Abstract
The pathogenic hallmarks of multiple sclerosis (MS) and neuromyelitis optica (NMO) are cellular and humoral inflammatory infiltrates and subsequent demyelination, or astrocytic cell death in NMO, respectively. These processes are accompanied by disruption of the blood–brain barrier as regularly observed by gadolinium enhancement on magnetic resonance imaging. The role of the l-arginine/nitric oxide (NO) pathway in the pathophysiology of neuroinflammatory diseases, such as MS and NMO, remains unclear. In the present study, we measured the concentrations of the nitric oxide (NO) metabolites nitrate and nitrite, the endogenous substrates of NO synthase (NOS) l-arginine (Arg) and l-homoarginine (hArg), and asymmetric dimethylarginine (ADMA), the endogenous inhibitor of NOS activity, in the serum and cerebrospinal fluid (CSF) of patients with MS, NMO or other neurologic diseases (OND). MS (551 ± 23 nM, P = 0.004) and NMO (608 ± 51 nM, P = 0.006) patients have higher ADMA concentrations in serum than healthy controls (HC; 430 ± 24 nM). For MS, this finding was confirmed in CSF (685 ± 100 nM in relapsing–remitting multiple sclerosis, RRMS; 597 ± 51 nM in secondary progressive multiple sclerosis, SPMS) compared with OND (514 ± 37 nM; P = 0.003). Serum concentrations of Arg (61.1 ± 9.7 vs. 63.6 ± 4.9 µM, P = 0.760), hArg (2.62 ± 0.26 vs. 2.52 ± 0.23 µM, P = 0.891), nitrate (38.1 ± 2.2 vs. 38.1 ± 3.0 µM) and nitrite (1.37 ± 0.09 vs. 1.55 ± 0.03 µM) did not differ between MS and OND. Also, CSF concentrations of hArg (0.685 ± 0.100 µM in RRMS, 0.597 ± 0.051 µM in SPMS, 0.514 ± 0.037 µM in OND), nitrate (11.3 ± 0.6 vs. 10.5 ± 0.3 µM) and nitrite (2.84 ± 0.32 vs. 2.41 ± 0.11 µM) did not differ between the groups. In NMO patients, however, serum Arg (117 ± 11 vs. 64 ± 4.9 μM, P = 0.004), nitrate (29 ± 2.1 vs. 38 ± 3 μM, P = 0.03), and nitrite (1.09 ± 0.02 vs. 1.55 ± 0.033 µM, P < 0.0001) were significantly different as compared to OND. Symmetric dimethylarginine (SDMA) concentration did not differ in serum between MS and HC (779 ± 43 vs. 755 ± 58 nM, P = 0.681) or in CSF between MS and OND patients (237 ± 11 vs. 230 ± 17 nM, P = 0.217). Our study suggests a potential role for ADMA and Arg in neuroinflammatory diseases with diverse functions in MS and NMO. Higher ADMA synthesis may explain reduced NO availability in NMO. hArg and SDMA seem not to play an important role in MS and NMO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitric oxide (NO) has various biological capabilities including inhibition of vascular inflammation and platelet aggregation, inhibition of immune cell adhesion, vasodilatation, and signal transduction in the neuronal system (Moncada and Higgs 1993; Leiper and Vallance 1999; Haghikia et al. 2007). l-Arginine (Arg) is the substrate of nitric oxide synthase (NOS) isoforms that include the neuronal NOS (nNOS), the endothelial NOS (eNOS) and the inducible NOS (iNOS). All NOS isoforms catalyse the conversion of Arg to NO and l-citrulline (Tsikas et al. 2000a). In humans, l-homoarginine (hArg), the homologue of Arg, is a non-essential, non-proteinogenic amino acid. Arg is a semi-essential proteinogenic amino acid and plays an important role in metabolism and nutrition in growth, health and disease (Wu et al. 2009). hArg may serve both as a substrate for NOS and as an inhibitor of NOS activity (Moali et al. 1998, 2000; Bretscher et al. 2003). Asymmetric dimethylarginine (ADMA) is a potent (IC50, 1.5 µM) endogenous inhibitor of nNOS activity (Tsikas et al. 2000a; Kielstein et al. 2007). Symmetric dimethylarginine (SDMA) turned out to be an endogenous inhibitor of nNOS activity (Tsikas et al. 2000a).
Changes in NO bioavailability and in circulating ADMA, SDMA and hArg concentrations have been intensively studied in cardiovascular diseases (CVD) in recent decades establishing high ADMA and low hArg concentrations as cardiovascular risk factors associated with cardiovascular and all-cause mortality (Böger et al. 2009; März et al. 2010; Pilz et al. 2011; Drechsler et al. 2011; Choe et al. 2013; Atzler et al. 2013, 2014; Pilz et al. 2014, 2015a, b). Moreover, recent studies on rheumatoid arthritis have demonstrated a positive association of inflammatory burden with ADMA levels independent of classical CVD risk factors (Sandoo et al. 2014), suggesting a pivotal role of altered NO metabolism in chronic inflammatory diseases. Yet, experimental studies have reported conflicting roles of NO in the pathophysiology of neuroimmunological diseases such as multiple sclerosis (MS) with pro- and anti-inflammatory properties (Mitrovic et al. 1994; Farias et al. 2007), leaving open the functional role of NO in this field.
In the present study, we aimed to evaluate the bioavailability of NO metabolites as well as the concentrations of the endogenous modulators of NOS activity, namely Arg, hArg, ADMA and SDMA, in the serum and cerebrospinal fluid of patients suffering from MS or neuromyelitis optica (NMO). Since the hArg-to-ADMA (hArg/ADMA) molar ratio is suggested to reflect the balance of NOS modulation with clinical relevance in CVD (Tsikas and Kayacelebi 2014), we included this parameter in our analyses.
MS is an autoimmune disorder of the central nervous system (CNS) with neurodegenerative features and the most common non-traumatic neurological disorder in the young adult among industrialized countries (Haghikia et al. 2013). Despite rapid progression in understanding the underlying autoimmune processes of MS, i.e., involvement of cellular and humoral components in demyelination and axonal damage in disease progression (Mahad et al. 2015), many aspects leading to the disease are still not well understood. That is the conflicting role of NO being controversially discussed in the field (Smith and Lassmann 2002). Although NMO shares many pathomechanisms with MS, it is distinct, since it is an antibody-mediated autoimmune disease of the CNS primarily directed against the astrocytic aquaporin (AQP)-4 water channel (Papadopoulos et al. 2014). The results of the present study may shed further light on the role of NO in the pathophysiology of MS and NMO.
Materials and methods
Patients
The characteristics of the subjects investigated in the present study are summarized in Tables 1 and 2. Cerebrospinal fluid (CSF) and serum samples for routine diagnostic and therapeutic purposes were obtained after written informed consent from 14 MS and 9 NMO patients, as well as from 11 healthy controls (HC). For this study, we assessed CSF samples from another 14 clinically well-defined MS patients and 26 patients with other neurologic diseases (OND). Hemorrhagic CSF samples were excluded. MS patients were subdivided into two groups according to the disease course, i.e., the relapsing–remitting MS (RRMS) group and the secondary progressive MS (SPMS) group. All samples were stored at −80 °C until analysis. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in an approval by the Ethics Committee of the Ruhr-University Bochum (Bochum, Germany).
Biochemical analyses and quality control
hArg (Kayacelebi et al. 2014a, 2015) and ADMA (Tsikas et al. 2003; Tsikas 2009) in serum and CSF samples were analyzed by GC–MS/MS. SDMA was analyzed in some serum and CSF samples by GC–MS/MS as reported recently for urinary SDMA (Tsikas et al. 2011). Arg in serum and CSF samples was determined by GC–MS (Tsikas et al. 2003). Nitrite and nitrate in serum and CSF samples were quantitated simultaneously by GC–MS (Tsikas 2000).
The serum samples of the study were analyzed in two runs alongside quality control (QC) of unspiked and spiked serum samples. Study samples were analyzed once only, whereas QC samples were analyzed in duplicate. Spiked concentrations were 0.5 µM for ADMA and SDMA, 1.5 µM for hArg, 60 µM for Arg, 2 and 4 µM for nitrite, and 20 and 40 µM for nitrate as described elsewhere (Tsikas 2000; Kayacelebi et al. 2015). The respective basal concentrations were (all in µM): 0.390 ± 0.014, 0.495 ± 0.007, 2.96 ± 0.30, 87.5 ± 3.7, 0.64 ± 0.08, and 7.29 ± 0.37. The results of the QC samples are summarized in Table 3. All biochemical parameters were measured in the QC samples with generally acceptable precision (relative standard deviation, RSD in %) and accuracy (recovery in %).
Statistical analysis and data presentation
Statistical analyses were performed on and graphs were constructed by Origin 7.5G and GraphPad Prism 5.04. Nonparametric correlation (Spearman) between two parameters was performed. Statistical significance was tested by the nonparametric Mann–Whitney t test. A P value ≤0.05 was considered significant. Data are presented as mean ± SEM.
Results
Amino acids
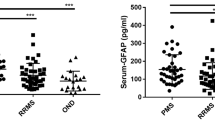
The serum concentrations of hArg, ADMA and Arg and the molar ratio hArg/ADMA in MS and NMO patients and in HC are shown in Fig. 1. The serum concentration of hArg did not significantly differ (P = 0.095) between MS (2.62 ± 0.26 µM) and NMO (3.39 ± 0.43 µM) or between HC (2.52 ± 0.23 µM) and MS (P = 0.891) or NMO (P = 0.053) (Fig. 1a). The serum concentration of ADMA was higher in MS (551 ± 23 nM, P = 0.004) and NMO (608 ± 51 nM, P = 0.006) patients compared to HC (430 ± 24 nM), with no statistical difference between MS and NMO patients (P = 0.614) (Fig. 1b). No significant difference in the hArg/ADMA ratio was seen between the MS (4.836 ± 0.552, P = 0.132) or NMO (5.697 ± 0.813, P = 0.762) group and the HC group (6.040 ± 0.639) (Fig. 1c). Also, the hArg/ADMA ratio did not differ between the MS and NMO groups (P = 0.329). MS patients and HC controls had very similar serum Arg concentrations (61.1 ± 9.7 vs. 63.6 ± 4.9 µM, P = 0.760). However, NMO patients had significantly higher serum Arg concentrations (117 ± 11 μM) than MS patients (P = 0.002) and HC (P = 0.001) (Fig. 1d). The serum SDMA concentration did not differ between MS and HC (779 ± 43 vs. 755 ± 58 nM, P = 0.681). There was no correlation between the ADMA and SDMA serum concentrations in the MS (r = 0.425, P = 0.129) and HC (r = 0.356, P = 0.286) groups. In serum of MS patients, ADMA (r = −0.772, P = 0.001) but not hArg (r = −0.464, P = 0.095) or SDMA (r = −0.330, P = 0.249) correlated with Arg. In serum of HC subjects, ADMA (r = −0.772, P = 0.001) but not hArg (r = −0.464, P = 0.095) or SDMA (r = −0.330, P = 0.249) correlated with Arg. In serum of the MS patients, ADMA, hArg (r = 0.538, P = 0.088) and SDMA did not correlate with Arg.
The CSF concentrations of hArg and ADMA and their molar ratio hArg/ADMA in MS and OND patients are shown in Fig. 2. The hArg concentration in the CSF samples did not differ between the groups (0.685 ± 0.100 µM in RRMS, 0.597 ± 0.051 µM in SPMS, and 0.514 ± 0.037 µM in OND). The CSF concentration of ADMA was higher in the MS patients (123 ± 19 nM in RRMS, 128 ± 11 nM in SPMS) compared to the OND patients (94.5 ± 5.1 nM). The ADMA concentration in CSF differed between SPMS and OND (P = 0.003). The hArg/ADMA ratio in CSF did not differ between the groups (6.10 ± 1.17 in RRMS, 4.78 ± 0.39 in SPMS, and 5.72 ± 0.48 in OND). The CSF concentrations of hArg and ADMA were lower than in serum, yet the hArg/ADMA ratio was comparable in both biological samples. Arg was not measured in CSF samples. The CSF concentration of SDMA did not differ between MS and OND patients (237 ± 11 vs. 230 ± 17 nM, P = 0.217). The SDMA concentration in CSF failed narrowly to reach statistical significance between SPMS and RRMS patients (247 ± 10 vs. 209 ± 25 nM, P = 0.059). ADMA and SDMA concentrations in CSF correlated in MS (r = 0.732, P = 0.003) and OND (r = 0.558, P = 0.003) patients.
Nitrite and nitrate
The serum concentration of nitrate and nitrite did not differ between MS patients (38.1 ± 2.15 µM nitrate; 1.37 ± 0.09 µM nitrite) and HC (38.1 ± 3.02 µM nitrate P = 0.763; 1.55 ± 0.03 µM nitrite, P = 0.476). In the NMO patients, nitrate (29 ± 2.1 μM, P = 0.03) and nitrite (1.09 ± 0.02 nM, P < 0.001) were significantly lower than in HC.
The CSF concentration of nitrate (11.3 ± 0.56 µM) and nitrite (2.84 ± 0.32 µM) in the MS patients and the CSF concentration of nitrate (10.5 ± 0.32 µM) and nitrite (2.41 ± 0.11 µM) in the OND patients were not significantly different (P = 0.201 and P = 0.767, respectively).
Relationship between nitrite, nitrate and hArg/ADMA in serum and CSF
There was no correlation between serum levels of nitrite and hArg/ADMA in the MS patients (Fig. 3a). In HC, a positive but not statistically significant correlation between serum nitrite and hArg/ADMA was observed (Fig. 3c). In the serum, nitrate correlated negatively with hArg/ADMA in the MS patients (Fig. 3b) and in the HC subjects (Fig. 3d), but narrowly failed to reach statistical significance (Fig. 3d). In CSF, nitrite but not nitrate correlated negatively with hArg/ADMA in the OND patients (Fig. 4).
Discussion
This is the first study to investigate the relationship between hArg and ADMA in serum and CSF samples of patients suffering from MS and in serum of patients with NMO. Our study shows that MS patients have higher CSF concentrations of ADMA as compared to OND and higher serum levels of ADMA HC. On average, MS and NMO patients had about 120 nM and 180 nM higher ADMA concentrations in serum than HC, respectively. From the quantitative point of view, these are remarkable differences (Horowitz and Heresztyn 2007), and are supportive of the involvement of ADMA in the pathology of MS and NMO. Although ADMA is a strong inhibitor of nNOS (IC50, 1.5 µM; Tsikas et al. 2000a), higher serum and CSF concentrations of ADMA in MS patients were not associated with lower concentrations of nitrite and nitrate in serum and CSF. This observation may indicate that ADMA, which is an endogenous inhibitor of constitutive and inducible NOS isoforms (Tsikas 2009; Tsikas et al. 2000a, b; Kielstein et al. 2007), is not necessarily involved in MS as an inhibitor of NO synthesis. This observation is in line with a previous study reporting higher concentrations of nitrate and nitrite in CSF of MS patients, suggesting a correlation between disease progression and levels of nitrate and nitrite (Redjak et al. 2004). However, in NMO higher ADMA levels were associated with reduced nitrate and nitrite concentrations indicating impaired NO availability presumably caused by the inhibitory action of ADMA on NOS activity. This observation points to diverse function of elevated ADMA in MS and NMO.
The serum concentration of hArg in MS patients was very close to that in the OND group of the present study. In CSF, there was a trend towards higher hArg concentrations in MS as compared to HC. Interestingly, both ADMA and hArg are present in CSF at lower concentrations than in serum or plasma and they are close to those measured in skeletal and adipose tissue of healthy humans (May et al. 2014, 2015).
Emerging evidence suggests that hArg may antagonize ADMA effects in the circulation and that the hArg/ADMA molar ratio may be a more suitable parameter to evaluate the relative effects of hArg and ADMA (Kayacelebi et al. 2014b; Tsikas and Kayacelebi 2014). The hArg/ADMA molar ratio may include a component related to NO. In theory, this is possible because ADMA is an inhibitor of NOS (Tsikas 2009; Tsikas et al. 2000a, b; Kielstein et al. 2007), and because hArg may serve both as substrate and inhibitor of NOS activity (Moali et al. 1998, 2000). Thus, one may assume that the hArg/ADMA molar ratio may correlate with nitrite and/or nitrate as NO metabolites. Despite some limitations, nitrite in serum (and plasma) may indicate NO bioavailability and thus NO-related function (Tsikas 2015). We did not observe a correlation between serum nitrite and the hArg/ADMA molar ratio, neither in HC nor in MS patients, but only a positive trend in HC. Interestingly, in our MS patients nitrite did not correlate with the hArg/ADMA molar ratio. Thus, the hArg/ADMA molar ratio may also relate to biological activities of ADMA and hArg beyond the Arg/NO pathway. In contrast to serum, nitrite in the CSF correlated negatively with the hArg/ADMA molar ratio both in the MS patients and OND patients. The same also applied to nitrate, whereas the extent of correlation appeared to be weaker for nitrate as compared to nitrite. The overall negative associations between hArg/ADMA and nitrate or nitrite in CSF observed in the present study remain to be established in larger studies. Studies in this area are very rare and their input debatable mainly because of analytical shortcomings with respect to ADMA, nitrite, nitrate and other NO metabolites (Stojanovic et al. 2012; Ljubisavljevic et al. 2012).
The results of the present study indicate a potential role for ADMA in both MS and NMO. However, differences in serum concentrations of nitrate and nitrite in MS and NMO suggest diverse effects of ADMA further supporting the pathomechanistic differences of these two otherwise similar neuroimmunological disorders.
Abbreviations
- ADMA:
-
Asymmetric dimethylarginine (N G, N G-dimethyl-l-arginine)
- AGAT:
-
Arginine:glycine amidinotransferase
- AQP:
-
Aquaporin
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluids
- GC–MS:
-
Gas chromatography–mass spectrometry
- GC–MS/MS:
-
Gas chromatography–tandem mass spectrometry
- hArg:
-
Homoarginine
- HC:
-
Healthy controls
- MS:
-
Multiple sclerosis
- NMO:
-
Neuromyelitis optica
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- OND:
-
Other neurologic diseases
- RRMS:
-
Relapsing–remitting multiple sclerosis
- SDMA:
-
Symmetric dimethylarginine (N G, N G′-dimethyl-l-arginine)
- SPMS:
-
Secondary progressive multiple sclerosis
References
Atzler D, Rosenberg M, Andersso M, Choe CU, Lutz M, Zugck C et al (2013) Homoarginine—an independent marker of mortality in heart failure. Int J Cardiol 168:4907–4909
Atzler D, Gore MO, Ayers CR, Choe CU, Böger RH, de Lemos JA, McGuire DK, Schwedhelm E (2014) Homoarginine and cardiovascular outcome in the population-based Dallas Heart Study. Arterioscler Thromb Vasc Biol 34:2501–2507
Böger RH, Maas R, Schulze F, Schwedhelm E (2009) Asymmetric dimethylarginine (ADMA) as a prospective marker of cardiovascular disease and mortality—an update on patient populations with a wide range of cardiovascular risk. Pharmacol Res 60:481–487
Bretscher LE, Li H, Poulos TL, Griffith OW (2003) Structural characterization and kinetics of nitric-oxide synthase inhibition by novel N5-(iminoalkyl)- and N5-(iminoalkenyl)-ornithines. J Biol Chem 278:46789–46797
Choe CU, Atzler D, Wild PS, Carter AM, Böger RH, Ojeda F, Simova O, Stockebrand M, Lackner K, Nabuurs C, Marescau B, Streichert T, Muller C, Luneburg N, De Deyn PP, Benndorf RA, Baldus S, Gerloff C, Blankenberg S, Heerschap A, Grant PJ, Magnus T, Zeller T, Isbrandt D, Schwedhelm E (2013) Homoarginine levels are regulated by l-arginine:glycine amidinotransferase and affect stroke outcome: results from human and murine studies. Circulation 128:1451–1461
Drechsler C, Meinitzer A, Pilz S, Krane V, Tomaschitz A, Ritz E, März W, Wanner C (2011) Homoarginine, heart failure, and sudden cardiac death in haemodialysis patients. Eur J Heart Fail 13:852–859
Farias AS, de la Hoz C, Castro FR, Oliveira EC, Ribeiro dos Reis JR, Silva JS, Langone F, Santos LM (2007) Nitric oxide and TNFalpha effects in experimental autoimmune encephalomyelitis demyelination. NeuroImmunoModulation 14:32–38
Haghikia A, Mergia E, Friebe A, Eysel UT, Koesling D, Mittmann T (2007) Long-term potentiation in the visual cortex requires both nitric oxide receptor guanylyl cyclases. J Neurosci 27:818–823
Haghikia A, Hohlfeld R, Gold R, Fugger L (2013) Therapies for multiple sclerosis: translational achievements and outstanding needs. Trends Mol Med 19:309–319
Horowitz JD, Heresztyn T (2007) An overview of plasma concentrations of asymmetric dimethylarginine (ADMA) in health and disease and in clinical studies: methodological considerations. J Chromatogr B 851:42–50
Kayacelebi AA, Beckmann B, Gutzki FM, Jordan J, Tsikas D (2014a) GC–MS and GC–MS/MS measurement of the cardiovascular risk factor homoarginine in biological samples. Amino Acids 46:2205–2217
Kayacelebi AA, Pham VV, Willers J, Hahn A, Stichtenoth DO, Jordan J, Tsikas D (2014b) Plasma homoarginine (hArg) and asymmetric dimethylarginine (ADMA) in patients with rheumatoid arthritis: is homoarginine a cardiovascular corrective in rheumatoid arthritis, an anti-ADMA? Int J Cardiol 176:1129–1131
Kayacelebi AA, Knöfel AK, Beckmann B, Hanff E, Warnecke G, Tsikas D (2015) Measurement of unlabeled and stable isotope-labeled homoarginine, arginine and their metabolites in biological samples by GC-MS and GC-MS/MS. Amino Acids. doi:10.1007/s00726-015-1984-3
Khalil AA, Tsikas D, Akolekar R, Jordan J, Nicolaides KH (2013) Asymmetric dimethylarginine, arginine and homoarginine at 11–13 weeks’ gestation and preeclampsia: a case-control study. J Hum Hypertens 27:38–43
Kielstein A, Tsikas D, Galloway GP, Mendelson JE (2007) Asymmetric dimethylarginine (ADMA)—a modulator of nociception in opiate tolerance and addiction? Nitric Oxide 17:55–59
Leiper J, Vallance P (1999) Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc Res 43:542–548
Leiper JM, Vallance P (2006) The synthesis and metabolism of asymmetric dimethylarginine (ADMA). Eur J Clin Pharmacol 26:33–38
Ljubisavljevic S, Stevanovic I, Pavlovic R, Sokolovic D, Pavlovic D, Cvetkovic T (2012) J Neurol Sci 318:106–111
Mahad DH, Trapp BD, Lassmann H (2015) Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol 14:183–193
März W, Meinitzer A, Drechsler C, Pilz S, Krane V, Kleber ME, Fischer J, Winkelmann BR, Böhm BO, Ritz E, Wanner C (2010) Homoarginine, cardiovascular risk, and mortality. Circulation 122:967–975
May M, Batkai S, Zörner AA, Tsikas D, Jordan J, Engeli S (2014) Clinical evaluation of extracellular ADMA concentrations in human blood and adipose tissue. Int J Mol Sci 15:1189–1200
May M, Kayacelebi AA, Batkai S, Jordan J, Tsikas D, Engeli S (2015) Plasma and tissue homoarginine concentrations in healthy and obese humans. Amino Acids. doi:10.1007/s00726-015-1922-4
Mitrovic B, Ignarro LJ, Montestruque S, Smoll A, Merrill JE (1994) Nitric oxide as a potential pathological mechanism in demyelination: its differential effects on primary glial cells in vitro. Neuroscience 3:575–585
Moali C, Boucher JL, Sari MA, Stuehr DJ, Mansuy D (1998) Substrate specificity of NO synthases: detailed comparison of l-arginine, homo-l-arginine, their N omega-hydroxy derivatives, and N omega-hydroxynor-l-arginine. Biochemistry 37:10453–10460
Moali C, Brollo M, Custot J, Sari MA, Boucher JL, Stuehr DJ, Mansuy D (2000) Recognition of alpha-amino acids bearing various C=NOH functions by nitric oxide synthase and arginase involves very different structural determinants. Biochemistry 39:8208–8218
Moncada S, Higgs A (1993) The l-arginine-nitric oxide pathway. New Engl J Med 329:2002–2012
Papadopoulos MC, Bennett JL, Verkman AL (2014) Treatment of neuromyelitis optica: state-of-the-art and emerging therapies. Nat Rev Neurol 10:493–506
Pilz S, Tomaschitz A, Meinitzer A, Drechsler C, Ritz E, Krane V, Wanner C, Bohm BO, März W (2011) Low serum homoarginine is a novel risk factor for fatal strokes in patients undergoing coronary angiography. Stroke 42:1132–1134
Pilz S, Teerlink T, Scheffer PG, Meinitzer A, Rutters F, Tomaschitz A, Drechsler C, Kienreich K, Nijpels G, Stehouwer CD, März W, Dekker JM (2014) Homoarginine and mortality in an older population: the Hoorn study. Eur J Clin Invest 44:200–208
Pilz S, Meinitzer A, Gaksch M, Grübler M, Verheyen N, Drechsler C, Hartaigh BÓ, Lang F, Alesutan I, Voelkl J, März W, Tomaschitz A (2015a) Homoarginine in the renal and cardiovascular systems. Amino Acids. doi:10.1007/s00726-015-1993-2
Pilz S, Putz-Bankuti C, Meinitzer A, März W, Kienreich K, Stojakovic T, Pieber TR, Stauber RE (2015b) Association of homoarginine and methylarginines with liver dysfunction and mortality in chronic liver disease. Amino Acids. doi:10.1007/s00726-015-2000-7
Redjak K, Eikelenboom MJ, Petzold A, Thompson EJ, Stelmasiak Z, Lazeron RHC, Barkhof F, Polman CH, Uitdehaag BMJ, Giovannoni G (2004) CSF nitrite oxide metabolites are associated with activity and progression of multiple sclerosis. Neurology 63:1439–1445
Sandoo A, Dimitroulas T, Hodson J, Smith JP, Douglas KM, Kitas GD (2014) Cumulative inflammation associates with asymmetric dimethylarginine in rheumatoid arthritis: a 6 year follow-up study. Rheumatology (Oxford). doi:10.1093/rheumatology/keu349
Smith KJ, Lassmann H (2002) The role of nitric oxide in multiple sclerosis. Lancet Neurol 1:232–241
Stojanovic I, Vojinovic S, Ljubisavljevic S, Pavlovic R, Basic J, Pavlovic D, Ilic A, Cvetkovic T, Stukalov M (2012) INF-β1b therapy modulates l-arginine and nitric oxide metabolism in patients with relapse remittent multiple sclerosis. J Neurol Sci 323:187–192
Tsikas D (2000) Simultaneous derivatization and quantification of the nitric oxide metabolites nitrite and nitrate in biological fluids by gas chromatography/mass spectrometry. Anal Chem 72:4064–4072
Tsikas D (2009) De novo synthesis of trideuteromethyl esters of amino acids for use in GC–MS and GC–tandem MS exemplified for ADMA in human plasma and urine: standardization, validation, comparison and proof of evidence for their aptitude as internal standards. J Chromatogr B 877:2308–2320
Tsikas D (2015) Circulating and excretory nitrite and nitrate: their value as measures of nitric oxide synthesis, bioavailability and activity is inherently limited. Nitric Oxide 45C:1–3
Tsikas D, Kayacelebi AA (2014) Do homoarginine and asymmetric dimethylarginine act antagonistically in the cardiovascular system? Circ J 78:2094–2095
Tsikas D, Böger RH, Sandmann J, Bode-Böger SM, Frölich JC (2000a) Endogenous nitric oxide synthase inhibitors are responsible for the l-arginine paradox. FEBS Lett 478:1–3
Tsikas D, Sandmann J, Savva A, Luessen P, Böger RH, Gutzki FM, Frölich JC (2000b) Assessment of nitric oxide synthase activity in vitro and in vivo by gas chromatography–mass spectrometry. J Chromatogr B 742:143–153
Tsikas D, Schubert B, Gutzki FM, Sandmann J, Frölich JC (2003) Quantitative determination of circulating and urinary asymmetric dimethylarginine (ADMA) in humans by gas chromatography–tandem mass spectrometry as methyl ester tri(N-pentafluoropropionyl) derivative. J Chromatogr B 798:87–99
Tsikas D, Beckmann B, Gutzki FM, Jordan J (2011) Simultaneous gas chromatography-tandem mass spectrometry quantification of symmetric and asymmetric dimethylarginine in human urine. Anal Biochem 413:60–62
Wu G, Bazer FW, Davis TA, Kim SW, Li P, Marc Rhoads J, Carey Satterfield M, Smith SB, Spencer TE, Yin Y (2009) Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37:153–168
Conflict of interest
The authors declare that they have no conflict of interest related to the present research.
Ethical standard
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in an approval by the Ethics Committee of the Ruhr-University Bochum (Bochum, Germany).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haghikia, A., Kayacelebi, A.A., Beckmann, B. et al. Serum and cerebrospinal fluid concentrations of homoarginine, arginine, asymmetric and symmetric dimethylarginine, nitrite and nitrate in patients with multiple sclerosis and neuromyelitis optica. Amino Acids 47, 1837–1845 (2015). https://doi.org/10.1007/s00726-015-2015-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-2015-0