Abstract
The aim of this study was to identify the relationship between metabolic effort, muscular damage/activity indices, and urinary amino acids profile over the course of a strenuous prolonged endurance activity, as a cycling stage race is, in order to identify possible fatigue markers. Nine professional cyclists belonging to a single team, competing in the Giro d’Italia cycling stage race, were anthropometrically characterized and sampled for blood and urine the day before the race started, and on days 12 and 23 of the race. Diet was kept the same over the race, and power output and energy expenditure were recorded. Sera were assayed for muscle markers (lactate dehydrogenase, aspartate aminotransferase, and creatine kinase activities, and blood urea nitrogen), and creatinine, all corrected for plasma volume changes. Urines were profiled for amino acid concentrations, normalized on creatinine excretion. Renal function, in terms of glomerular filtration rate, was monitored by MDRD equation corrected on body surface area. Creatine kinase activity and blood urea were increased during the race as did serum creatinine while kidney function remained stable. Among the amino acids, taurine, glycine, cysteine, leucine, carnosine, 1-methyl histidine, and 3-methyl histidine showed a net decreased, while homocysteine was increased. Taurine and the dipeptide carnosine (β-alanyl-l-histidine) were significantly correlated with the muscle activity markers and the indices of effort. In conclusion, the metabolic profile is modified strikingly due to the effort. Urinary taurine and carnosine seem useful tools to evaluate the muscle damage and possibly the fatigue status on a long-term basis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability to maintain or even increase the physiological performance throughout consecutive competitions is crucial in determining the outcome of the competition itself. However, it is often observed, in different competitive sport disciplines, a decline in physical, as well as in mental, performances in the later stages of the event (Ra et al. 2014). This could be particularly true in competitions developed throughout consecutive days, as cycling stage races are (Corsetti et al. 2012). The study of the underlying biological mechanisms of fatigue along with the search for biochemical indexes of fatigue has stimulated the efforts of many research groups. However, these aspects have not yet been fully elucidated (Halson 2014).
Although central and psychological fatigue sensing is surely a key determinant of the performance outcome, an important role is covered by the fatigue due to subpathological muscle injuries consequent to muscle overuse (Nedelec et al. 2012). Indeed, strong muscle fiber activity is associated with muscle injury and muscle overuse induces protein catabolism and, consequently, the muscle fiber content is released in blood (Colombini et al. 2012b; Grasso et al. 2015). Of the different cellular components releasable into the circulation, following muscle fiber damage, proteins, peptides, and amino acids (aa) are among the most represented (Nagasawa et al. 1998; Tiao et al. 1997).
Plasma aa concentration changes during exercise mainly depend on type, intensity, and duration of exercise (Meeusen 2014). Indeed, while following short-term exercise, there is an increase in most aa, prolonged exercise causes an overall decrease in aa concentrations. This latter evidence is directly related to the central fatigue: some aa are precursors of neurotransmitters and their reduced availability is associated with fatigue sensation (Blomstrand 2001). Moreover, aa metabolism, release of both aa and their catabolites in the circulation, and their reuptake by other tissues are parts of complex metabolic pathways aimed in maintaining the energetic homeostasis (i.e., alanine cycle, urea cycle, tricarboxylic acid cycle) (DeBerardinis and Cheng 2010).
Kidneys play a key role in the homeostasis of body amino acid pools through synthesis, degradation, filtration, reabsorption, and urinary excretion of aa and peptides. For example, the kidney is the main actor in glutamine and proline disposal from blood, as well as for the in situ synthesis of serine, tyrosine, and arginine (Garibotto et al. 2010). In the kidney, aa (50–70 g/day) are efficiently recovered from the primary filtrate: 95–99 % of all aa are resorbed in the proximal convoluted tubule and proximal straight tubule. This is a well-conserved key role of the kidney proximal tubule. Additionally, kidney metabolism of aa participates in the whole body disposition of these compounds (Garibotto et al. 2010; Makrides et al. 2014; Pinto et al. 2013).
The aim of the present study was to investigate the possible existence of relationships between metabolic effort, muscular damage/activity indices, and urinary aa profile over the course of a strenuous prolonged endurance activity, in order to identify easy assessable fatigue-associated markers. Indeed, to our knowledge, only a few researches studied exercise-associated changes in aa profile, independently from the sample matrix, and they mainly focused on modifications consequent to single exercise bouts.
Materials and methods
Subjects
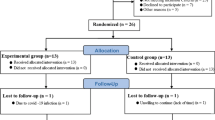
Nine professional cyclists belonging to the Liquigas-Cannondale team, competing in the 95th edition of Giro d’Italia 2012, were recruited and over the whole race, from May 5th to May 27th. Out of the 198 participating athletes, the cyclists recruited were ranked between the 5th and the 117th position. The race covered 3503.8 km over 21 stages, with only 2 days of rest. Table 1 illustrates the main features of the race.
The athletes’ age ranges 24.6–34.5 years. Weight and height, on fasting conditions, and body mass index (BMI) were calculated as weight/(height)2; anthropometric parameters are summarized in Table 2.
In the context of the no-needle policy, none of the athletes took drugs or supplements influencing the iron metabolism; although admitted the only administration of non-steroidal anti-inflammatory drugs and antibiotics, when needed, none of the recruited subject was treated.
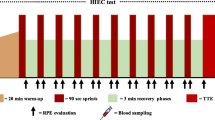
Blood and urine were collected the day before the race started (T0) then on days 12 (T1) and 23 (T2) of the race (Table 1). On T0, athletes underwent to 3 h of light training at 55 % VO2max, including a short bout (30 min) of medium–high subthreshold work at 60 % VO2max.
The protocol was approved by the reference IRB (ASL Milano 1), and the participants before giving a written consent were informed about the nature of the study.
Diet regimen
Based on the expected requirements, diet was approved by the team physician with the following composition: 6000 kcal per day, 45 % carbohydrates, 36 % proteins, and 19 % lipids. The team physician was responsible for checking the adherence of the participant to the diet regimen.
Power output and net energy expenditure derivation
The individual power output was measured, for each stage, using the PowerMeter™ (SRM GmbH, Jülich, D, EU) power sensor integrated into the bike pedal (sensitivity ± 2 %) and normalized on body weight. The total net energy expenditure (kcal), for each stage, was derived from the power output (Grasso et al. 2015).
Blood and urine samples
Union Cycliste Internationale (UCI) (UCI 2012) and World Anti-Doping Agency (WADA) (WADA 2011), as well as academic guidelines for pre-analytical warnings (Banfi and Dolci 2003) about collection and transport of specimens, were followed. Blood and urine samples were transported in sealed boxes at 4 °C and analyzed within the 36 h, as ruled out by WADA (WADA 2011). Evacuated tubes (BD SSTII™ Advance, BD Vacutainer® Systems, Becton–Dickinson, Franklin Lakes, NJ, USA) and EDTA spray-coated tubes (BD K2EDTA3.5 mL tubes) were used for analyte measurement. After drawing, tubes were inverted 10 times and stored in a sealed box at 4 °C. Following arrival in the laboratory, sera were separated by centrifugation (1300×g, 10 min, 4 °C) and immediately analyzed, while K2 EDTA-anticoagulated blood was homogenized for 15 min prior to being analyzed, as recommended by WADA (WADA 2011). The urine collections, in sterile boxes, were performed in a standard way, on midstream, immediately before blood samplings.
Muscle activity and kidney function
Lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and creatine kinase (CK) activities, and blood urea nitrogen (BUN), and creatinine (sCr) concentrations were measured in serum on ADVIA® 1200 Chemistry System. The reported total imprecision was <2.8 %, while the intra-assay CV % was <1.8 %.
Hemoglobin concentration ([Hb]) and hematocrit (Ht) were measured on a Sysmex XE 2100 (Sysmex, Kobe, Japan), with an imprecision of <2 %. They were used to determine the plasma volume shift, and thus to correct the blood parameters, as already described (Grasso et al. 2015).
During the study, the analyzers were regularly calibrated and checked following internal and external quality control schemes.
Renal function was checked by the means of the MDRD equation for the estimation of glomerular filtration rate (eGFR) (Colombini et al. 2012a):
Being the eGFR set on a theoretical body surface area (BSA) of 1.73 m2, the eGFR, calculated for each subject at each time point, has been corrected for the relative real BSA by the means of the equation by Haycock and Schwarz as recommended by the British Nuclear Medicine Society (Lamb et al. 2005):
Thus, corrected eGFR was calculated as follows:
Amino acid analysis
The concentrations of the following aa were measured in urine: creatinine (uCr), taurine (Tau), threonine (Thr), serine (Ser), asparagine (Asn), glutamate (Glu), glutamine (Gln), proline (Pro), glycine (Gly), alanine (Ala), citrulline (Cit), alpha-aminobutirric acid (AABA), valine (Val), cysteine (Cys), methionine (Met), cystathionine, isoleucine (Ile), leucine (Leu), tyrosine (Tyr), phenylalanine (Phe), homocysteine (Hey), gamma-aminobutirric acid (GABA), ornithine (Orn), lysine (Lys), 1-methylhistidine (1-MH), histidine (His), 3-methylhistidine (3-MH), arginine (Arg), and the dipeptide carnosine (Carn).
Free aa concentrations were determined by a Biochrom 30plus Amino Analyzer (Biochrom Ltd., Cambridge, UK, EU), a cation-exchange chromatography system (Mondino et al. 1972). In brief, aa were purified mixing the plasma samples 1:1 v/v with 10 % sulfosalicylic acid (Sigma Aldrich Corp., St. Louis, MO, USA) containing the internal standard norleucine 500 μmol/L (Sigma Aldrich Corp.) and adding 2 volumes of Lithium Citrate Loading Buffer pH 2.20 (Mondino et al. 1972). After strong agitation and cooling at 4 °C for 5 min, the mixtures were centrifuged 8 min at 14,000 rpm. Supernatants were filtered by syringe filter PVDF membranes (Carlo Erba Reagenti S.r.l., Rodano, I, EU) and 100 μL were the operative injection volumes. Postcolumn derivatization with ninhydrin allowed the detection of aa at the wavelength of 570 nm, while 440 nm for Pro. Standard Lithium Citrate buffers with pH 2.80, 3.00, 3.15, 3.50, and 3.55 and ninhydrin reagents utilized during separation were provided ready to use by Biochrom Ltd.
One hundred-twenty five μmol/L aa standard solution was prepared by mixing physiological basis with acids and neutrals and internal standard solution (Sigma Aldrich Corp.).
Data analysis was performed by EZChrome software (Agilent Technologies, Santa Clara, CA, USA). Areas of the peaks were used to determine aa concentrations and they were expressed in terms of μmol/L.
Statistical analysis
Data about urinary aa concentrations are presented corrected on creatinine excretion. Statistical analysis was performed with GraphPad Prism v6.0 software (GraphPad Software Inc., La Jolla, CA, USA). Based on the guidelines from Simundic (Simundic 2012), due to the small sample size, all the data have been presented and treated as not normally distributed. Thus, in the descriptive analysis, all values are expressed as median (range). Friedman’s test, with Dunn’s post hoc analysis, was applied to compare data over time. Correlation analysis was based on the two-tailed Spearman’s test, which was also used to analyze the correlation between the patterns of these parameters across time points. The level of significance was set at p < 0.05.
Results
Anthropometry and physical effort
Weight and BMI were not significantly modified throughout the race, while the efforts spent over the two half of the race differed. Particularly, while both the mean PO/m and the mean NEE were significantly increased, the PO/m and NEE accumulated over the first half were significantly greater than those accumulated in the second part (p < 0.01 for all comparisons). Data are summarized in Table 2.
Muscle activity and kidney function
Data about LDH, AST, and CK activities, corrected on plasma volume changes, in this population have been previously published (Grasso et al. 2015). Briefly, consequently to hemodilution, the only CK was significantly increased over the race. These data are reported in Table 3.
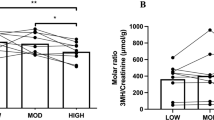
BUN (Fig. 1) was significantly increased from 18.6 (15.3–26.3) at T0, to 21.5 (19.0–29.2) at T1 (p < 0.001) and 25.2 (21.2–37.2) to T2 (p < 0.001 vs. T0 and p = 0.019 vs. T1).
Despite a significant increase in sCr between T1 and T2 (p = 0.015), kidney function, in terms of eGFR, did not show any significant change neither raw nor corrected on BSA (Table 4).
Urinary amino acids concentration
Beside the significant increase in uCr (Fig. 2), clearly reflecting the parallel increase in sCr, there were decreases in Tau, from T0 to T1 and T2, in Gly, from T0 to T2, in Cys, from T0 to T2 and from T1 to T2, Leu, from T1 to T2, and in Lys from T0 to T1 and T2. Carn was reduced of one-tenth from the value recorded at T0 and T1 to T2. 1-MH showed a one-third increase from T0 to T1 and then decreased to a one-third of the basal value at T2, while 3-MH decreased constantly, with the significance reach in comparison T0 vs. T2. Among the tested aa, only Hey showed a net significant increase from T0 to T1 and T2. The summary of the changes in urinary concentrations of the entire aa panel is illustrated in Fig. 3.
Among the significant correlation found (Table 5), here we outline that Tau modifications over the race are good to strongly inversely related to BUN, mean and accumulated PO, and mean accumulated NEE. Ala shows a direct correlation with both AST and BUN. Carn was directly related with BUN and mean PO and NEE, while Hey was fairly correlated with mean PO and NEE. The correlation summary is reported in Table 5.
Discussion
In the present study, we show that over 3 weeks of continuous endurance performance, along with an increase in CK, BUN, and uCr, the urinary profile of aa is substantially modified as a direct consequence of the metabolic effort. Particularly, urinary concentrations of Tau, Gly, Cys, 1-, 3-MH are decreased, while those of Carn are increased, despite a unchanged kidney function (Colombini et al. 2012a, b).
The strong muscular effort, marked by the steep increase in CK serum activity (Colombini et al. 2012b) and in BUN (Mashiko et al. 2004), and the metabolic effort, indexed by PO and NEE, show correlations, with the urinary aa concentrations.
Fatigue could be considered a symptom for muscular overuse and damage (Nedelec et al. 2012) and it represents an important determinant of the performance outcome. The availability of a tool, e.g., a marker, able to mark, or even to predict, the fatigue could be helpful in the monitoring of athletes during training and competitions. On the other hand, the matrix in which this ideal markers should be tested might be easily collected (i.e., not invasive sampling), in order to allow serial measurements, and easily assayable. Noteworthy, changes of these putative markers should be carefully interpreted. Indeed, these changes, other than the acute consequence of the exercise, also represent the specific result of an adaptation to homeostatic demands (Sanchis-Gomar and Lippi 2014).
Possible markers in fatigue monitoring could be represented by aa. Indeed, as stated above, heavy muscle activity is associated with muscle fiber injury. This is translated in muscle protein catabolism and, consequently, in the release of the protein catabolic products into the bloodstream (Colombini et al. 2012b; Grasso et al. 2015; Nagasawa et al. 1998; Tiao et al. 1997). As previously shown, changes in aa concentration in blood depend on exercise type, intensity, and duration with short-term exercise increasing concentrations of most aa and prolonged exercise causing a general decrease in aa concentration with a direct correlation with central fatigue (Blomstrand 2001). This latter phenomenon is linked to the fact that some aa are precursors of neurotransmitters (Blomstrand 2001).
Urine is, surely, a preferable matrix for monitoring tools testing, since for most analytes, the kidney physiology is well known. Indeed, for what concern aa, the amount released in urine represents a very small fraction of the circulating one but it reflects the situation in plasma, since the current knowledge suggests that physical activity has no effects on intra-renal aa metabolism (Garibotto et al. 2010; Makrides et al. 2014; Pinto et al. 2013).
We and other authors have previously used/proposed other testing matrices to assay metabolites related to metabolic efforts and fatigue in athletes. Particularly, an emerging matrix is represented by saliva which is somehow easier accessible than urine (Grasso et al. 2015; Ra et al. 2014). Recently, Ra and colleagues have tested, among other metabolites, salivary aa, pointing out their possible usefulness in monitoring fatigue (Ra et al. 2014).
Although very interesting as a sample matrix compared to urine, saliva use is still limited by the few knowledge about the saliva physiology, availability of saliva-specific assays, and the number of conditions affecting the ultrafiltration of the different metabolites in saliva (Gatti and De Palo 2011; Pfaffe et al. 2011).
In the present study, despite the stability of most of the aa tested, the recorded variation is of great importance in terms of metabolic response to the effort.
Carnosine, which is increased over the race, is a cytoplasmic dipeptide synthesized from β-alanine and histidine present at highest concentrations in skeletal muscles (Sale et al. 2013) where its main function is to activate myosin ATPase, along with its role as an intramuscular buffer (10–20 % of the total buffering capacity in type I and II muscle fibers) and reactive oxygen species (ROS) scavenger (Sale et al. 2013; Tiedje et al. 2010). Although there are several evidences about its involvement in olfaction, neuroprotection, and neurogenesis, there is no full support, instead, to the role of Carn as neurotransmitter (Bonfanti et al. 1999). To our knowledge, there are no published studies investigating the changes in plasma or urine concentrations of Carn following exercise. However, in equines, it has been demonstrated that, while acute exercise does not induce muscular Carn loss, a raise in its plasma concentrations follows exercise-induced muscle damage (Harris et al. 2012). Thus, whether the role as a putative marker of fatigue should be demonstrated, it is clear that its increase during a performance, such as represents a sign of muscular damage.
Taurine is a sulfur-containing aa (2-aminoethane sulfonic acid) abundant in several cell types and tissues, although it is highly present in skeletal and myocardial muscles. Characteristically, it is not incorporated into proteins but it directly intervenes in many and essential cell processes (development, signaling, membrane stability, Ca2+-dependent contraction, oxidants scavenger, membrane depolarization) as well as in whole body homeostasis (glucose tolerance, insulin sensitivity) (Spriet and Whitfield 2015). Rodents studies demonstrated the essential role of Tau in contractile function of the skeletal muscle; however, human studies did not supported these findings (Spriet and Whitfield 2015). Metabolomics analysis of saliva in soccer players, after 3 consecutive days of matches, showed an increase in Tau (Ra et al. 2014). These data are in line with the trend previously observed by Cuisinier and colleagues who found a tendency to increase (without achieving the significance) for urinary Tau immediately and after 24 h after a marathon (Cuisinier et al. 2001). This aa-like compound is released in blood from contracting muscles in order to scavenge ROS (Ortenblad et al. 2003). In our long-term observation, instead, urinary Tau concentrations are reduced of two-third at the end of the race and, interestingly, the high inter-individual variability observed at baseline is considerably reduced after 12 days and even more at 23 days indicating a tendency of the intervention (the physical effort) to make homogenous the homeostatic response. This decline could be intended as a depletion of body Tau which could mark an impairment of skeletal muscle function as demonstrated by the correlation with muscle activity/damage markers we tested but, on the other hand, it could also be linked to an impairment of the psychological feelings of athletes, since Tau is involved in the endogenous anti-depressant cerebral loops (Iio et al. 2012). In this view, urinary Tau can be a possible marker for both muscle and central fatigue.
Although significant, changes in glycine, in our study, are very limited. Gly is highly present in collagen molecules and it is also a precursor of porphyrins and purines. Moreover, a role of Gly as antioxidant, within the folate recycle, is known (Amelio et al. 2014). Thus, despite, the lack of knowledge about its metabolism in physical exercise, if excluded is role as excitatory neurotransmitter (Harvey and Yee 2013), the slight decline we observed could be ascribed to the effort. The same situation, for what its concern behavior over the race and the link to the ROS scavenging, can be depicted for cysteine.
While no information are available about the metabolism of 1-methyl histidine in exercise, urinary 3-methyl histidine is considered a direct index of muscle protein breakdown (Nagasawa et al. 1998; Tiao et al. 1997). We observed a decrease in 3-MH, throughout our observation, but it not correlates with any of the muscle activity/damage markers. Based on the previous data, the modifications in 3-MH we have recorded could mark a shift of protein metabolism toward anabolism to satisfy the really high requirements, despite a generalized muscular damage.
Diet is one of the main determinants of plasma, and thus urinary, concentrations of aa. Modifications in dietary regimen cause changes in the circulating aa profile (Forslund et al. 2000). In the present context, however, the nutrient intake through diet has been kept, practically, unchanged along the race and the adhesion to diet has been strikingly checked. Thus, the modifications observed during the study are due to the physical activity performed.
In conclusion, with the present study, we have shown that following a long-term endurance activity, in highly selected and homogenous professional athletes, the metabolic profile is modified strikingly due to the effort. The evaluation of muscular activity and damage throughout the classical muscle markers, and particularly CK, cannot explain the overall integrated homeostatic response of the muscle. Here we have shown that due to their physiological roles, the monitoring of both taurine and carnosine, and particularly in urine as an easily accessible matrix for serial evaluation, represents a useful tool to evaluate the muscle damage and possibly the fatigue status on a long-term basis.
References
Amelio I, Cutruzzola F, Antonov A, Agostini M, Melino G (2014) Serine and glycine metabolism in cancer. Trends Biochem Sci 39:191–198. doi:10.1016/j.tibs.2014.02.004
Banfi G, Dolci A (2003) Preanalytical phase of sport biochemistry and haematology. J Sports Med Phys Fitness 43:223–230
Blomstrand E (2001) Amino acids and central fatigue. Amino Acids 20:25–34
Bonfanti L, Peretto P, De Marchis S, Fasolo A (1999) Carnosine-related dipeptides in the mammalian brain. Progr Neurobiol 59:333–353
Colombini A, Corsetti R, Graziani R, Lombardi G, Lanteri P, Banfi G (2012a) Evaluation of creatinine, cystatin C and eGFR by different equations in professional cyclists during the Giro d’Italia 3-weeks stage race. Scand J Clin Lab Invest 72:114–120. doi:10.3109/00365513.2011.642305
Colombini A, Corsetti R, Machado M, Graziani R, Lombardi G, Lanteri P, Banfi G (2012b) Serum creatine kinase activity and its relationship with renal function indices in professional cyclists during the Giro d’Italia 3-week stage race. Clin J Sports Med 22:408–413. doi:10.1097/JSM.0b013e31825e66cc
Corsetti R, Lombardi G, Lanteri P, Colombini A, Graziani R, Banfi G (2012) Haematological and iron metabolism parameters in professional cyclists during the Giro d’Italia 3-weeks stage race. Clin Chem Lab Med 50:949–956
Cuisinier C, Ward RJ, Francaux M, Sturbois X, de Witte P (2001) Changes in plasma and urinary taurine and amino acids in runners immediately and 24 h after a marathon. Amino Acids 20:13–23
DeBerardinis RJ, Cheng T (2010) Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 29:313–324. doi:10.1038/onc.2009.358
Forslund AH et al (2000) Inverse relationship between protein intake and plasma free amino acids in healthy men at physical exercise. Am J Physiol Endocrinol Metab 278:E857–E867
Garibotto G, Sofia A, Saffioti S, Bonanni A, Mannucci I, Verzola D (2010) Amino acid and protein metabolism in the human kidney and in patients with chronic kidney disease. Clin Nutr 29:424–433. doi:10.1016/j.clnu.2010.02.005
Gatti R, De Palo EF (2011) An update: salivary hormones and physical exercise. Scand J Med Sci Sports 21:157–169. doi:10.1111/j.1600-0838.2010.01252.x
Grasso D et al (2015) Bone-muscle unit activity, salivary steroid hormones profile, and physical effort over a 3-week stage race. Scand J Med Sci Sports 25:70–80
Halson SL (2014) Monitoring training load to understand fatigue in athletes. Sports Med 44:S139–S147. doi:10.1007/s40279-014-0253-z
Harris RC, Wise JA, Price KA, Kim HJ, Kim CK, Sale C (2012) Determinants of muscle carnosine content Amino Acids 43:5–12. doi:10.1007/s00726-012-1233-y
Harvey RJ, Yee BK (2013) Glycine transporters as novel therapeutic targets in schizophrenia, alcohol dependence and pain. Nat Rev Drug Discov 12:866–885. doi:10.1038/nrd3893
Iio W, Matsukawa N, Tsukahara T, Toyoda A (2012) The effects of oral taurine administration on behavior and hippocampal signal transduction in rats. Amino Acids 43:2037–2046. doi:10.1007/s00726-012-1282-2
Lamb EJ, Tomson CR, Roderick PJ (2005) Estimating kidney function in adults using formulae. Ann Clin Biochem 42:321–345. doi:10.1258/0004563054889936
Makrides V, Camargo SM, Verrey F (2014) Transport of amino acids in the kidney. Compr Physiol 4:367–403. doi:10.1002/cphy.c130028
Mashiko T, Umeda T, Nakaji S, Sugawara K (2004) Effects of exercise on the physical condition of college rugby players during summer training camp. Br J Sports Med 38:186–190
Meeusen R (2014) Exercise, nutrition and the brain. Sports Med 44:S47–S56. doi:10.1007/s40279-014-0150-5
Mondino A, Bongiovanni G, Fumero S, Rossi L (1972) An improved method of plasma deproteination with sulphosalicylic acid for determining amino acids and related compounds. J Chromatogr 74:255–263
Nagasawa T, Hirano J, Yoshizawa F, Nishizawa N (1998) Myofibrillar protein catabolism is rapidly suppressed following protein feeding. Biosci Biotechnol Biochem 62:1932–1937. doi:10.1271/bbb.62.1932
Nedelec M, McCall A, Carling C, Legall F, Berthoin S, Dupont G (2012) Recovery in soccer: part I—post-match fatigue and time course of recovery. Sports Med 42:997–1015. doi:10.2165/11635270-000000000-00000
Ortenblad N, Young JF, Oksbjerg N, Nielsen JH, Lambert IH (2003) Reactive oxygen species are important mediators of taurine release from skeletal muscle cells. Am J Physiol Cell Physiol 284:C1362–C1373. doi:10.1152/ajpcell.00287.2002
Pfaffe T, Cooper-White J, Beyerlein P, Kostner K, Punyadeera C (2011) Diagnostic potential of saliva: current state and future applications. Clin Chem 57:675–687. doi:10.1373/clinchem.2010.153767
Pinto V, Pinho MJ, Soares-da-Silva P (2013) Renal amino acid transport systems and essential hypertension. FASEB J 27:2927–2938. doi:10.1096/fj.12-224998
Ra SG, Maeda S, Higashino R, Imai T, Miyakawa S (2014) Metabolomics of salivary fatigue markers in soccer players after consecutive games. Appl Physiol Nutr Metab 39:1120–1126. doi:10.1139/apnm-2013-0546
Sale C, Artioli GG, Gualano B, Saunders B, Hobson RM, Harris RC (2013) Carnosine: from exercise performance to health. Amino Acids 44:1477–1491. doi:10.1007/s00726-013-1476-2
Sanchis-Gomar F, Lippi G (2014) Physical activity—an important preanalytical variable. Biochem Med 24:68–79. doi:10.11613/BM.2014.009
Simundic AM (2012) Practical recommendations for statistical analysis and data presentation in Biochemia Medica journal. Biochem Med 22:15–23
Spriet LL, Whitfield J (2015) Taurine and skeletal muscle function. Curr Opin Clin Nutr Metab Care 18:96–101. doi:10.1097/MCO.0000000000000135
Tiao G, Hobler S, Wang JJ, Meyer TA, Luchette FA, Fischer JE, Hasselgren PO (1997) Sepsis is associated with increased mRNAs of the ubiquitin-proteasome proteolytic pathway in human skeletal muscle. J Clin Invest 99:163–168. doi:10.1172/JCI119143
Tiedje KE, Stevens K, Barnes S, Weaver DF (2010) Beta-alanine as a small molecule neurotransmitter. Neurochem Int 57:177–188. doi:10.1016/j.neuint.2010.06.001
UCI (2012) Union Cycliste Internationale. UCI Anti-doping procedural guidelines 7.0. Storage and Transport of samples. http://www.uci.ch/Modules/BUILTIN/getObject.asp?MenuId=MTI1NzE&ObjTypeCode=FILE&type=FILE&id=Nzc2ODE&LangId=1. Accessed February 2012
WADA (2011) World Anti-doping Agency. Requirements for passport operation from international standard for testing and international standard for laboratories. http://www.wada-ama.org/Documents/Resources/Guidelines/WADA_ABP_OperatingGuidelines_EN_2.1.pdf. Accessed January 2012
Acknowledgments
The authors are indebted with Mr. Massimiliano Casavecchia who helped in data storage and handling and with the Cannondale pro-cycling team for availability and logistics. This study has been partially supported by the ITALIAN MINISTRY OF HEALTH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling Editor: E. Rawson.
Rights and permissions
About this article
Cite this article
Corsetti, R., Barassi, A., Perego, S. et al. Changes in urinary amino acids excretion in relationship with muscle activity markers over a professional cycling stage race: in search of fatigue markers. Amino Acids 48, 183–192 (2016). https://doi.org/10.1007/s00726-015-2077-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-2077-z