Abstract
Eight male subjects performed leg press exercise, 4 × 10 repetitions at 80% of their maximum. Venous blood samples were taken before, during exercise and repeatedly during 2 h of recovery. From four subjects, biopsies were taken from the vastus lateralis muscle prior to, immediately after and following one and 2 h of recovery. Samples were freeze-dried, individual muscle fibres were dissected out and identified as type I or type II. Resistance exercise led to pronounced reductions in the glutamate concentration in both type I (32%) and type II fibres (70%). Alanine concentration was elevated 60–75% in both fibre types and 29% in plasma. Glutamine concentration remained unchanged after exercise; although 2 h later the concentrations in both types of fibres were reduced 30–35%. Two hours after exercise, the plasma levels of glutamate and six of the essential amino acids, including the branched-chain amino acids were reduced 5–30%. The data suggest that glutamate acts as an important intermediate in muscle energy metabolism during resistance exercise, especially in type II fibres.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Resistance exercise alters the turnover of muscle protein, with both the rates of synthesis and breakdown being enhanced in the period of recovery following even a single bout of resistance exercise (Biolo et al. 1995, 1997; Tipton et al. 1999). This elevation in protein turnover is associated with changes in the concentration of free amino acids in the muscle cell. For example, the intracellular concentration of leucine increases immediately after resistance exercise, but decreases during the subsequent 2-h recovery period to a level lower than that present prior to the exercise (Dreyer et al. 2006). However, it is not known whether these changes in human skeletal muscle occur in slow-twitch type I fibres, which possess high-oxidative capacity, and/or in fast-twitch type II fibres, where glycolysis is utilized to a greater extent to produce energy (Essén et al. 1975; Garnett et al. 1979).
In experimental animals, slow- and fast-twitch muscles demonstrate differences in their amino acid contents. For example, in the rat the levels of glutamate and glutamine are both three to fivefold higher in the slow-twitch soleus muscle than in the fast-twitch plantaris and gastrocnemius muscles. Similarly, the level of taurine is 50–100% higher in the slow-twitch soleus muscle; whereas glycine and alanine levels are 30–40% higher in the fast-twitch muscles (Turinsky and Long 1990). Furthermore, in the middle gluteal muscle of horses and camels, taurine levels are two to tenfold higher in type I than in type II fibres (Dunnett et al. 1992, 1997). In the human vastus lateralis muscle, the concentration of taurine is fourfold higher in type I than in type II fibres (Harris et al. 1998), although no such difference was observed in individuals who perform endurance training (Essén-Gustavsson and Blomstrand 2002). At the same time, in the latter study, the levels of glutamate, aspartate and arginine were shown to be 10, 13 and 33% higher, respectively, in the type II fibres.
Moreover, sustained endurance exercise leads to a pronounced reduction in the concentration of glutamate along with an elevation of tyrosine in both type I and type II fibres, with no alterations with respect to taurine and glutamine (Essén-Gustavsson and Blomstrand 2002). These observations indicate that amino acid metabolism plays an important role in both types of fibres during endurance exercise, but the influence of resistance exercise on amino acid concentrations remains to be elucidated. Therefore, the present investigation was designed to examine the effects of an acute session of resistance exercise on amino acid concentrations in both plasma and in pools of type I and type II fibres in the skeletal muscle of moderately trained individuals. Our hypothesis was that resistance exercise should influence both fibre types, especially the type II fibres leading to more pronounced changes in amino acid concentrations in type II fibres due to their larger involvement in the exercise.
Materials and methods
Subjects
Eight healthy men with a mean (±SE) age of 25 ± 1 years, height of 178 ± 2 cm, weight of 74 ± 3.5 kg, and maximal oxygen uptake of 3.78 ± 0.20 L min−1, were recruited for this study. These subjects all reported that they performed endurance or resistance exercise training once or twice weekly. They were fully informed about the purpose of the investigation as well as the possible risks involved. All subjects gave their informed consent prior to their participation in the study. This study was approved by the Ethical Committee at the Karolinska Institutet. Seven of these eight subjects also participated in an investigation described previously (Karlsson et al. 2004).
Preparatory tests
Prior to the experiment, the subjects carried out two preparatory tests. The first of these was designed to determine their maximum one-repetition load (1 RM) and involved leg press exercise from a knee angle of 90° to full-extended 180°, with successive increases in the load until the subject could not perform more than a single repetition. The subjects were allowed to rest for an unlimited period between successive trials in order to avoid muscle fatigue and all reached their maximum load after five or six trials. The second preparatory test, carried out at least 2 weeks prior to the actual collection of data, involved performance of the standardized exercise routine in order to familiarize the subjects with the intensity and frequency of repetitions associated with the study task itself.
Maximal oxygen uptake was determined on a mechanically braked cycle ergometer (Monark 816E, Vansbro, Sweden). The work rate was gradually increased until exhaustion as described by Astrand and Rodahl (1986). Oxygen uptake was measured continuously utilizing an on-line system (Amis 2001 Automated Metabolic Cart, Innovision A/S, Odense, Denmark).
Experimental protocol
The subjects were requested to refrain from vigorous physical activity for at least 2 days prior to the start of the experiment, and to fast overnight before reporting to the laboratory in the morning. The subject was asked to lie down and a catheter was then inserted into the antecubital vein for taking blood samples. With one subject, technical difficulties were encountered and therefore no blood samples were collected from this subject. Muscle biopsies were obtained from the lateral portion of the right and left quadriceps (vastus lateralis) muscle employing a Weil-Blakesley conchotome (AB Wisex, Mölndal, Sweden), as described by Henriksson (1979).
To warm up, the subjects pedalled a cycle ergometer at 100 W for 10 min. Thereafter, they were seated in the leg press apparatus to perform resistance exercise at a load corresponding to 80% of their one RM. A metronome beating 50 times per minute, together with verbal guidance, were used to enable the subjects to perform each repetition at a set pace, with the concentric and eccentric phase each lasting for 2.4 s. A short pause (approximately 1.2 s long) at both the extended (180°) and bent (90°) knee angles enhanced the subjects’ ability to control the force and speed of their movement. Each subject performed four sets of ten repetitions with a 5-min rest between consecutive sets, yielding a total protocol time of 20 min. This experimental procedure has been described in more detail previously (Karlsson et al. 2004).
Blood samples were taken at rest prior to the warm-up, immediately before beginning the resistance exercise, following two sets of ten repetitions each, immediately after termination of the exercise and following 15, 30, 60, 90 and 120 min of recovery. Muscle biopsies were taken at rest (prior to the warm-up), immediately after termination of the resistance exercise and following 1 and 2 h of recovery, with the first and third biopsies being taken from the right vastus lateralis muscle and the second and fourth from the left vastus lateralis muscle. The second biopsy in each leg was taken approximately 5 cm proximal to the first one. Muscle samples from four of the eight subjects were used for analysis of amino acid content in pools of type I and type II fibres. Each subject drank 150 ml of water prior to the warm-up, immediately before beginning the resistance exercise, after 15 min of exercise and following 15, 30, 60, and 90 min of recovery.
Analysis of free amino acids in venous blood
The blood samples were drawn from the venous catheter into heparinized tubes and then centrifuged (9,000g for 2 min) and the plasma stored at −80°C. The plasma samples were deproteinized by precipitation with 5% trichloric acid (1:5), centrifuged at 9,000g for 2 min and the supernatant stored at −80°C. The concentration of amino acids in the supernatant was measured by reversed-phase high performance liquid chromatography (HPLC) according to Pfeifer et al. (1983), with orthophthaldehyde (OPA) as the derivatizing agent.
Preparation of single muscle fibres and measurement of their amino acid contents
The muscle biopsies were freeze-dried and fragments of single muscle fibres were dissected out under a dissection microscope (with a magnification of 40–80×) and a segment of each fragment stained histochemically for myofibrillar ATPase after preincubation for 15 min at pH 10.35 at 37°C. On the basis of this staining, fibres were classified and pooled into groups of type I and type II fibres (Essén et al. 1975). The pools of fibres were then weighed on an automatic electrobalance (Cahn 25, Ventron Corp., Paramount, Calif., USA); the average weight (range) of the fibre pools was 102.3 (40.3–214.7) μg. These pools of the two types of fibres were then extracted with ice-cooled 0.4 M perchloric acid (PCA; approx. 1:100); the extract was centrifuged at 9,000g for 2 min and the resulting supernatant stored at −80°C until being analysed for free amino acids as described above for the plasma samples.
Statistical analyses
Parametric statistical procedures were employed to calculate the means, standard errors of the mean (SE) and linear correlation coefficients (r). Unless indicated, the values presented in the text are mean ± SE. A one-way analysis of variance (ANOVA) was used to evaluate changes in the plasma concentrations of amino acids with time and a two-factorial (time, fibre type) ANOVA to evaluate differences over time and between the two types of fibres. When statistical significance was found, Fisher LSD was utilized to verify where the difference occurred. Significance was accepted at P < 0.05.
Results
The subjects’ performance
Six of the subjects managed to perform the stipulated four sets of ten repetitions each at 80% of their one RM. One subject carried out two repetitions less during the final set and one performed a total of 30 repetitions during the four sets.
Plasma concentrations of free amino acids
As shown in Table 1, the plasma concentration of alanine increased by 29% during exercise and remained elevated during the first 30 min of recovery, but decreased thereafter. The corresponding concentrations of glutamate and glutamine were not altered significantly during exercise, but the level of glutamate was reduced by 30% after 2 h of recovery. The levels of tyrosine and phenylalanine remained unchanged during exercise, but were reduced by 10% following 2 h of recovery. The plasma concentrations of leucine and isoleucine were attenuated by 6% during exercise, whereas the levels of all three branched-chain amino acids (BCAA) decreased during recovery to values that were 10–20% lower than prior to exercise. The concentration of tryptophan was reduced by 10% during exercise and remained at this lower level throughout the 2-h recovery period. Otherwise, the plasma concentrations of the remaining amino acids did not change during or following resistance exercise, except in the case of methionine and serine, the concentrations of which also decreased significantly during recovery.
Amino acids in pools of type I and type II muscle fibres
At rest
In Tables 2 and 3 the concentrations of free amino acids in pools of type I and type II muscle fibres at rest prior to exercise, after resistance exercise and following one and 2 h of recovery are documented. At rest, the concentration of taurine was 2.4-fold higher in type I than in type II fibres, whereas that of methionine was 40% higher in type II fibres. No other significant differences were observed.
Following exercise
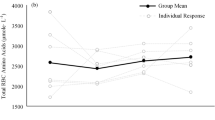
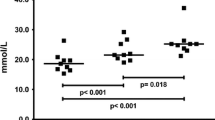
Immediately following resistance exercise, the concentration of glutamate was reduced 32 and 70% in type I- and type II-fibres, respectively, with this more pronounced decrease in the type II fibres being present in all four subjects examined. However, after only 1 h of recovery, the levels had returned to the basal value in both types of fibres (Fig. 1a). The reduction in glutamate concentration during exercise was positively correlated to the corresponding level prior to exercise in both type I (r = 0.94) and type II (r = 0.98) fibres. The alanine concentration was elevated 60–75% in both type I and type II fibres following exercise, resuming pre-exercise levels after 2 h of recovery (Fig. 1b). The levels of glutamine and taurine were unaltered immediately after exercise, but 2 h later the glutamine level was attenuated 30–35% in both types of fibres (Fig. 1c).
The concentration of tyrosine in type I fibres was elevated in all four subjects (27%) after resistance exercise, as was the concentration of phenylalanine in both type I and type II fibres (30 and 18%, respectively) (Tables 2, 3). However, according to the ANOVA, no significant effect over time for tyrosine and phenylalanine was achieved, although there was a tendency for interaction between time and fibre type (P = 0.065 and P = 0.078 for tyrosine and phenylalanine, respectively). The concentration of BCAA in the type I fibres remained unaltered during and after exercise, but was elevated 37% in the type II fibres immediately after exercise (Fig. 1d). No significant changes in the concentrations of any of the remaining amino acids were detected during the experimental period (Tables 2, 3).
Discussion
The major findings reported here are that (1) a single session of leg press exercise results in alterations in the concentration of free amino acids in both type I and type II fibres, (2) resistance exercise was found to cause only minor changes in plasma levels of free amino acids, with the exception of a 30% enhancement in the level of alanine and (3) during the recovery period there was a decrease in the plasma levels of glutamate and some of the essential amino acids, including the BCAA.
The pronounced changes in the concentration of glutamate detected here in both type I and type II fibres following exercise indicate that both fibre types were involved in the resistance exercise. Furthermore, the fact that the reduction in glutamate level was twice as great in type II than in type I fibres suggests that the former make a larger contribution to the development of force, i.e. that all or, at least relatively many of the type II fibres were recruited in order to produce the force required. The decrease in glutamate level may be due to an increased rate of the reaction between glutamate and pyruvate giving rise to oxoglutarate and alanine. Furthermore, glutamate is also an important ammonia acceptor during exercise producing glutamine, which may contribute to the reduction in glutamate content. A third possible mechanism behind the decrease in glutamate is that glutamate is transaminated with oxaloacetate to form aspartate, a substrate for the purine nucleotide cycle (Graham et al. 1995). The observed elevation of alanine in both fibre types after exercise support the involvement of the first reaction, whereas no increases were seen in glutamine and aspartate in any fibre type suggesting that the latter two reactions are less important during the type of resistance exercise employed.
A decrease in glutamate concentration also occurred during endurance exercise, but in this case it was similar in both types of fibres, which may reflect the fact that power output during cycling is less than that associated with resistance exercise (Essén-Gustavsson and Blomstrand 2002). It is noteworthy that the reported levels of glutamate in both type I and type II fibres of trained cyclists are 40% higher than in our present subjects (compare Tables 2, 3 with Essén-Gustavsson and Blomstrand 2002). A similar difference has been observed in the muscle glutamate content of endurance-trained individuals and sedentary controls (Henriksson 1991; Graham et al. 1995). In agreement with the findings on endurance exercise, we observed a correlation between the initial concentration of glutamate in type I and type II fibres and the extent of reduction in this concentration during resistance exercise. This observation constitutes additional support for an involvement of glutamate in energy metabolism in muscle cells during both endurance and resistance exercise.
The tendency for a larger increase in tyrosine and phenylalanine in the type I fibres during exercise (Tables 2, 3) suggests an enhanced rate of protein degradation in these fibres, even though the plasma levels of these amino acids did not increase. Indeed, if anything, the plasma levels of tyrosine and phenylalanine were lowered during the recovery period. Together, these observations indicate that the aromatic amino acids produced by enhanced protein degradation are not released from the muscle cells, but kept available for protein synthesis. The concentration of BCAA was elevated only in the type II fibres following exercise, which may suggest an uptake of these amino acids, particularly in these fibres. Indeed, the leucine concentration in whole muscle has been reported to be enhanced as a result of resistance exercise (Dreyer et al. 2006).
The conclusion that both types of fibres are recruited during resistance exercise gains further support from the observation that the concentration of alanine in both type I and type II fibres was shown here to increase following such exercise. This indicates that the rate of glycolysis, the consequent production of pyruvate and its subsequent conversion to alanine were all enhanced. As a result of this elevated production of alanine in muscle, the plasma level of this amino acid also increased markedly in association with the exercise and remained high during at least 1 h of recovery. In fact, plasma levels of lactate have been reported to be elevated following this same type of resistance exercise, providing a further indication that glycolysis is stimulated and makes a significant contribution to the metabolic generation of energy (Mascher et al. 2008).
The subjects in the present study performed dynamic exercise to warm-up prior to the resistance exercise. It cannot be ruled out that the warm up exercise has influenced the amino acid level in the muscle. However, at this short-term and relatively light dynamic exercise, it is unlikely that amino acid metabolism is affected to any major extent. This is supported by the fact that no changes in plasma amino acid concentrations were detected before and after the warm-up exercise (Table 1).
Taurine and glutamine, the two amino acids present at highest concentrations in skeletal muscle, have been proposed to play a role in the regulation of muscle volume (Parry-Billings et al. 1991; Huxtable 1992; Cuisinier et al. 2002). In addition, the pool of free glutamine in muscle may serve as an important source of this substrate for the immune system (Newsholme and Parry-Billings 1990). Thus, the reduction in glutamine concentration in both type I and type II fibres observed here following 2 h of recovery from exercise may possibly reflect release of this amino acid from muscle cells as a means of avoiding impairment of immunological functions (Parry-Billings et al. 1990; Castell 2003).
Minor changes in plasma amino acids were found during resistance exercise, only the level of alanine increased significantly. In contrast, during the 2-h recovery period after exercise a reduction in the level of several of the essential amino acids, including the BCAA occurred. A decrease in BCAA 1 h after resistance exercise has been reported previously (Mero et al. 2008), but in addition, the present data also show a reduction in other essential amino acids. Such a reduction in the level of essential amino acids may diminish the anabolic effect of resistance exercise as an elevated supply of essential amino acids further stimulates the rate of protein synthesis and leads to a positive protein balance, i.e. the rate of muscle protein synthesis is larger than the rate of degradation (Biolo et al. 1997; Tipton et al. 1999). In the subgroup of subjects from whom the single fibres were analysed, the same pattern was seen in plasma amino acid changes as for the whole group of subjects.
Our observation that taurine was present at a markedly higher level in type I than in type II muscle fibres is in agreement with two previous studies involving relatively untrained subjects (Harris et al. 1998; Tallon et al. 2007), but in contrast to our own previous finding that in endurance-trained subjects the level of this amino acid is the same in both types of fibres (Essén-Gustavsson and Blomstrand 2002). It is worth emphasizing that the level of taurine in type I fibres was similar in the untrained and endurance-trained subjects, whereas the corresponding level in type II fibres was higher in the endurance trained subjects, perhaps as a consequence of years of regular endurance exercise. Moreover, higher concentrations of taurine have also been observed in whole muscle of endurance-trained individuals in comparison to sedentary controls (Henriksson 1991; Graham et al. 1995). Also of interest in this context are the reports that elevated levels of taurine improve physical endurance, whereas reduced levels are associated with lower force output in experimental animals (Yatabe et al. 2003; Hamilton et al. 2006). Furthermore, taurine intake seems to have an impact on muscle amino acid metabolism during 2 h of submaximal exercise in human subjects, even though carbohydrate and fat oxidation was not altered (Galloway et al. 2008).
In summary, a single session of leg press exercise was found to cause alterations in the concentrations of certain free amino acids in both type I and type II muscle fibres and in plasma. The most pronounced change involved glutamate, the concentration of which decreased in both types of fibres but to a much greater extent in type II fibres. At the same time, the level of alanine was elevated in both types of fibres with the level of BCAA being elevated in type II fibres only following exercise. The levels of taurine and glutamine remained unchanged, but after 2 h of recovery from exercise the glutamine level in both fibre types was reduced. Resistance exercise caused only minor changes in plasma levels of amino acids, except for alanine that increased, whereas during the 2-h recovery period the level of several essential amino acids decreased.
References
Astrand P-O, Rodahl K (1986) Textbook of work physiology. McGraw Hill, New York, p 358
Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR (1995) Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab 268:E514–E520
Biolo G, Williams B, Fleming R, Wolfe R (1997) An abundant supply of amino acids enhances the metabolic effects of exercise on muscle protein. Am J Physiol Endocrinol Metab 273:E122–E129
Castell L (2003) Glutamine supplementation in vitro and in vivo, in exercise and in immunodepression. Sports Med 33:323–345
Cuisinier C, de Welle JM, Verbeeck RK, Poortmans JR, Ward R, Sturbois X, Francaux M (2002) Role of taurine in osmoregulation during endurance exercise. Eur J Appl Physiol 87:489–495
Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB (2006) Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 576:613–624
Dunnett M, Harris RC, Sewell DA (1992) Taurine content and distribution in equine skeletal muscle. Scand J Clin Lab Invest 52:725–730
Dunnett M, Harris RC, Soliman MZ, Suwar AA (1997) Carnosine, anserine and taurine contents in individual fibres from the middle gluteal muscle of the camel. Res Vet Sci 62:213–216
Essén-Gustavsson B, Blomstrand E (2002) Effect of exercise on concentrations of free amino acids in pools of type I and type II fibres in human muscle with reduced glycogen stores. Acta Physiol Scand 174:275–281
Essén B, Jansson E, Henriksson J, Taylor AW, Saltin B (1975) Metabolic characteristics of fibre types in human skeletal muscle. Acta Physiol Scand 95:153–165
Garnett RA, O’Donovan MJ, Stephens JA, Taylor A (1979) Motor unit organization of human medial gastrocnemius. J Physiol 287:33–43
Galloway SD, Talanian JL, Shoveller AK, Heigenhauser GJ, Spriet LL (2008) Seven days of oral taurine supplementation does not increase muscle taurine content or alter substrate metabolism during prolonged exercise in humans. J Appl Physiol 105:643–651
Graham TE, Turcotte LP, Kiens B, Richter EA (1995) Training and muscle ammonia and amino acid metabolism in humans during prolonged exercise. J Appl Physiol 78:725–735
Hamilton EJ, Berg HM, Easton CJ, Bakker AJ (2006) The effect of taurine depletion on the contractile properties and fatigue in fast-twitch skeletal muscle of the mouse. Amino Acids 31:273–278
Harris RC, Dunnett M, Greenhaff PL (1998) Carnosine and taurine contents in individual fibres of human vastus lateralis muscle. J Sport Sci 16:639–643
Henriksson J (1991) Effect of exercise on amino acid concentrations in skeletal muscle and plasma. J Exp Biol 160:149–165
Henriksson KG (1979) “Semi-open” muscle biopsy technique. A simple outpatient procedure. Acta Neurol Scand 59:317–323
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72:101–163
Karlsson HKR, Nilsson PA, Nilsson J, Chibalin AV, Zierath JR, Blomstrand E (2004) Branched-chain amino acids increase p70S6k phosphorylation in human skeletal muscle after resistance exercise. Am J Physiol Endocrinol Metab 287:E1–E7
Mascher H, Tannerstedt H, Brink-Elfegoun T, Ekblom B, Gustafsson T, Blomstrand E (2008) Repeated resistance exercise training induces different changes in mRNA expression of MAFbx and MuRF-1 in human skeletal muscle. Am J Physiol Endocrinol Metab 294:E43–E51
Mero A, Leikas A, Rinkinen N, Huhta P, Hulmi JJ, Pitkänen H, Knuutinen J (2008) Effect of strength training session on plasma amino acid concentration following oral ingestion of arginine or taurine in men. Amino Acids 35:99–106
Newsholme EA, Parry-Billings M (1990) Properties of glutamine release from muscle and its importance for the immune system. J Parenter Enteral Nutr 14:63S–67S
Parry-Billings M, Bevan SJ, Opara E, Newsholme EA (1991) Effects of changes in cell volume on the rates of glutamine and alanine release from rat skeletal muscle in vitro. Biochem J 276:559–561
Parry-Billings M, Blomstrand E, McAndrew N, Newsholme EA (1990) A communicational link between skeletal muscle, brain, and cells of the immune system. Int J Sports Med 11:S122–S128
Pfeifer R, Korpi J, Burgoyne R, McCourt D (1983) Practical application of HPLC to amino acid analyses. Am Lab 15:77–84
Tallon MJ, Harris RC, Maffulli N, Tarnopolsky MA (2007) Carnosine, taurine and enzyme activities of human skeletal muscle fibres from elderly subjects with osteoarthritis and young moderately active subjects. Biogerontology 8:129–137
Tipton KD, Ferrando AA, Phillips SM, Doyle D, Wolfe RR (1999) Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol Endocrinol Metab 276:E628–E634
Turinsky J, Long CL (1990) Free amino acids in muscle: effect of muscle fiber population and denervation. Am J Physiol 258:E485–E491
Yatabe Y, Miyakawa S, Miyazaki T, Matsuzaki Y, Ochiai N (2003) Effects of taurine administration in rat skeletal muscles on exercise. J Orthop Sci 8:415–419
Acknowledgments
This study was supported financially by the Swedish National Centre for Research in Sports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blomstrand, E., Essén-Gustavsson, B. Changes in amino acid concentration in plasma and type I and type II fibres during resistance exercise and recovery in human subjects. Amino Acids 37, 629–636 (2009). https://doi.org/10.1007/s00726-008-0182-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-008-0182-y