai S, Jordan J, Tsikas D, Engeli S (2015) Plasma and tissue homoarginine concentrations in healthy and obese humans. Amino Acids. doi:10.1007/s00726-015-1922-4 (in press)
Moali C, Boucher JL, Sari MA, Stuehr DJ, Mansuy D (1998) Substrate specificity of NO synthases: detailed comparison of l-arginine, homo-l-arginine, their N omega-hydroxy derivatives, and N omega-hydroxynor-l-arginine. Biochemistry 37:10453–10460
Moali C, Brollo M, Custot J, Sari MA, Boucher JL, Stuehr DJ, Mansuy D (2000) Recognition of alpha-amino acids bearing various C=NOH functions by nitric oxide synthase and arginase involves very different structural determinants. Biochemistry 39:8208–8218
Pentyala J, Rao SLN (1999) Sustained nitric oxide generation with l-homoarginine. Res Commun Biochem Cell Mol Biol 3:223–232
Pilz S, Tomaschitz A, Meinitzer A, Drechsler C, Ritz E, Krane V, Wanner C, Böhm BO, März W (2011a) Low serum homoarginine is a novel risk factor for fatal strokes in patients undergoing coronary angiography. Stroke 42:1132–1134
Pilz S, Meinitzer A, Tomaschitz A, Drechsler C, Ritz E, Krane V, Wanner C, Boehm BO, März W (2011b) Low homoarginine concentration is a novel risk factor for heart disease. Heart 97:1222–1227
Pilz S, Teerlink T, Scheffer PG, Meinitzer A, Rutters F, Tomaschitz A, Drechsler C, Kienreich K, Nijpels G, Stehouwer CD, März W, Dekker JM (2014) Homoarginine and mortality in an older population: the Hoorn study. Eur J Clin Invest 44:200–208
Radomski MW, Palmer RM, Moncada S (1990) An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Nat Acad Sci USA 87:5193–5197
Ravani P, Maas R, Malberti F, Pecchini P, Mieth M, Quinn R, Tripepi G, Mallamaci F, Zoccali C (2013) Homoarginine and mortality in pre-dialysis chronic kidney disease (CKD) patients. PLoS One 8:e72694
Reczkowski RS, Ash DE (1994) Rat liver arginase: kinetic mechanism, alternate substrates, and inhibitors. Arch Biochem Biophys 312:31–37
Valtonen P, Laitinen T, Lyyra-Laitinen T, Raitakari OT, Juonala M, Viikari JS, Heiskanen N, Vanninen E, Punnonen K, Heinonen S (2008) Serum l-homoarginine concentration is elevated during normal pregnancy and is related to flow-mediated vasodilatation. Circ J 72:1879–1884
van der Zwan LP, Davids M, Scheffer PG, Dekker JM, Stehouwer CDA, Teerlink T (2013) l-Homoarginine and l-arginine are antagonistically related to blood pressure in an elderly population: the Hoorn study. J Hypertens 31:1114–1123
Weinhaus AJ, Poronnik P, Tuch BE, Cook DI (1997) Mechanisms of arginine-induced increase in cytosolic calcium concentration in the beta-cell line NIT-1. Diabetologia 40:374–382
White MF, Christensen HN (1982) The two-way flux of cationic amino acids across the plasma membrane of mammalian cells is largely explained by a single transport system. J Biol Chem 257:10069–10080
White MF, Gazzola GC, Christensen HN (1982) Cationic amino acid transport into cultured animal cells, part I. Influx into cultured human fibroblasts. J Biol Chem 257:4443–4449
Acknowledgments
This work was supported by an Else-Kröner Memorial Stipendium from the Else-Kröner Fresenius Stiftung to C.C. and grants from the Deutsche Forschungsgemeinschaft (DFG) (CH872/1-1 to C.C., A.N. and D.I., and IS63/3-1/2 to D.I.). D.A. received funding from the European Union under a Marie Curie Intra-European Fellowship for Career Development. We wish to thank H. Voss and U. Wolters for expert animal care.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
All experimental procedures were in accordance with institutional guidelines and approved by the local animal Ethics Committee.
Author information
Authors and Affiliations
Corresponding author
Additional information
E. Schwedhelm and C. Choe contributed equally.
Rights and permissions
About this article
Cite this article
Stockebrand, M., Hornig, S., Neu, A. et al. Homoarginine supplementation improves blood glucose in diet-induced obese mice. Amino Acids 47, 1921–1929 (2015). https://doi.org/10.1007/s00726-015-2022-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-2022-1